Abstract
Importance
Breast magnetic resonance imaging (MRI) is increasingly used for breast cancer screening, diagnostic evaluation, and surveillance However, we lack data on national patterns of breast MRI use in community practice.
Objective
To describe 2005–2009 patterns of breast magnetic resonance imaging (MRI) use in U.S. community practice.
Design
Observational cohort study
Setting
Data collected from 2005–2009 on breast MRI and mammography from five national Breast Cancer Surveillance Consortium registries.
Participants
Data included 8931 breast MRI examinations and 1,288,924 screening mammograms from women aged 18–79 years.
Main measures
We calculated the rate of breast MRI examinations per 1000 women with breast imaging within the same year and described the clinical indications for the breast MRI examinations by year and age. We compared women screened with breast MRI to women screened with mammography alone for patient characteristics and lifetime breast cancer risk.
Results
The overall rate of breast MRI from 2005 through 2009 nearly tripled from 4.2 to 11.5 examinations per 1000 women with the most rapid rise from 2005–2007 (p=0.02). The most common clinical indication was diagnostic evaluation (40.3%), followed by screening (31.7%). Compared to women who received screening mammography alone, women who underwent screening breast MRI were more likely to be <50 years, white non-Hispanic, nulliparous, and have extremely dense breast tissue, a family history of breast cancer, and a personal history of breast cancer. The proportion of women screened by breast MRI at high lifetime risk for breast cancer (>20%) increased during the study period from 9% in 2005 to 29% in 2009.
Conclusions and relevance
Use of breast MRI for screening in high-risk women is increasing. However, our findings suggest there is a need to improve appropriate utilization, including among women who may benefit from screening breast MRI.
INTRODUCTION
In 2012, women in the United States underwent nearly 39 million mammograms.1,2 However, breast magnetic resonance imaging (MRI) is increasingly used for breast cancer screening, diagnostic evaluation, and surveillance.3,4 Mammography remains the key imaging tool for population-based screening5 and for work-up of women experiencing breast symptoms,6 but breast MRI is becoming more common in community settings.3,7,8 The benefit of breast MRI includes high sensitivity for identifying clinically occult breast malignancy.6 However, compared to mammography, breast MRI has a modest specificity that leads to higher false positive rates;9,10 it is also more expensive and requires intravenous contrast medium.
National guidelines support breast MRI for particular clinical indications. The most widely accepted guideline, from the American Cancer Society (ACS) in 2007, is to screen asymptomatic women at high risk for breast cancer defined as: 1) known BRCA gene mutation carriers; 2) first-degree relatives of a known BRCA gene mutation carrier who are themselves untested; or 3) women with >20% lifetime risk of breast cancer, according to risk assessment tools based on family history of breast cancer.11,12 The ACS lacked sufficient evidence to make recommendations for women in other risk subgroups. The National Comprehensive Cancer Network (NCCN) recommends considering the use of pre-operative breast MRI for women with a new breast cancer diagnosis to determine the extent of disease before surgery in occult tumors, although there is not a broad consensus.6
Despite the rapid expansion of breast MRI in different settings and for multiple clinical applications, most published reports on its use are from single institutions3,7 and focus only upon specific populations.7,8 We lack data on national patterns of breast MRI use in community practice.
Our purpose was to evaluate patterns of breast MRI use among community-based facilities across the U.S.. Using data from 2005 through 2009 from five registries in the Breast Cancer Surveillance Consortium (BCSC),13 we evaluated rates and distributions of clinical indications for breast MRI temporally and by age. We also compared characteristics of women screened with breast MRI to women who were screened with mammography alone.
METHODS
Study registries
The BCSC is a collaboration of breast imaging registries in community-based settings with linkages to tumor and/or pathology registries. The BCSC is supported by a Statistical Coordinating Center (SCC). The goals of the BCSC are to assess the delivery and quality of U.S. breast cancer imaging and patient outcomes. This study used data from five registries: Carolina Mammography Registry, Group Health Cooperative (Washington State), New Hampshire Mammography Network, San Francisco Mammography Registry, and Vermont Breast Cancer Surveillance System.13 The data from these five registries reflect mammography practice as it is performed in the community and are located in counties that contain slightly more than 5% of the nation's population.14 Each registry and the SCC received institutional review board approval for either active consent or passive permission or a waiver of consent to enroll participants, link study data, and perform analytic studies. All procedures are Health Insurance Portability and Accountability Act compliant. All registries and the SCC have received a Federal Certificate of Confidentiality and other protection for the identities of women, physicians, and facilities that are subjects of this research.
BCSC data are collected as part of routine clinical care at the time of imaging from patients and radiologists. Mammography data include indication for the mammogram and American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) assessment and breast density.15 Each registry site sends their data to the SCC for pooling and statistical analysis. All data undergo rigorous quality control checks.
