Abstract
Background
Whole body cryotherapy (WBC) is the therapeutic application of extreme cold air for a short duration. Minimal evidence is available for determining optimal exposure time.
Purpose
To explore whether the length of WBC exposure induces differential changes in inflammatory markers, tissue oxygenation, skin and core temperature, thermal sensation and comfort.
Method
This study was a randomised cross over design with participants acting as their own control. Fourteen male professional first team super league rugby players were exposed to 1, 2, and 3 minutes of WBC at −135°C. Testing took place the day after a competitive league fixture, each exposure separated by seven days.
Results
No significant changes were found in the inflammatory cytokine interleukin six. Significant reductions (p<0.05) in deoxyhaemoglobin for gastrocnemius and vastus lateralis were found. In vastus lateralis significant reductions (p<0.05) in oxyhaemoglobin and tissue oxygenation index (p<0.05) were demonstrated. Significant reductions (p<0.05) in skin temperature were recorded. No significant changes were recorded in core temperature. Significant reductions (p<0.05) in thermal sensation and comfort were recorded.
Conclusion
Three brief exposures to WBC separated by 1 week are not sufficient to induce physiological changes in IL-6 or core temperature. There are however significant changes in tissue oxyhaemoglobin, deoxyhaemoglobin, tissue oxygenation index, skin temperature and thermal sensation. We conclude that a 2 minute WBC exposure was the optimum exposure length at temperatures of −135°C and could be applied as the basis for future studies.
Introduction
Whole body cryotherapy (WBC) is the therapeutic application of extremely cold dry air, usually between −110°C and −140°C [1], [2]. WBC is becoming popular amongst athletes, coaches and clinicians across a variety of sports in order to prevent injury and promote recovery. Typically the timescale of exposure is reported as between 2–3 minutes [2]. Although a number of studies [1], [3]–[8] have investigated the physiological effects of WBC, the optimal WBC protocol required to initiate beneficial physiological responses is unknown [2], [9]. This is due to the lack of randomised controlled clinical studies investigating either exposure duration or number of treatment cycles [2], [9]. One of the more commonly reported beneficial changes following WBC is a reduction in inflammatory markers [2], [10], [11], [12]. Recently the acceleration of recovery from exercise induced muscle damage (EIMD) was reported after three WBC exposures [13]. However Costello and colleagues [1] reported ineffective results of WBC when administered 24 hours following eccentric exercises in order to help alleviate muscle soreness. Furthermore the enhancement of muscle force recovery was demonstrated to be ineffective in this study [1], [3]. One previous study has evaluated various physiological changes in professional rugby players, demonstrating changes in haematological profiles, following a protocol of 5 WBC exposures over 5 consecutive days [14]. However this study lacked a control group, therefore it is difficult to discriminate WBC effects compared to the cumulative effects of training. Rugby league is a contact sport, with a match consisting of two, forty minute halves of play where players engage in high intensity activity, intermittent with periods of low activity [15]. Due to the physical demands placed upon rugby league players which result in symptoms of fatigue and muscle performance decrements [15], WBC has recently become very popular as a recovery modality within this sport. The purpose of this study was to determine optimal WBC exposure duration and to measure physiological and perceptual responses following a 1, 2 and 3 minute WBC exposure at −135°C in elite rugby league players the day after a competitive fixture.
Materials and Methods
Ethics Statement
The study was conducted according to the Declaration of Helsinki (WMA, 2008), was approved by UCLan Built, Sport and Health Ethics Committee (BuSH 128) and by the Club Player Welfare officer. Participants provided written consent to take part in the study.
Participants
Fourteen (24 years; mean weight 77.6 kg; mean height 183.2 cm) professional rugby league players from Wigan Warriors RLFC volunteered, all were participating at first team level mid competitive season.
Experimental Protocol
This study was a randomised cross over design with participants acting as their own control. Three WBC exposures, separated by seven days following a competitive fixture the previous evening. Each WBC exposure consisted of 30 seconds precooling at −60°C then randomised (randomization.com) exposure for either 1, 2, or 3 minutes at −135°C in a liquid nitrogen cryochamber installed on a trailer (JUKA, Poland), owned and operated by BOC Linde. Prior to each WBC session, participants were prepared according to the standard BOC Linde operating protocol [16].
Inflammatory Markers
Baseline venous blood samples were taken three days prior to the first WBC exposure. Venous blood samples were collected 20 minutes prior to and 20 minutes post WBC exposure (Table 1). All samples were collected from the antecubital vein using standard venepuncture techniques and the S-Monovette blood collection system (Sarstedt). The anticoagulant was potassium EDTA. Samples were centrifuged at 1200× g for 10 minutes and plasma was removed and stored as aliquots at −20°C until analysis. Plasma levels of interleukin six (IL-6) were analysed using a quantitative sandwich enzyme immunoassay as per manufacturer's instructions (Aviva Systems Biology), using a Perkin Elmer Enspire plate reader. All standards and samples were analysed in duplicate and interleukin six (IL-6) levels were interpolated from the standard curve using 4 parameter logistic curve fit. The immunoassay had a sensitivity to detect a concentration of at least 1 pg/mL.
