Abstract
We have cloned the gene coding for the 1.3S biotin-containing subunit of transcarboxylase (EC 2.1.3.1) from Propionibacterium shermanii. Transcarboxylase is a well-characterized enzyme composed of 30 polypeptides of three different types: twelve 1.3S biotinyl subunits, six 5S dimeric outer subunits, and one 12S hexameric central subunit. In propionic acid fermentation, the enzyme catalyzes the transfer of a carboxyl group from methylmalonyl-CoA to pyruvate in two partial reactions. The 1.3S subunit binds the outer and central subunits of the enzyme together, and its biotin serves as carboxyl carrier between subsites on the central and outer subunits where each partial reaction occurs. The cloned gene has been expressed in Escherichia coli, and the 1.3S subunit accumulates to 7% of total cellular protein. The foreign protein is recognized and biotinated by biotin holoenzyme synthetase of E. coli. The identifications of the gene and its product were confirmed by four independent approaches: DNA sequence analysis, immunoprecipitation, incorporation of labeled biotin, and measurement of enzymatic activity in the first partial reaction.
Full text
PDF


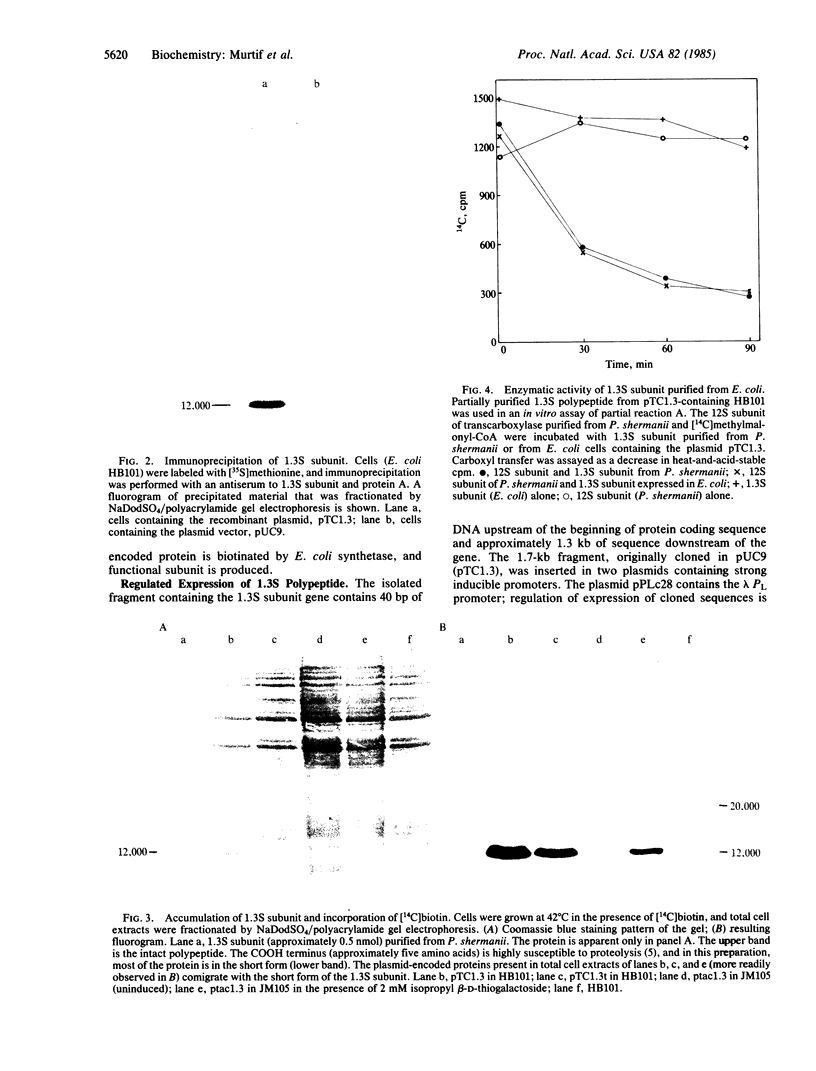

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad F., Jacobson B., Chuang M., Brattin W., Wood H. G. Isolation of peptides from the carboxyl carrier subunit of transcarboxylase. Role of the non-biotinyl peptide in assembly. Biochemistry. 1975 Apr 22;14(8):1606–1611. doi: 10.1021/bi00679a010. [DOI] [PubMed] [Google Scholar]
- Berger M., Wood H. G. Immunochemistry of the subunits of transcarboxylase. J Biol Chem. 1976 Nov 25;251(22):7021–7034. [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Caruthers M. H., Beaucage S. L., Efcavitch J. W., Fisher E. F., Goldman R. A., deHaseth P. L., Mandecki W., Matteucci M. D., Rosendahl M. S., Stabinsky Y. Chemical synthesis and biological studies on mutated gene-control regions. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):411–418. doi: 10.1101/sqb.1983.047.01.048. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chuang M., Ahmad F., Jacobson B., Wood H. G. Evidence that the two partial reactions of transcarboxylation are catalyzed by two dissimilar subunits of transcarboxylase. Biochemistry. 1975 Apr 22;14(8):1611–1619. doi: 10.1021/bi00679a011. [DOI] [PubMed] [Google Scholar]
- Craft D. V., Goss N. H., Chandramouli N., Wood H. G. Purification of biotinidase from human plasma and its activity on biotinyl peptides. Biochemistry. 1985 May 7;24(10):2471–2476. doi: 10.1021/bi00331a012. [DOI] [PubMed] [Google Scholar]
- Fall R. R., Alberts A. W., Vagelos P. R. Analysis of bacterial biotin-proteins. Biochim Biophys Acta. 1975 Feb 27;379(2):496–503. doi: 10.1016/0005-2795(75)90156-7. [DOI] [PubMed] [Google Scholar]