Breast MRI data
We used data on breast MRI conducted in 2005–2009 on women aged 18–79 years old. Data were obtained from registries retrospectively and prospectively using electronic radiology data systems (64%), chart abstraction of radiology reports (20%), completion of advanced imaging form at time exam (16%), and billing codes (0.02%). For each examination, standardized information was recorded including the clinical indication for the breast MRI, BI-RADS assessment, and clinical recommendations (e.g., routine mammographic follow-up, follow-up breast MRI in 6 months, or biopsy). To evaluate potential bias due to incomplete data capture from BCSC facilities, the registries surveyed BCSC facilities to estimate data capture for breast MRI examinations. Facilities estimated completeness of recording breast MRI data as none, <50%, 50–89%, or ≥90%.
The majority of examinations were coded with a single indication (89%). In the 11% of breast MRI examinations with >1 indication (up to 3 could be coded), we categorized clinical indication using the following hierarchy to isolate true screening breast MRI examinations in asymptomatic women: 1) evaluation of extent of disease in patients with a recent breast cancer diagnosis; 2) evaluation of response to neoadjuvant chemotherapy; 3) axillary adenopathy (malignant) of unknown primary; 4) additional evaluation of recent mammogram or other non-MRI breast imaging; 5) evaluation of breast problem (i.e., symptomatic); 6) recurrence vs. scar; 7) short interval follow-up of prior breast MRI examination; 8) screening (i.e., asymptomatic); 9) evaluation of implants; and 10) other. For example, if an examination was coded as additional evaluation of a recent mammogram and screening, the indication was coded as additional evaluation of a recent mammogram. For all examinations, primary indications were combined into four broader groups: cancer staging/treatment (groups 1–3), diagnosis (groups 4–6), screening including surveillance (group 8), and other (groups 7, 9–10).
Patient characteristics
At the time of mammography, women completed a questionnaire to ascertain age, race/ethnicity, first-degree family history of breast cancer, history of breast procedures, and other risk factors. For breast MRI examinations, women typically did not complete an additional patient questionnaire. Therefore, we linked the breast MRI examination to the most recent patient questionnaire completed at a mammography visit within the prior 12 months (median number of days=87).
History of prior mammography within 12 months was calculated based on mammography receipt within BCSC data. A personal history of breast cancer was documented through either self-report or linkage with BCSC pathology and cancer registry data. BI-RADS breast density was obtained from the most recent mammogram. If breast density measures were missing from the most recent mammogram, the next mammogram within 18 months informed the missing breast density measure and no change in hormone therapy use and incident breast cancer diagnosis. Finally, for each woman without a personal history of breast cancer, we calculated lifetime breast cancer risk based on the National Cancer Institute Breast Cancer Risk Assessment Tool (BCRAT; http://www.cancer.gov/bcrisktool/).16–20 The BCRAT includes age, race, previous breast biopsies, presence of atypia, age at menarche, age at first live birth, and history of breast cancer in first-degree relatives. Risk was categorized as <15%, 15–20%, and >20%, to correspond to cutoffs in the ACS guidelines for breast screening with MRI.11
Statistical Analysis
We identified 9537 breast MRI examinations in 2005–2009. We excluded 606 (6.4%) examinations with missing data on clinical indication. The final sample was 8931 breast MRI examinations from 6777 individual women (range 1–13 exams per woman). About 92% of the breast MRI data are from facilities with >90 data capture. We calculated the total number of breast MRI examinations by year and the distribution by clinical indication, year of exam, and 10-year age groups. We calculated annual breast MRI rates as the number of breast MRI examinations per 1000 women with any breast imaging within the same year from facilities reporting >90% data capture of breast MRI examinations. Rates were calculated for overall breast MRI use and according to clinical indication. We used a linear regression model to test for trends in the rates of breast MRI use over time by 2005–2007 and 2007–2009.
We compared patient characteristics, combinations of characteristics and BCRAT lifetime risk scores among women screened with breast MRI (n=2,831 examinations) to women screened with mammography alone (n=1,288,924 examinations). We present the distribution of patient characteristics by all women and restricted to women with no prior breast cancer history. Differences in patient characteristics were calculated using a chi-squared test. In a sensitivity analysis, we restricted imaging data to facilities reporting >90% data capture of breast MRI examinations.. As the results were similar, we present the overall findings for all imaging examinations rather than the restricted sample. All analyses were performed using SAS® Version 9.2 (SAS Institute, Cary, NC), and two-sided p<0.05 was considered statistically significant.