Table 1. Timing of data collection.
| Data | Base line | Pre exposure | Immediately post exposure | →5 minutes | 5 minutes post exposure | →10 minutes | 10 minutes post exposure | →15 minutes | 15 minutes post exposure | →20 minutes | 20 minutes post exposure |
| Core Temperature | • | • | • | ||||||||
| Thermal Image (Tsk) | • | • | • | • | • | • | |||||
| Thermal Sensation | • | • | • | • | • | • | |||||
| Thermal Comfort | • | • | • | • | • | • | |||||
| Blood Sample* | • | • | • | ||||||||
| NIRO | • | • | • | • | • | • |
• = Parameter measurement protocol at each time point of testing.
Baseline blood sampling taken three days prior to the first exposure as a normative comparison for 20 minutes post WBC exposures blood sampling.
Tissue Oxygenation
A dual-channel continuous wave near-infrared spectrometer (NIRS) (NIRO-200 Oxygenation Monitor, Hamamatsu, Japan), with a sampling rate of 6 Hz, assessed for changes in muscle oxygenation. To evaluate muscle oxygen content, 1 emitting laser diode and 2 detecting photodiodes, absorbencies of 775, 810, and 850 nm, were used to measure the ratio of oxygenated haemoglobin to total haemoglobin [17]. Tissue oxygen saturation and haemoglobin content were determined using a modified Beer–Lambert law [18]. Measurement sites were wiped with an alcohol wipe prior to optode placement. One optode was attached to the muscle belly of the left gastrocnemius at the widest point and one optode was placed on the left vastus lateralis (VL) midway between the promixal patella and the inguinal crease. Optodes were held in place using transparent double sided hypoallergenic sticky tape and covered with a bandage. An optode spacing of 4 cm was used and the differential path length factor applied for the gastrocnemius and vastus lateralis were 4.65 and 5.99 respectively (17). Key measurements were change in oxyhaemoglobin (O2Hb); deoxyhaemoglobin (HHb) in µmol·L−1 from an arbitrary zero value; tissue oxygenation index (TOI) expressed as a percentage value. Data was sampled and averaged from 3×5 minute intervals pre and, every 5 minutes up to twenty minutes post WBC exposure (Table 1).
Skin Temperature
Skin temperature (Tsk) was measured via noncontact, digital, infrared thermal imaging (TI). A ThermoVision A40M Thermal Imaging Camera (Flir systems, Danderyd, Sweden) emissivity set at 0.97–0.98 was used according to standard medical protocols [19]. The camera was mounted on a tripod height 1.2 m; distance 3.5 m from participants and connected to a laptop running Thermacam Researcher Pro 2.8 software (Flir systems, Danderyd, Sweden). To define regions of interest (ROI) wooden markers were attached mid clavicle and anterior superior iliac spine [20]. Four ROI were defined: anterior triangle of the neck (ROIA); torso (ROIB); lower abdomen (ROIC); back (ROID). Thermal images were recorded pre, immediately post and every five minutes post WBC exposure, up to 20 minutes (Table 1).
Core Temperature
Core temperature was recorded via ingestion of a core temperature pill (CorTemp® Wireless Ingestible Temperature Sensor-Product No. HT150002. HQInc. Florida. USA.) Core temperature data was then recorded by a hand held device (CorTemp® Data Recorder-Product No. HT150001) (Table 1). Participants arrived 40 minutes prior to their allocated WBC exposure, to swallow the core temperature pill. Core temperature pills are normally swallowed 6 hours prior to measurement, however the club did not consent to this, correspondence with the pill manufacturers' confirmed that data would still be valid.
Thermal Sensation and Thermal Comfort Questionnaires
Each participant recorded thermal sensation ratings [21] once pre, immediately following WBC and every 5 minutes up to 20 minutes (Table 1). Participants were asked ‘How are you feeling now?’ and answered by pointing to a scale from −4 to 4. (−4 = very cold, −3 = cold, −2 = cool, −1 = slightly cool, 0 = neutral, +1 = slightly warm, +2 = slightly hot +3 = hot, +4 = very hot). Thermal comfort [22] was also assessed prior to and immediately after WBC using a five point scale. Participants were asked ‘Do you find this’ and answered by choosing: 0 = comfortable; 1 = slightly comfortable; 2 = uncomfortable; 3 = very uncomfortable; 4 = extremely uncomfortable.
Statistical Analysis
Continuous data were analysed using a mixed method model SPSS (version 19.0, SPSS Inc, Chicago, IL), using the data pre exposure as a covariate to determine changes from pre and all other time points, applying least significant difference pairwise comparisons. With the fixed factors being time points and exposure. The distributions of the data values about the mean were assessed and the data found to be suitable for parametric statistical testing. The use of an adjustment for multiple comparison was considered to be too aggressive for this exploratory study. Nominal data from the questionnaires were analysed with Friedman Tests to explore differences between all three exposures and Wilcoxon sign rank tests were used as a post hoc comparison between exposures and time points.
Results
Inflammatory Markers
No significant changes were recorded for the inflammatory cytokine IL-6 (Table 2).