- Freytag S. O., Collier K. J. Molecular cloning of a cDNA for human pyruvate carboxylase. Structural relationship to other biotin-containing carboxylases and regulation of mRNA content in differentiating preadipocytes. J Biol Chem. 1984 Oct 25;259(20):12831–12837. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- KOSOW D. P., HUANG S. C., LANE M. D. Propionyl holocarboxylase synthesis. I. Preparation and properties of the enzyme system. J Biol Chem. 1962 Dec;237:3633–3639. [PubMed] [Google Scholar]
- Kumar G. K., Bahler C. R., Wood H. G., Merrifield R. B. The amino acid sequences of the biotinyl subunit essential for the association of transcarboxylase. J Biol Chem. 1982 Nov 25;257(22):13828–13834. [PubMed] [Google Scholar]
- LANE M. D., YOUNG D. L., LYNEN F. THE ENZYMATIC SYNTHESIS OF HOLOTRANSCARBOXYLASE FROM APOTRANSCARBOXYLASE AND (+)-BIOTIN. I. PURIFICATION OF THE APOENZYME AND SYNTHETASE; CHARACTERISTICS OF THE REACTION. J Biol Chem. 1964 Sep;239:2858–2864. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maloy W. L., Bowien B. U., Zwolinski G. K., Kumar K. G., Wood H. G., Ericsson L. H., Walsh K. A. Amino acid sequence of the biotinyl subunit from transcarboxylase. J Biol Chem. 1979 Nov 25;254(22):11615–11622. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McAllister H. C., Coon M. J. Further studies on the properties of liver propionyl coenzyme A holocarboxylase synthetase and the specificity of holocarboxylase formation. J Biol Chem. 1966 Jun 25;241(12):2855–2861. [PubMed] [Google Scholar]
- Miyoshi K., Arentzen R., Huang T., Itakura K. Solid-phase synthesis of polynucleotides. IV. Usage of polystyrene resins for the synthesis of polydeoxyribonucleotides by the phosphostriester method. Nucleic Acids Res. 1980 Nov 25;8(22):5507–5517. doi: 10.1093/nar/8.22.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Rylatt D. B., Keech D. B., Wallace J. C. Pyruvate carboxylase: isolation of the biotin-containing tryptic peptide and the determination of its primary sequency. Arch Biochem Biophys. 1977 Sep;183(1):113–122. doi: 10.1016/0003-9861(77)90425-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutton M. R., Fall R. R., Nervi A. M., Alberts A. W., Vagelos P. R., Bradshaw R. A. Amino acid sequence of Escherichia coli biotin carboxyl carrier protein (9100). J Biol Chem. 1977 Jun 10;252(11):3934–3940. [PubMed] [Google Scholar]
- Tucci A., Goldberger G., Whitehead A. S., Kay R. M., Woods D. E., Colten H. R. Biosynthesis and postsynthetic processing of human C-reactive protein. J Immunol. 1983 Nov;131(5):2416–2419. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., Harmon F. R., Wühr B., Hübner K., Lynen F. Comparison of the biotination of apotranscarboxylase and its aposubunits. Is assembly essential for biotination? J Biol Chem. 1980 Aug 10;255(15):7397–7409. [PubMed] [Google Scholar]
- Wood H. G. The anatomy of transcarboxylase and the role of its subunits. CRC Crit Rev Biochem. 1979 Dec;7(2):143–160. doi: 10.3109/10409237909105430. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]