Results
Patterns of breast MRI use, 2005–2009
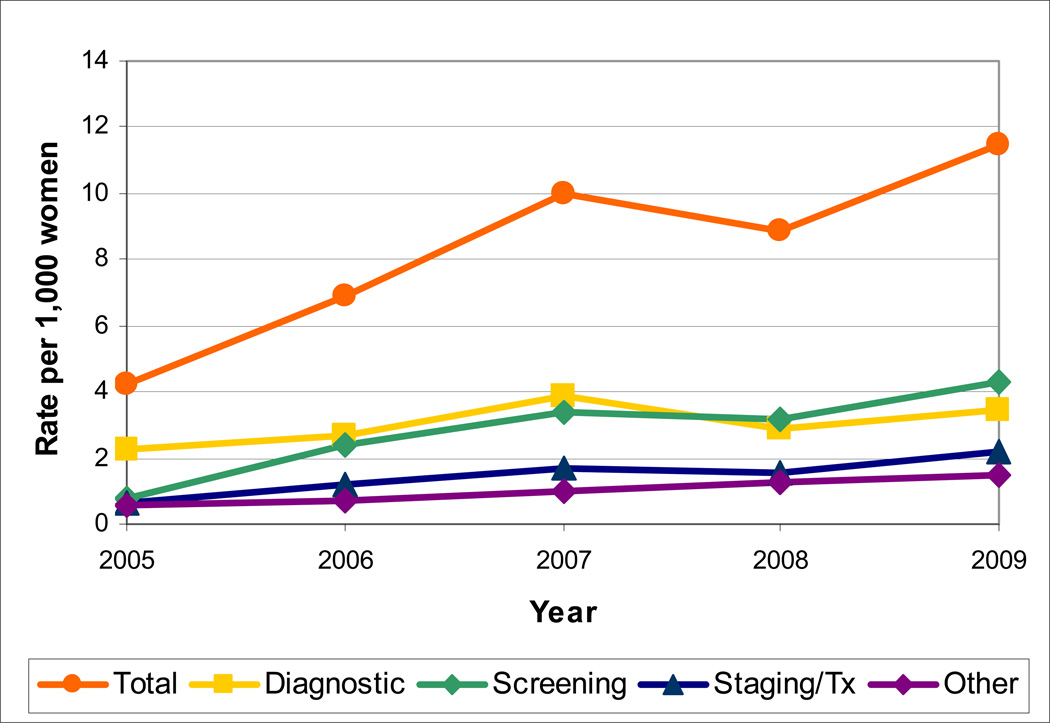
From 2005 through 2009, the rate of breast MRI use increased from 4.2 to 11.5 examinations per 1000 women (Figure 1). The steepest rise in use occurred from 2005–2007 with an increase of 5.8 MRI examinations per 1000 women per year (p=0.02) compared to only 1.5 additional examinations per year from 2007–2009 (p=0.45). Through 2007, the most common use of breast MRI was diagnostic work-up for a positive finding. The rate of screening breast MRI increased more than four-fold between 2005 and 2007 from 0.8 to 3.4 per 1000 women, and then remained fairly stable at 4.3 breast MRI examinations per 1000 women in 2009.
FIGURE 1.
Temporal trends in breast MRI rates per 1000 women overall and by clinical indication (from the Breast Cancer Surveillance Consortium, 2005–2009).
The total number of breast MRI examinations per year increased from 863 in 2005 to 2264 in 2007. From 2007–2009, the number of exams per year remained stable at approximately 2150 (Table 1). Across the five-year study period, the most common indications were diagnostic work-up of a non-MRI or clinical finding (40.3%), screening (31.7%), cancer staging/treatment (16.3%) and other (11.8%).
TABLE 1.
Clinical indications for breast MR imaging examination by year, 2005 through 2009.
| Year |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | Total | |||||||
| Indication for exam | N | % | N | % | N | % | N | % | N | % | N | % |
| DIAGNOSTIC | 459 | (53.2) | 581 | (39.5) | 938 | (41.4) | 871 | (39.8) | 747 | (34.8) | 3596 | (40.3) |
| Additional evaluation | 417 | (48.3) | 495 | (33.6) | 756 | (33.4) | 627 | (28.7) | 575 | (26.8) | 2870 | (32.1) |
| Evaluation of breast problem | 35 | (4.1) | 78 | (5.3) | 156 | (6.9) | 214 | (9.8) | 122 | (5.7) | 605 | (6.8) |
| Recurrence vs. scar | 7 | (0.8) | 8 | (0.5) | 26 | (1.1) | 30 | (1.4) | 50 | (2.3) | 121 | (1.4) |
| SCREENING | 156 | (18.1) | 494 | (33.6) | 742 | (32.8) | 699 | (32.0) | 740 | (34.5) | 2831 | (31.7) |
| BREAST CANCER STAGING/TREATMENT | 128 | (14.8) | 254 | (17.3) | 359 | (15.8) | 321 | (14.7) | 389 | (18.2) | 1451 | (16.3) |
| Evaluation of extent of disease in recent breast cancer diagnosis | 121 | (14.0) | 241 | (16.4) | 340 | (15.0) | 310 | (14.2) | 364 | (17.0) | 1376 | (15.4) |
| Evaluation of response to neoadjuvant chemotherapy | 4 | (0.5) | 9 | (0.6) | 14 | (0.6) | 9 | (0.4) | 25 | (1.2) | 61 | (0.7) |
| Axillary adenopathy (malignant), unknown primary | 3 | (0.3) | 4 | (0.3) | 5 | (0.2) | 2 | (0.1) | 0 | (0.0) | 14 | (0.2) |
| OTHER | 120 | (13.9) | 143 | (9.7) | 225 | (9.9) | 295 | (13.4) | 270 | (12.6) | 1053 | (11.8) |
| Other | 88 | (10.2) | 82 | (5.6) | 146 | (6.4) | 156 | (7.1) | 96 | (4.5) | 568 | (6.4) |
| Short interval follow-up of prior MR | 20 | (2.3) | 49 | (3.3) | 67 | (3.0) | 134 | (6.1) | 158 | (7.4) | 428 | (4.8) |
| Evaluation of implants | 12 | (1.4) | 12 | (0.8) | 12 | (0.5) | 5 | (0.2) | 16 | (0.7) | 57 | (0.6) |
| TOTAL | 863 | (100.0) | 1,472 | (100.0) | 2,264 | (100.0) | 2,186 | (100.0) | 2,146 | (100.0) | 8,931 | (100.0) |
The proportion of examinations with an indication of diagnostic work-up decreased from 53.2% in 2005 to 34.8% in 2009. The single most common indication contributing to the diagnostic work-up classification was evaluation of a prior non-MRI finding. The proportion of examinations attributed to screening increased from <20% in 2005 to 34.5% in 2009. Evaluation for cancer staging and treatment, primarily among newly diagnosed women, remained fairly constant over the study period at 15–18% of all examinations.