Table 2. Comparison between WBC exposure times for all testing parameters and significant values.
| Measurement | WBC Exposure Time (minutes) | Compared to WBC exposure time (minutes) | Mean Difference | Sig. |
| Interleukin Six (IL-6) (pg/mL) | 1 | 2 | 0.37 | 0.81 |
| 1 | 3 | 0.14 | 0.93 | |
| 2 | 3 | −0.23 | 0.89 | |
| Tissue Oxygenation Gastrocnemius HHB (µmol·L−1) | 1 | 2 | −2.50 | 0.58 |
| 1 | 3 | −0.41 | 0.93 | |
| 2 | 3 | 2.09 | 0.64 | |
| Tissue Oxygenation Gastrocnemius TOI (µmol·L−1) | 1 | 2 | −0.87 | 0.45 |
| 1 | 3 | −1.98 | 0.08 | |
| 2 | 3 | −1.11 | 0.33 | |
| Tissue Oxygenation VL O2HB (µmol·L−1) | 1 | 2 | 2.24 | 0.37 |
| 1 | 3 | 5.93 | 0.02 | |
| 2 | 3 | 3.70 | 0.14 | |
| Tissue Oxygenation VL HHB (µmol·L−1) | 1 | 2 | −3.82 | 0.14 |
| 1 | 3 | −2.53 | 0.33 | |
| 2 | 3 | 1.29 | 0.62 | |
| Tissue Oxygenation VL TOI (µmol·L−1) | 1 | 2 | −0.28 | 0.84 |
| 1 | 3 | 1.78 | 0.19 | |
| 2 | 3 | 2.06 | 0.13 | |
| Tsk (°C) ** | 1 | 2 | 1.21 | 0.00 |
| 1 | 3 | 1.70 | 0.00 | |
| 2 | 3 | .50 | 0.00 | |
| Core Temperature (°C) * | 1 | 2 | 0.51 | 0.20 |
| 1 | 3 | 0.72 | 0.08 | |
| 2 | 3 | 0.20 | 0.62 |
Core Temperature (°C) average mean difference for all time points (pre up to 20 minutes post WBC exposure).
Tsk (°C) average mean difference for all regions, for all time points (pre up to 20 minutes post WBC exposure).
Tissue Oxygenation
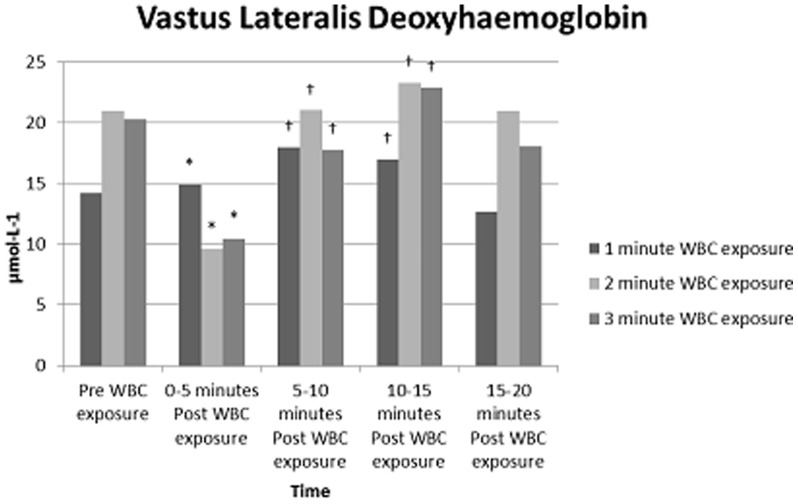
The mixed methods model showed significant reductions (p<0.05) in HHb occurred in gastrocnemius, between pre and 0–5 minutes post WBC (Table 3). Gastrocnemius HHb demonstrated a significant increase (p<0.05) between 0–5, 5–10, 10–15 minutes post WB compared to pre WBC. VL O2Hb demonstrated a significant reduction (p<0.05) when comparing a 1 minute to a 3 minute WBC exposure (Table 2). Significant differences (p<0.05) were found in HHb for VL when comparing pre WBC and 0–5 minutes post WBC and comparing 0–5 post WBC and 5–10, 10–15 minutes post WBC (Table 3). A significant reduction (p<0.05) in TOI for VL, between pre and 0–5 minutes post WBC occurred.
Table 3. Comparison of parameters between time points, pre, immediately post, +5, +10, +15 and +20 minutes post WBC exposures for all exposures.