Across all ages, use of breast MRI for diagnostic purposes was the most common indication, with the highest proportion in the oldest women in our study (70–79 years) (Table 2). Screening accounted for 34.3% of MRI examinations in women <50 years compared to 17.8% in women aged 70–79 years. Proportions for other indications were similar across age groups.
TABLE 2.
Proportion of 2005–2009 breast MRI examinations by clinical indication and stratified by age.
| Total | ||
|---|---|---|
| N | (%) | |
| Age <40 | 940 | |
| Diagnostic | 370 | (39.4) |
| Screen | 322 | (34.3) |
| Staging/Treatment | 136 | (14.5) |
| Other | 112 | (11.9) |
| Age 40–49 | 2,655 | |
| Diagnostic | 1,026 | (38.6) |
| Screen | 924 | (34.8) |
| Staging/Treatment | 385 | (14.5) |
| Other | 320 | (12.1) |
| Age 50–59 | 2,943 | |
| Diagnostic | 1,123 | (38.2) |
| Screen | 968 | (32.9) |
| Staging/Treatment | 483 | (16.4) |
| Other | 369 | (12.5) |
| Age 60–69 | 1,742 | |
| Diagnostic | 756 | (43.4) |
| Screen | 501 | (28.8) |
| Staging/Treatment | 300 | (17.2) |
| Other | 185 | (10.6) |
| Age 70–79 | 651 | |
| Diagnostic | 321 | (49.3) |
| Screen | 116 | (17.8) |
| Staging/Treatment | 147 | (22.6) |
| Other | 67 | (10.3) |
Comparison of patient characteristics by screening breast MRI vs. mammography alone
Compared to women screened for breast cancer by mammography alone, women screened by breast MRI were significantly more likely to be younger (<50 years), white non-Hispanic, or nulliparous; they were more likely to have a first degree relative with breast cancer, prior breast biopsy, or extremely dense breast tissue (Table 3). Women who received screening breast MRI were also more likely to have a personal history of breast cancer (44.9%) compared to women who received mammography alone (4.7%) (p<0.0001). When we restricted our evaluation to women without a personal history of breast cancer, the distribution of characteristics did not substantially change with two exceptions. Among women without a personal history of breast cancer, screening breast MRI was used by significantly fewer women with a prior breast biopsy but significantly more women with a family history of breast cancer compared to women who received mammography alone.
TABLE 3.
Patient characteristics of women receiving breast cancer screening by breast MRI or mammography alone, 2005–2009.^
| Total | Women without prior breast cancer diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breast MRI | Mammography only | Breast MRI | Mammography only | |||||||
| N | (%) | N | (%) | p-value | N | (%) | N | (%) | p-value | |
| Total | 2,831 | 1,288,924 | 1,559 | 1,228,812 | ||||||
| Age group | <0.0001 | <0.0001 | ||||||||
| <40 | 322 | (11.4) | 30,940 | (2.4) | 254 | (16.3) | 30,426 | (2.5) | ||
| 40–49 | 924 | (32.6) | 361,998 | (28.1) | 585 | (37.5) | 356,708 | (29.0) | ||
| 50–59 | 968 | (34.2) | 435,526 | (33.8) | 490 | (31.4) | 419,104 | (34.1) | ||
| 60–69 | 501 | (17.7) | 296,764 | (23.0) | 195 | (12.5) | 276,086 | (22.1) | ||
| 70–79 | 116 | (4.1) | 163,696 | (12.7) | 35 | (2.2) | 146,488 | (11.9) | ||
| Race/ethnicity | <0.0001 | <0.0001 | ||||||||
| White, non-Hispanic | 2,347 | (85.3) | 936,313 | (75.0) | 1,293 | (85.9) | 888,808 | (74.7) | ||
| Black, non-Hispanic | 32 | (1.2) | 33,534 | (2.7) | 19 | (1.3) | 32,222 | (2.7) | ||
| Hispanic | 95 | (3.5) | 68,360 | (5.5) | 62 | (4.1) | 66,199 | (5.6) | ||
| Asian | 189 | (6.9) | 174,915 | (14.0) | 79 | (5.2) | 169,077 | (14.2) | ||
| Other* | 89 | (3.2) | 35,414 | (2.8) | 52 | (3.5) | 33,860 | (2.8) | ||
| Missing | 79 | 40,388 | 54 | 38,646 | ||||||
| Age at menarche | 0.18 | 0.85 | ||||||||
| <11 | 525 | (29.2) | 237,428 | (29.4) | 280 | (28.6) | 229,361 | (29.3) | ||
| 12–13 | 851 | (47.3) | 374,563 | (45.4) | 444 | (45.4) | 355,300 | (45.3) | ||
| ≥14 | 424 | (23.6) | 208,205 | (25.2) | 255 | (26.0) | 198,854 | (25.4) | ||
| Missing | 1,031 | 463,728 | 580 | 445,297 | ||||||
| Age at first birth | <0.0001 | <0.0001 | ||||||||
| Nulliparous | 656 | (33.4) | 237,681 | (25.6) | 337 | (32.9) | 227,018 | (25.7) | ||
| <20 | 144 | (7.3) | 139,005 | (14.9) | 76 | (7.4) | 131,818 | (14.9) | ||
| 20–24 | 309 | (15.7) | 217,071 | (23.3) | 157 | (15.3) | 204,374 | (23.1) | ||
| 25–29 | 347 | (17.7) | 138,948 | (14.9) | 181 | (17.7) | 131,450 | (14.9) | ||
| >30 | 510 | (25.9) | 197,379 | (21.2) | 272 | (26.6) | 189,081 | (21.4) | ||
| Missing | 865 | 358,840 | 536 | 345,071 | ||||||
| Mammography in prior 12 months | ||||||||||
| No | 449 | (15.9) | 237 | (15.2) | ||||||
| Yes | 2,382 | (84.1) | 1,322 | (84.8) | ||||||
| Personal history of breast cancer | ||||||||||
| No | 1,559 | (55.1) | 1,228,812 | (95.3) | <0.0001 | |||||
| Yes | 1,272 | (44.9) | 60,112 | (4.7) | ||||||
| First degree family history of breast cancer1 | <0.0001 | <0.0001 | ||||||||
| No | 1,078 | (47.8) | 953,510 | (82.6) | 376 | (29.6) | 912,974 | (83.0) | ||
| Yes | 1,177 | (52.2) | 201,325 | (17.4) | 895 | (70.4) | 187,641 | (17.0) | ||
| Missing | 576 | 134,089 | 288 | 128,197 | ||||||
| “Missing” for MRI mainly due to no self-reported patient information | ||||||||||
| Prior breast biopsy (not benign only, includes FNA) | <0.0001 | <0.0001 | ||||||||
| No | 906 | (32.0) | 984,808 | (76.4) | 1,017 | (65.2) | 981,500 | (79.9) | ||
| Yes | 1,925 | (68.0) | 304,116 | (23.6) | 542 | (34.8) | 247,312 | (20.1) | ||
| BI-RADS breast density1,2 | <0.0001 | <0.0001 | ||||||||
| Fatty | 90 | (5.0) | 106,888 | (11.5) | 66 | (5.9) | 101,453 | (11.5) | ||
| Scattered | 505 | (28.3) | 369,748 | (39.9) | 315 | (28.1) | 350,640 | (39.7) | ||
| Heterogeneous | 795 | (44.6) | 359,743 | (38.9) | 470 | (41.9) | 343,443 | (38.9) | ||
| Extremely dense | 394 | (22.1) | 89,247 | (9.6) | 271 | (24.2) | 86,759 | (9.8) | ||
| Missing | 1,047 | 363,298 | 437 | 346,517 | ||||||
| Combinations of risk factors | ||||||||||
| Positive family history and extreme breast density | ||||||||||
| 204 | (12.9) | 14,449 | (1.7) | <0.0001 | 166 | (16.8) | 13,927 | (1.7) | <0.0001 | |
| Prior breast biopsy and positive family history | ||||||||||
| 693 | (30.7) | 62,897 | (5.4) | <0.0001 | 415 | (32.7) | 49,739 | (4.5) | <0.0001 | |
| Prior breast biopsy and extreme breast density | ||||||||||
| 262 | (14.7) | 23,917 | (2.6) | <0.0001 | 141 | (12.6) | 21,544 | (2.4) | <0.0001 | |
| Positive family history, extreme breast density and prior breast biopsy | ||||||||||
| 122 | (7.7) | 4,834 | (0.6) | <0.0001 | 85 | (8.6) | 4,329 | (0.5) | <0.0001 | |
Restricted facilities are those with 90% or higher data capture of MRI exams.
Other race/ethnicity includes Native Hawaiian or other Pacific Islander, American Indian or Alaskan Native, mixed (>1) race, as well as race reported as other.
Missing information at time of MRI exam was filled in using information from the most recent mammogram in the prior 12 months.