| Time Point* | to Time Point | Core Temp °C** | Sig. | Tsk °C*** | Sig. | Blood IL-6 | Sig. | NIRO G O2HB | Sig. | NIRO G HHB | Sig. | NIRO G TOI | Sig. | NIRO VL O2HB | Sig. | NIRO VL HHB | Sig. | NIRO VL TOI | Sig. |
| 0 | 1 | −0.50 | 0.21 | 8.85 | 0.00 | 4.28 | 0.31 | 12.79 | 0.03 | 2.36 | 0.11 | −1.70 | 0.60 | 6.85 | 0.04 | 3.81 | 0.03 | ||
| 0 | 2 | 3.40 | 0.00 | −2.15 | 0.61 | −3.05 | 0.60 | 1.78 | 0.23 | −3.74 | 0.25 | −0.43 | 0.90 | 2.20 | 0.21 | ||||
| 0 | 3 | 2.59 | 0.00 | −1.62 | 0.70 | −7.83 | 0.18 | 1.19 | 0.42 | −1.80 | 0.58 | −2.59 | 0.44 | 2.44 | 0.16 | ||||
| 0 | 4 | 2.19 | 0.00 | 2.33 | 0.58 | −6.52 | 0.27 | 0.95 | 0.52 | −1.18 | 0.71 | 1.24 | 0.71 | 1.54 | 0.38 | ||||
| 0 | 5 | −0.38 | 0.35 | 2.01 | 0.00 | 0.15 | 0.91 | ||||||||||||
| 1 | 2 | −5.45 | 0.00 | −6.43 | 0.13 | −15.85 | 0.01 | −0.58 | 0.69 | −2.04 | 0.53 | −7.28 | 0.03 | −1.61 | 0.35 | ||||
| 1 | 3 | −6.26 | 0.00 | −5.90 | 0.16 | −20.62 | 0.00 | −1.17 | 0.43 | −0.10 | 0.98 | −9.44 | 0.01 | −1.38 | 0.43 | ||||
| 1 | 4 | −6.66 | 0.00 | −1.95 | 0.64 | −19.31 | 0.00 | −1.41 | 0.34 | 0.52 | 0.87 | −5.61 | 0.10 | −2.28 | 0.19 | ||||
| 1 | 5 | 0.12 | 0.76 | −6.84 | 0.00 | ||||||||||||||
| 2 | 3 | −0.81 | 0.00 | 0.53 | 0.90 | −4.77 | 0.41 | −0.59 | 0.69 | 1.95 | 0.55 | −2.16 | 0.52 | 0.24 | 0.89 | ||||
| 2 | 4 | −1.21 | 0.00 | 4.49 | 0.29 | −3.46 | 0.55 | −0.83 | 0.57 | 2.56 | 0.43 | 1.67 | 0.62 | −0.66 | 0.70 | ||||
| 2 | 5 | −1.39 | 0.00 | ||||||||||||||||
| 3 | 4 | −0.40 | 0.09 | 3.96 | 0.35 | 1.31 | 0.82 | −0.24 | 0.87 | 0.61 | 0.85 | 3.83 | 0.26 | −0.90 | 0.60 | ||||
| 3 | 5 | −0.58 | 0.02 | ||||||||||||||||
| 4 | 5 | −0.18 | 0.46 |
Time points for all lengths of WBC exposure: 0 = pre WBC exposure, 1 = Immediately post WBC exposure, 2 = 5 minutes post WBC exposure, 3 = 10 minutes post WBC exposure, 4 = 15 minutes WBC exposure and 5 = 20 post WBC exposure.
Core temperature (°C) average mean difference.
Tsk (°C) average mean difference.
Skin Temperature
Significant reductions (p<0.05) occurred in average Tsk when comparing 1, 2 and 3 minute WBC exposures (Table 2), when using a mixed methods model. When comparing average Tsk changes over time, significant differences (p<0.05) were found between pre exposure and all post exposure time points (Table 3). Significant differences (p<0.05) in average Tsk were found when comparing ROIC (lower abdomen) with ROI A, B and D. No significant differences were found in average Tsk between ROIB and ROID.
Core Temperature
No significant differences were found in core temperature.
Thermal Sensation and Thermal Comfort Scoring
The Friedman tests for the mixed method model demonstrated significant changes in thermal sensation (p = .008) and thermal comfort (p = .004) The Wilcoxson post-hoc comparison between 1 and 2 minute exposure indicated a significant reduction (p<0.05) in thermal sensation (Table 2). No significant differences were reported when comparing 1 and 3 minutes or 2 and 3 minutes. Comparison of pre and immediately post thermal sensation scores for all exposure times demonstrated significant reductions (p<0.05). No significant differences were found in thermal comfort when comparing WBC exposure times.
Adverse Incident
On the first day of testing a Samoan player with intolerance to ice packs underwent a three minute WBC exposure. The player did not disclose his cold intolerance to the study team or BOC personnel. He suffered a mild superficial skin burn bilaterally on the mid portion of the anterior thigh. The skin damage consisting of erythema and minor blistering appeared in a horizontal strip approximately 2 cm high and 10 cm wide the day following WBC. The player was able to train normally and played competitively the following week, although willing to continue in the study, he was excluded by the study team. This adverse incident occurred despite the following safety measures. 1. The study information sheet explicitly stated that there would be an exposure to extremely cold air (−110°C to −140°C). 2. The study consent form asked about medical conditions that may affect participation. 3. The BOC screening protocol included a specific question about intolerance to cold. This incident raises an important issue, consequently we recommend that all participants receiving WBC are screened, acknowledge the contraindications of WBC and are made aware of the potential risks of exposure.
Discussion
To our knowledge, this is the first study which has examined duration of exposure time of WBC at −135°C and effects on inflammatory marker (IL-6), tissue oxygenation, skin and core temperature, thermal sensation and comfort.