Breast density was not collected at time of MRI. Missing data on breast density was filled in for both MRI and mammography from a mammogram within +/− 18 months if no change in hormone therapy use and no breast cancer diagnosis was noted.
Women with combinations of prior breast biopsy, positive family history, and extremely dense breast tissue were more likely to be screened by breast MRI. About one-third of all breast MRI for screening was in women with both a prior breast biopsy and positive family history (p<0.0001), regardless of personal history of breast cancer.
Among women who received screening breast MRI, 25% had a >20% lifetime risk of developing breast cancer according to the BCRAT model and 53% had <15% lifetime risk, compared to women who received mammography alone, where 2% had a lifetime risk of >20% and 92% had a lifetime risk of <15% (Table 4). The proportion of women at high risk for breast cancer who received breast MRI for screening increased from 9% in 2005 to 29% in 2009 (test for trend p<0.0001). No change by risk status over the study period was seen in women screened by mammography alone. Over the five year study period, 25,237 women who were screened with mammography alone had a >20% lifetime breast cancer risk.
TABLE 4.
Breast Cancer Risk Assessment Tool scores for lifetime breast cancer risk for women screened by breast MRI and women screened by mammography alone, 2005–2009.
| Year | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | Total | |||||||
| BRCAT lifetime risk score | N | % | N | % | N | % | N | % | N | % | N | % |
| Exams of women who received breast MRI for screening* | ||||||||||||
| <15% | 57 | (74) | 147 | (50) | 227 | (55) | 194 | (53) | 202 | (50) | 827 | (53) |
| 15–20% | 13 | (17) | 85 | (29) | 84 | (20) | 79 | (21) | 87 | (21) | 348 | (22) |
| >20% | 7 | (9) | 60 | (21) | 101 | (25) | 96 | (26) | 119 | (29) | 383 | (25) |
| Exams of women who received screening mammography only | ||||||||||||
| <15% | 234,209 | (92) | 229,157 | (92) | 228,708 | (92) | 222,685 | (92) | 216,402 | (92) | 1,131,161 | (92) |
| 15–20% | 15,120 | (6) | 14,480 | (6) | 14,590 | (6) | 14,560 | (6) | 13,657 | (6) | 72,407 | (6) |
| >20% | 5,153 | (2) | 5,110 | (2) | 5,033 | (2) | 5,021 | (2) | 4,920 | (2) | 25,237 | (2) |
Test for trend comparing total proportion at >20% high risk to <20% (p<0.0001)
DISCUSSION
Our study describes the patterns of breast MRI use in U.S. community practice, the setting in which most women receive breast imaging. Our results confirm a rise in the use of breast MRI from 2005–2007. Up to 2009, rates of use remained constant overall and across all clinical indications. Other reports demonstrated an increase in use of breast MRI3,7 without observing a plateau in utilization rates.
During our study period, the most common use of breast MRI was for diagnostic evaluation of a non-MRI finding. We could not determine if these examinations were performed to avoid biopsy of a suspicious clinical finding or to plan for a subsequent biopsy. Use of breast MRI to avoid biopsy does not have a sufficiently high negative predictive value to warrant its use for this purpose, particularly given the relatively low pretest probability of malignancy (∼2%) above which biopsy is performed as standard practice.21 Over the study period, the use of breast MRI for diagnostic work-up diminished. By 2009, the rate of breast MRI for diagnosis was similar to the rate for screening, which was the second most common indication overall.
We determined that several patient characteristics were associated with use of screening with breast MRI, which confirms findings by Miller et al22 suggesting increased use among women with a family history or personal history of breast cancer. We detected that younger, white women were more likely to receive breast MRI for screening, which differed from Miller et al who found that older, black women were more likely to receive breast MRI.22 Differences in our results might be explained by our examination of screening breast MRI compared to Miller’s attention to breast MRI for any clinical indications Also, women who had a prior breast biopsy and a positive family history were more likely to receive breast MRI than mammography alone, demonstrating two factors which influence patient care.
Of the women screened with breast MRI, 45% had a personal history of breast cancer. For the remaining 55%, we used the BCRAT model to determine whether breast MRI use reflected the national recommendations that women at high risk lifetime of breast cancer receive screening breast MRI as an adjunct to mammography. We determined that only 25% of women screened with breast MRI were considered high lifetime risk (>20%). The majority of women receiving screening breast MRI were at intermediate lifetime breast cancer risk (15–20%), for which evidence for breast MRI screening is insufficient, or average risk (<15%), for which screening breast MRI is not recommended. No evidence suggested that the overall rate of screening breast MRI increased after the ACS recommendation in 2007, although the proportion of women at high risk for breast cancer who received breast MRI for screening increased over the five-year study period. Overall, <5% of women with >20% lifetime risk received breast MRI for screening. Current patterns suggest there was improvement in clinical alignment with breast screening guidelines.