Inflammatory Markers
Strenuous exercise is known to induce a rapid, exponential, increase in IL-6 [23]. In agreement with previous research [24] our findings revealed elevated levels of IL-6, 10–16 hours post-game. An interesting observation from our IL-6 data was the individual variability in IL-6 levels demonstrated by the high standard deviations. This variance may be explained by the lack of similarity in player movement and loading experienced during rugby league play. Several studies have shown that there are differences in player activities, and loading, as defined by position [25], [26], [27]. An appreciation of positional differences is recommended when further investigating rugby league. In the present study, a single WBC exposure, irrespective of duration, did not significantly alter IL-6 levels. This is in line with previous literature [4] investigating a 3 minute exposure, at −110°C. Our 20 minute post exposure collection point was the shortest used when compared to similar studies [7], [13], [28]. Blood sampling at 20 minutes post WBC may have been too early for any changes to have occurred in the haematological markers, and this warrants further investigation. A previous study [28] reported that blood samples were taken 30 minutes post 3 minute WBC exposure, with no reports of inability to collect blood samples. An interesting challenge to the collection of venous blood samples was the partial ‘shutdown’ of the peripheral vasculature, particularly the median veins of the antecubital fossa. It appeared that vascular shunting from upper to lower limbs took place especially following 3 minute WBC exposure. Despite the target veins being visibly distended, as expected in very lean, muscular athletes; venous blood flow was severely impaired in several participants. Venepuncture was successful in all cases, yet an appropriate level of peripheral blood flow was often compromised, particularly following the 3 minute WBC exposure. This led to incomplete inflammatory myokine profiles being generated for some participants.
Tissue Oxygenation
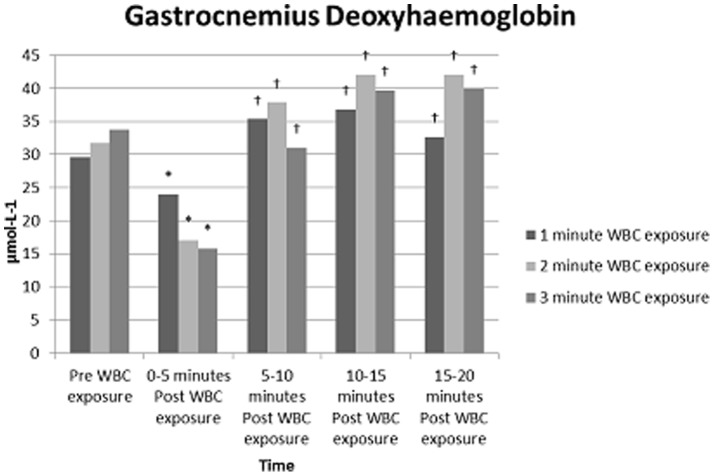
NIRO data demonstrate that a 3 minute WBC exposure differed significantly to a 1 or 2 minute WBC exposure. Results suggest localised tissue hypoxia in VL occurred as O2Hb decreased with a subsequent increase in HHb. (Figure 1). In gastrocnemius, a decrease in blood volume, 0–5 minutes post WBC occurred (Figure 2). O2Hb and HHb both increased to above pre WBC exposure measures over the remaining 15 minutes, indicative of venous pooling. These findings are supported by previous research that suggests extreme cold exposure reduces cardiac output and therefore produces a shift in blood volume to the venous circulation leading to pooling and reduced venous return [29]. Results indicate that gastrocnemius was more susceptible to pooling at all exposures than VL, possibly due to a weaker vasoconstriction response rather than active vasodilation. Vasoconstriction of peripheral blood vessels to maintain core temperature is generally observed with moderate cold exposure [30]. Initial responses during the present study demonstrate this physiological response. However, under extreme cold exposure the body endeavours to protect the extremities. As deeper muscles lose heat to the surface, cold-induced vasodilation (CIVD) can occur [31]. The exact mechanisms leading to CIVD are not fully understood. However, an increase in peripheral blood flow/volume followed by pooling of the blood has been observed [32] as was evident following a 3 minute WBC exposure in the present study. CIVD is thought to initiate when core temperatures rise above 36°C [31]. In the current study, core temperature changes although insignificant demonstrate a rise to 37°C immediately post WBC, this would potentially lend support to the concept of CIVD response. Although supporting previous literature [33], caution is needed when using CIVD as an explanation of our data as we did not have continuous data on blood flow, Tsk or core temperature.
Figure 1. Mean NIRO values for VL, pre and post WBC exposure for all three WBC time exposures (1, 2 and 3 minutes).
Values are means (N = 9). Statistical significance (P<0.05) observed over a period of time (pre, immediately post exposure, +5 minutes, +10 Minutes and, +15 minutes post exposure. * indicates significantly different to pre WBC; †indicates significantly different to 0–5 min post WBC.
Figure 2. Tissue deoxygenation for gastrocnemius, pre and post WBC exposure for all three WBC time exposures (1, 2 and 3 minutes).
Values are means (N = 9). Statistical significance (P<0.05) observed over a period of time (pre, immediately post exposure, +5 minutes, +10 Minutes, +15 minutes and +20 minutes post exposure. * indicates significantly different to pre WBC; †indicates significantly different to 0–5 min post WBC.
NIRO is sensitive to small movements and as a result of participants standing for 10 minutes pre and 20 minutes post WBC, some contraction of the muscles would have occurred. It is impossible to avoid movement, however some participants may have moved around more than others, affecting the results. Future research should take this in to account and have participants resting on a plinth.