Several possibilities might explain why women with only an average lifetime breast cancer risk are screened by breast MRI. Some women with lifetime breast cancer risk scores <20% might have indications for screening breast MRI that were not captured in our data (e.g., BRCA mutation, first-degree relative with BRCA mutation, or prior chest radiation). However, these are unlikely to account for a substantial portion of the women at lower risk but undergoing screening MRI, as they are relatively rare in the general population. Another possible reason is misperception about breast cancer risk. Both women and physicians overestimate a woman’s lifetime risk of breast cancer,23,24 and inaccurate perceptions about risk can arise in the absence of clear risk counseling. Both younger and older women estimate their lifetime risk of breast cancer as approximately 18–20% higher than their actual risk,23 and women who are at average risk are more likely to overestimate their risk than high-risk women.24 Physicians might base their estimate of high-risk on report of family history alone without using a risk calculator.25 Also, women with dense breast might also be receiving breast MRI due to low sensitivity of screening mammography in dense breast tissue.26 Our data suggest increased use of breast screening MRI by combinations of risk factors, which are easily assessed by providers.
Also, clinically important were the women—about 25,200 in our study sample—who had a >20% lifetime risk of breast cancer but were screened by mammography alone. ACS and NCCN guidelines recommend breast MRI screening with screening mammography for women at high-risk for breast cancer. Brinton et al. documented that women with a high lifetime breast cancer risk do not fully adopt screening with breast MRI, and have a low overall rate of adherence to guidelines.27 Berg and colleagues evaluated reasons that high-risk women refuse breast MRI and found the most common were claustrophobia, time constraints, financial concerns, a physician who did not provide referral or believe MRI was warranted, and lack of patient interest.28 Additional issues could be availability of breast MRI outside of urban/academic areas or providers who do not assess breast cancer risk. Our study did not address why women received a particular type of imaging.
Strengths of our study are that it provides the only multi-facility evaluation of breast MRI utilization patterns across US community practice. Although data were from five BCSC registries from a variety of community-based practice facilities across the United States, there remain some limitations. First, women from BCSC registries might have different patient characteristics than women outside the BCSC; however, when we restricted to facilities with sufficient data capture, there were no differences in the distribution of patient characteristics in our sensitivity analysis. Second, our data are reported in aggregate and we did not evaluate geographic variation. Therefore, we could not evaluate disparities in access to care, which contribute to determining who receives advanced technologies.8 The reporting of indication for breast MRI examinations could differ across contributing registries, which could lead to heterogeneity in examination indication. To address this, we reviewed the indications for exams and created a hierarchy to identify true screening. Our study data included documented clinical indication, an improvement over studies using claims-based registries with no data on exam indication. Finally, we used the BCRAT model to estimate lifetime risk in our population. Although the ACS recommends classification with a familial-based model, this was not possible with our data. There are discrepancies in the reporting of lifetime risk of breast cancer as reported by Ozanne et al. who demonstrated inconsistency in three familial-based risk models that put women in different categories with differential overlap.29
Our rapidly changing healthcare environment demands that we frequently, accurately, and comprehensively evaluate the diffusion of new technology into community practice, including how often technology is being used and for what clinical indications Our findings suggest there have been improvements in appropriate use of breast MRI, with fewer exams performed for further evaluation of abnormal mammograms and symptomatic patients, and more breast MRI performed for high risk screening. We also identified a need for more appropriate risk-based use of breast MRI as a screening examination, as we identified women at average risk receiving MRI and women at high risk not receiving MRI. Our findings suggest there is a need for improvement in use of diagnostic and screening breast MRI for women most likely to benefit from this imaging tool.
ACKNOWLEDGEMENTS
Dr. Lehman has been a paid consultant to GE Healthcare, Bayer Healthcare, and Philips Healthcare. All other authors have no conflicts of interest to declare.
This work was supported by the National Cancer Institute-funded Breast Cancer Surveillance Consortium co-operative agreement (U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, U01CA70040) and the National Cancer Institute-funded grants RC2CA148577 and P01 CA154292. The collection of cancer data used in this study was supported in part by several state public health departments and cancer registries throughout the U.S. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html.
The design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at: http://breastscreening.cancer.gov/. In addition, Dr. Wernli was supported in part by the Agency for Healthcare Research and Quality (K12 HS019482).
Laura Ichikawa had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of interest disclosure: Dr. Lehman has been a paid consultant to GE Healthcare, Bayer Healthcare, and Philips Healthcare. All other authors have no conflicts of interest to disclose.
REFERENCES
- 1.U.S. Food and Drug Administration. [Accessed 10/9/2012];MQSA National Statistics. http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/FacilityScorecard/ucm113858.htm.