Skin and Core Temperature
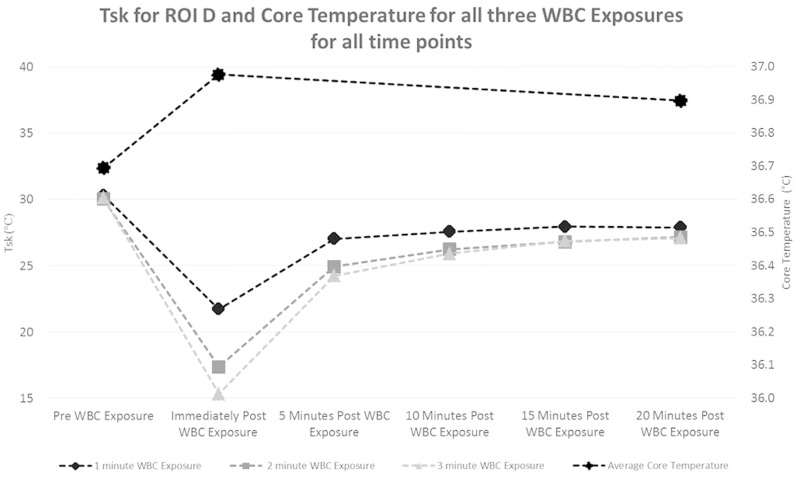
All exposure times of WBC reduced Tsk in all four ROI. Previous studies have shown the effectiveness of WBC in the reduction of Tsk [21]. The lowest Tsk recorded in the current study was 12.1°C in two out of the nine participants, following a three minute exposure in ROI ‘C’ (lower abdomen). As reported in previous studies, an extended phase of rewarming occurred following WBC exposure, no significant changes in Tsk occurred beyond 10 minutes post exposure and mean Tsk at 20 minutes post WBC did not reach mean Tsk pre WBC [34], [35] (Figure 3). Although core temperature did not show any significant changes following WBC, core temperature displays a predictable relationship with Tsk; where the maximum post WBC drop in Tsk occurred, a small rise in core temperature was observed for all exposure times (Figure 3). The core temperature pill was ingested 40 minutes prior to WBC exposure and although a longer time is normal, results did demonstrate change in core measurement temperatures. The pattern of change in Tsk and the slight rise in core temperature suggests vascular shunting to maintain the functions of vital organs.
Figure 3. Core and Abdomen skin temperature before and after WBC exposure (1, 2 and 3 minutes).
Y-axis on the left measures skin temperature and Y-axis on the right measures core temperature. Values are means (N = 9). for timepoints, pre, immediately post exposure, +5 minutes, +10 Minutes, +15 minutes and +20 minutes post exposure.
Thermal Sensation and Thermal Comfort Scoring
Prior to WBC participants rated their thermal sensation as ‘neutral’ or ‘slightly warm’. Following all WBC exposure times significant reductions in thermal sensation were noted, in most cases participants rated themselves as ‘very cold’ or ‘cold’. No significant differences occurred between 2 and 3 minutes exposures for thermal sensation, however between 1 and 2 minute exposures there was a significant reduction (p<0.05) in thermal sensation scores. Immediately post 1 minute WBC exposure participants reported feeling ‘slightly cool’ compared 2 minutes where participants reported feeling ‘cold’ or ‘very cold’. The differences in perceived temperature sensation suggest that 1 minute may not be a long enough WBC exposure to initiate perceptual changes in temperature sensation.
Limitations
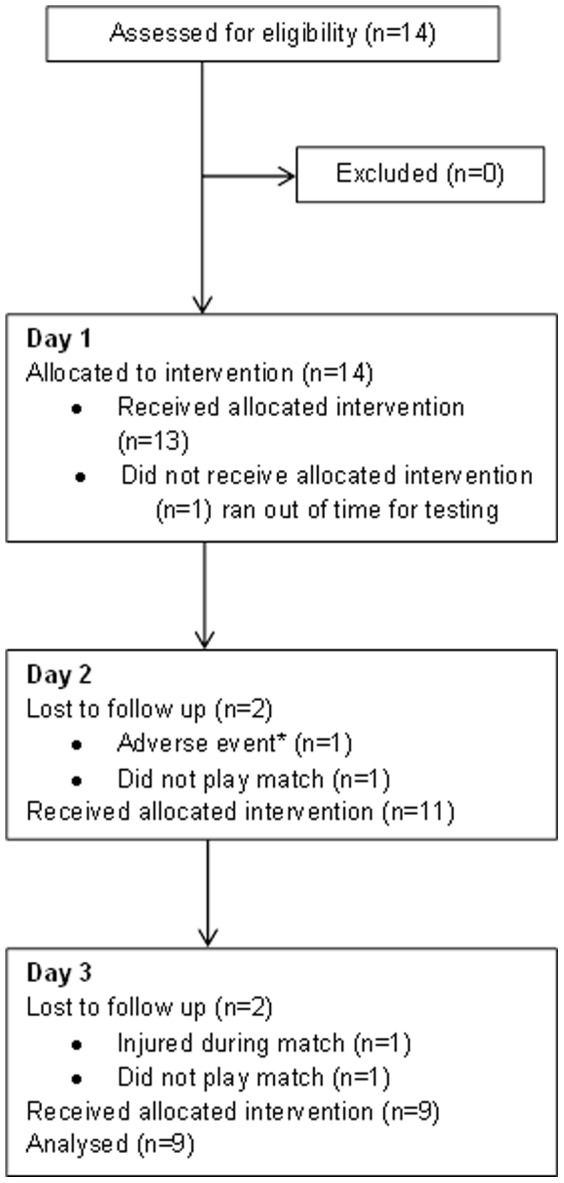
Although ecologically valid, with elite players participating in competitive fixtures mid-season, there was potential for players to drop out due to injury or non-selection to play (Figure 4). The training ground environment in which the testing took place was also a limitation, as a temperature controlled room was not available. We also acknowledge that although the temperature of the cryochamber was set at −135°C, fluctuations occurred during the exposure times, which is common with prolonged use and any thermal stimulation [36]. No skin surface temperatures were measured during WBC exposure periods neither was air flow, in order to observe convective strength of WBC. This was due to the lack of equipment suitable to measure such parameters. We were unable to measure internal recommended temperature as reported by Savic et al (2013) therefore differences in actual and reported temperatures may vary. The core temperature pill should ideally be ingested approximately 6 hours prior to testing, future studies should take this into consideration.