- 2.Howlader N, Noone AM, Krapcho M, et al. [Accessed May 12, 2012];SEER Cancer Statistics Review, 1975–2008, based on November 2010 SEER data submission, posted to the SEER web site, 2011. 2011 http://seer.cancer.gov/csr/1975_2008/
- 3.Stout NK, Nekhlyudov L. Early uptake of breast magnetic resonance imaging in a community-based medical practice, 2000–2004. J Womens Health (Larchmt) 2011 Apr;20(4):631–634. doi: 10.1089/jwh.2010.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmore L, Margenthaler JA. Breast MRI surveillance in women with prior curative-intent therapy for breast cancer. J Surg Res. 2010 Sep;163(1):58–62. doi: 10.1016/j.jss.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010 Mar-Apr;60(2):99–119. doi: 10.3322/caac.20063. [DOI] [PubMed] [Google Scholar]
- 6.Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw. 2009 Feb;7(2):193–201. doi: 10.6004/jnccn.2009.0013. [DOI] [PubMed] [Google Scholar]
- 7.Elmore L, Margenthaler JA. Use of breast MRI surveillance in women at high risk for breast cancer: a single-institutional experience. Ann Surg Oncol. 2010 Oct;17(Suppl 3):263–267. doi: 10.1245/s10434-010-1236-4. [DOI] [PubMed] [Google Scholar]
- 8.Sommer CA, Stitzenberg KB, Tolleson-Rinehart S, Carpenter WR, Carey TS. Breast MRI utilization in older patients with newly diagnosed breast cancer. J Surg Res. 2011 Sep;170(1):77–83. doi: 10.1016/j.jss.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kriege M, Brekelmans CT, Obdeijn IM, et al. Factors affecting sensitivity and specificity of screening mammography and MRI in women with an inherited risk for breast cancer. Breast Cancer Res Treat. 2006 Nov;100(1):109–119. doi: 10.1007/s10549-006-9230-z. [DOI] [PubMed] [Google Scholar]
- 10.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005 May 21–27;365(9473):1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 11.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007 Mar-Apr;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 12.Lehman CD, Smith RA. The role of MRI in breast cancer screening. J Natl Compr Canc Netw. 2009 Nov;7(10):1109–1115. doi: 10.6004/jnccn.2009.0072. [DOI] [PubMed] [Google Scholar]
- 13.Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol. 1997 Oct;169(4):1001–1008. doi: 10.2214/ajr.169.4.9308451. [DOI] [PubMed] [Google Scholar]
- 14.Breast Cancer Surveillance Consortium. BCSC: Working together to advance breast cancer research. http://breastscreening.cancer.gov/
- 15.American College of Radiology. American College of Radiology (ACR) Breast Imaging Reporting and Data System Atlas (BI-RADS® Atlas) Reston, VA: 2003. [Google Scholar]
- 16.Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999 Sep 15;91(18):1541–1548. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 17.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989 Dec 20;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 18.Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007 Dec 5;99(23):1782–1792. doi: 10.1093/jnci/djm223. [DOI] [PubMed] [Google Scholar]
- 19.Matsuno RK, Costantino JP, Ziegler RG, et al. Projecting individualized absolute invasive breast cancer risk in Asian and Pacific Islander American women. J Natl Cancer Inst. 2011 Jun 22;103(12):951–961. doi: 10.1093/jnci/djr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA, et al. Validation of the Gail model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001 Mar 7;93(5):358–366. doi: 10.1093/jnci/93.5.358. [DOI] [PubMed] [Google Scholar]
- 21.Bluemke DA, Gatsonis CA, Chen MH, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA. 2004 Dec 8;292(22):2735–2742. doi: 10.1001/jama.292.22.2735. [DOI] [PubMed] [Google Scholar]
- 22.Miller JW, Sabatino S, Thompson TD, et al. Breast MRI Use Uncommon among U.S. Women. Cancer Epidemiol Biomarkers Prev. 2012 Nov 15; doi: 10.1158/1055-9965.EPI-12-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buxton JA, Bottorff JL, Balneaves LG, et al. Women's perceptions of breast cancer risk: are they accurate? Can J Public Health. 2003 Nov-Dec;94(6):422–426. doi: 10.1007/BF03405078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas JS, Kaplan CP, Des Jarlais G, Gildengoin V, Perez-Stable EJ, Kerlikowske K. Perceived risk of breast cancer among women at average and increased risk. J Womens Health (Larchmt) 2005 Nov;14(9):845–851. doi: 10.1089/jwh.2005.14.845. [DOI] [PubMed] [Google Scholar]
- 25.Haas JS, Kaplan CP, Gregorich SE, Perez-Stable EJ, Des Jarlais G. Do physicians tailor their recommendations for breast cancer risk reduction based on patient's risk? J Gen Intern Med. 2004 Apr;19(4):302–309. doi: 10.1111/j.1525-1497.2004.30280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004 Dec;233(3):830–849. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 27.Brinton JT, Barke LD, Freivogel ME, Jackson S, O'Donnell CI, Glueck DH. Breast cancer risk assessment in 64,659 women at a single high-volume mammography clinic. Acad Radiol. 2012 Jan;19(1):95–99. doi: 10.1016/j.acra.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010 Jan;254(1):79–87. doi: 10.1148/radiol.2541090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozanne E, Drohan B, Bosinoff P, et al. Which risk model to use? Clinical implications of the ACS MRI screening guidelines. Cancer Epidemiol Biomarkers Prev. 2012 Oct 23; doi: 10.1158/1055-9965.EPI-12-0570. [DOI] [PubMed] [Google Scholar]