Figure 4. CONSORT diagram showing participant flow and retention.
Summary
A pattern in the results from this study emerged suggesting that a 2 minute WBC exposure at −135°C following 30 seconds pre-cooling at −60°C was the optimum WBC exposure for first team professional rugby league players. A 2 minute WBC exposure induces potentially beneficial physiological and perceptual changes, greater than those achieved following a 1 minute WBC exposure but without any of the negative effects demonstrated by a 3 minute exposure. Whilst we have not demonstrated support for a single bout of WBC, other studies have shown the potential of WBC in mediating muscular recovery by attenuating the inflammatory process [4]. Successful use of WBC to ‘blunt’ the inflammatory response appears to be effective when administered immediately post exercise, with repeat exposures. This supports recent literature, suggesting that physiological changes are dependent on the number of sessions of WBC [28]. It is therefore suggested that future research could follow a similar protocol to this study, using a 2 minute WBC exposure at −135°C this would facilitate the development of a stronger evidence base for WBC. These studies should focus on determining the optimum number of sessions per day/week and also over what time period exposure cycles should take place.
What are the new findings?
30 seconds at −60°C followed by 2 minutes WBC at around −135°C appear to be an optimum WBC exposure time.
2 minute WBC exposure produces physiological changes in core and skin temperature, tissue oxygenation in vastus lateralis and gastrocnemius muscles and thermal sensation responses.
Professions working within elite sport can be advised on applying an optimal WBC exposure time of 2 minutes at around −135°C for physiological changes to occur.
How might it impact on clinical practice in the near future?
A duration time of 2 minutes of WBC exposure at −135°C has been established as a safe protocol for future application for male elite rugby league players.
Clinicians should utilise the protocol from this study to compare future research which will help now determine the number of required WBC exposure sessions to initiate a larger physiological responses.
Research investigating WBC on other elite athletes and women would be advantageous to broaden the knowledge on WBC across a variety of sports and gender.
Funding Statement
Jill Alexander was funded to work on the project by a University of Central Lancashire innovation voucher. The use of the mobile Cryochamber, liquid nitrogen and specialist operator were provided free of charge by BOC Linde throughout the testing. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Costello JT, Algar L, Donnelly A (2012) Effects of whole-body cryotherapy (−110°C) on proprioception and indices of muscle damage. Scand J Med Sci Sports 22: 190–198. [DOI] [PubMed] [Google Scholar]
- 2. Banfi G, Lombardi G, Columbini A, Melegati G (2010) Whole-body cryotherapy in athletes. Sports Med 7: 1–9. [DOI] [PubMed] [Google Scholar]
- 3. Costello JT, Culliga K, Selfe J, Donnelly A (2012) Muscle, skin and core temperature after −110°C cold air and 8°C water treatment. PloS ONE 11: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pournot H, Bieuzen F, Louis J, Mounier R, Fillard JR, et al. (2011) Time-course of changes in inflammatory responses after whole-body cryotherapy multi exposures following severe exercise. PLos ONE 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Costello JT, McInerney CD, Bleakley C, Selfe J, Donnelly A (2012) The use of thermal imaging in assessing skin temperature following cryotherapy: A review. J Therm Bio 37: 103–110. [Google Scholar]
- 6.Costello JT, Selfe J, Karki A, McInerney CD, Donnelly A (2013) Comparison of the effects of cold-air (−110°C) and water (8°C) cryotherapy on knee skin temperature. Conference paper ACSM.
- 7. Fonda B, Sarabon N (2013) Effects of whole-body cryotherapy on recovery after hamstring damaging exercise: A crossover study. Scand J Med Sci Sports 23: 270–278. [DOI] [PubMed] [Google Scholar]
- 8. Ziemann E, Olek A, Kujach S, Grzywacz T, Antosiewicz J, et al. (2012) Five-day whole body cryostimulation, blood inflammatory markers and performance in high-ranking professional tennis players. J Ath Training 47: 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lubkowska A, Banfi G, Dolegowska B, d'Eril G, Luczak J, et al. (2010) Changes in lipid profile in response to three different protocols of whole-body cryostimulation treatments. Cryobiology 61: 22–25. [DOI] [PubMed] [Google Scholar]
- 10. Banfi G, Melegati G, Barassi A, Dogliotti G, d'Eril G, et al. (2009) Effects of whole-body cryotherapy on serum mediators of inflammation and serum muscle enzymes in athletes. J Ther Bio 34: 55–59. [Google Scholar]
- 11. Wozniak A, Wozniak B, Drewa G, Mila-Kierzenkowska C, Rakowski A (2007) The effect of whole-body cryostimulation on lysosomal enzyme activity in kayakers during training. 100: 137–142. [DOI] [PubMed] [Google Scholar]
- 12. Lubkowska A, Szygula Z, Klimek A, Torii M (2010) Do sessions of cryostimulation have influence on white blood cell count, level of IL6 and total oxidative and antioxidative status in healthy men? Eur J Appl Physiol 1: 67–72. [DOI] [PubMed] [Google Scholar]
- 13. Hausswirth C, Louis J, Bieuzen F, Pournot H, Fournier J, et al. (2011) Effects of whole-body cryotherapy vs. far-infrared vs. passive modalities on recovery from exercise-induced muscle damage in highly-trained runners. PLoS ONE 12: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lombardi G, Lanteri P, Porcelli S, Mauri C, Columbini A, et al. (2013) Hematological profile and martial status in rugby players during whole body cryostimulation. PLos ONE 8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Twist C, Waldron M, Highton J, Burt D, Daniels M (2012) Neuromuscular, biochemical and perceptual post-match fatigue in professional rugby league forwards and backs. J Sports Sci 30: 359–367. [DOI] [PubMed] [Google Scholar]
- 16.BOC (2013) Available: http://www.youtube.com/watch?v=MPuLYIYsrm8&feature=share&list=PLOvWstFPDR3AaYI-1omrVPh-sIMXW9Tl6. Accessed 2013 Dec 8.
- 17. Delpy DT, Cope M (1997) Quantification in tissue near-infrared spectroscopy. Philos Trans R Soc Lond Ser B 352: 649–659. [Google Scholar]
- 18. Murkin JM, Arango M (2009) Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anesth 103: 3–13. [DOI] [PubMed] [Google Scholar]
- 19. Ring EFJ, Ammer K (2000) The technique of infrared imaging in medicine. Thermology Int 10: 7–14. [Google Scholar]
- 20. Selfe J, Hardaker N, Thewlis D, Karki A (2006) An accurate and reliable method of thermal data analysis in thermal imaging of the anterior knee for use in cryotherapy research. Arch Phys Med Rehabil 12: 1630–1635. [DOI] [PubMed] [Google Scholar]
- 21. Cholewka A, Drzazga Z, Sieron A (2012) Thermography study of skin response due to whole-body cryotherapy. Skin Res Technol 18: 180–187. [DOI] [PubMed] [Google Scholar]
- 22.ISO. Ergonomics of the thermal environment-assessment of the influence of the thermal environment using subjective judgement scales. International Organization for Standardization, Geneva 1995.
- 23. Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev 88: 1379–1406. [DOI] [PubMed] [Google Scholar]
- 24. Pedersen BK (2011) Muscles and their myokines. J Exp Biol 214: 337–346. [DOI] [PubMed] [Google Scholar]
- 25. Austin DJ, Kelly SJ (2013) Positional differences in professional rugby league match play through the use of global positioning systems. J Strength Cond Res 27: 14–19. [DOI] [PubMed] [Google Scholar]
- 26. Austin DJ, Gabbett TJ, Jenkins DJ (2011) Repeated high-intensity exercise in a professional rugby league. J Strength Cond Res 25: 1898–1904. [DOI] [PubMed] [Google Scholar]
- 27. Gabbett TJ, Jenkins DG, Abernethy B (2012) Physical demands of professional rugby league training and competition using microtechnology. J Sci Med Sport 15: 80–86. [DOI] [PubMed] [Google Scholar]
- 28. Lubkowska A, Dolegowska B, Szygula Z (2012) Whole-body Cryostimulation – Potential Beneficial Treatment for Improving Antioxidant capacity in Healthy Men – Significance of the Number of Sessions. PLos ONE 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fouris AD, Cheung SS (2009) Influence of thermal balance on cold-induced vasodilation. J Appl Physiol 106: 1264–1271. [DOI] [PubMed] [Google Scholar]
- 30. Stocks JM, Taylor NAS, Tipton MJ, Greenleaf J (2004) Human physiological responses to cold exposure. Aviation Space Environ Med 75: 444–457. [PubMed] [Google Scholar]
- 31. Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, et al. (2007) S-nitrosohemoglobin deficiency: A mechanism for loss of physiological activity in banked blood. PNAS 104: 17058–17062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flouris AD, Westwood DA, Mekjavic IB, Cheung S (2008) Effect of body temperature on cold induced vasodilation. Eur J App Phys 104: 491–499. [DOI] [PubMed] [Google Scholar]
- 33. Gregson W, Black M, Jones H, Milson J, Morton J, et al. (2011) Influence of cold water immersion on limb and cultaneous blood flow at rest. Am J Sports Med 6: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 34. Karki A, Karppi P, Ekberg J, Selfe J (2004) A thermographic investigation of skin temperature changes in response to a thermal washout of the knee in healthy young adults. Thermol Int 14: 137–41. [Google Scholar]
- 35. Selfe J, Sutton C, Hardaker N, Greenhalgh S, Karki A, et al. (2010) Anterior knee pain and cold knees: A possible association in women. The Knee 17: 319–323. [DOI] [PubMed] [Google Scholar]
- 36. Savic M, Fonda B, Sarabon (2013) Actual temperature during and thermal response after whole-body cryotherapy in cryo-cabin. Journal of Thermal Biology 38: 186–191. [Google Scholar]