Introduction
The neuroprotective function of the blood-brain barrier (BBB) presents a major challenge for drug delivery to the central nervous system. Critical to this function, BBB membrane transporters include ATP-binding cassette (ABC) transporters that limit drug penetration across the BBB, and the less well studied solute carrier (SLC) transporters. Here, expression profiling of 359 SLC transporters, comparative expression analysis with kidney and liver, and immunoassays in brain microvessels identified previously unknown transporters at the human BBB.
Background
The blood-brain barrier (BBB) regulates exchange between the blood and the central nervous system (CNS) through the unique properties of the endothelial cells forming the CNS vasculature, and prevents most small molecule drugs from permeating the CNS (1). The two primary routes by which most drugs pass through the BBB are transcellular passive diffusion and carrier-mediated transport. Furthermore, both paths require overcoming the various metabolic enzymes and ATP-binding cassette (ABC) transporters expressed in CNS endothelial cells, the latter of which have well- established roles in restricting drug penetration across the BBB (2). Accordingly, most BBB transporter expression profiling and functional studies have focused on ABCs, leaving the solute carrier (SLC) transporters as a group largely overlooked.
In this study, we isolated human brain microvessels (BMV) to identify SLC transporters expressed at the human BBB. The transporter expression profile was determined using a real-time PCR (RT-PCR) array, and selected transporters were followed up with immunoassays to determine transporter protein expression in BMVs. These studies suggest that the BBB is rich in expression of SLC transporters and imply an important role for this superfamily in CNS homeostasis and drug delivery.
Validation of BMV enrichment
Human cerebral cortex samples (Table S1) from donors 1-3 were used to prepare BMV enriched fractions for human BBB transporter identification by mRNA expression profiling. BMV enrichment was determined by visual inspection (Figure S1A) and measuring expression of pan-endothelial (PECAM1 and VEC), brain endothelial (GLUT1), neuronal (SYP), and astrocytic (GFAP) cell markers (Figure S1B). Visual inspection indicated that the majority of blood vessels in BMV fractions were ≤10 μm diameter. Cell marker analysis revealed that all measured endothelial cell genes increased from 3- to 45-fold, while SYP and GFAP were both reduced approximately 2-fold in BMV enriched fractions relative to paired whole cerebral cortex samples (see Supplemental Information for materials and methods). These results indicate that the BMV fraction was successfully enriched with the blood vessels comprising the BBB.
Transporter mRNA expression profile in BMVs
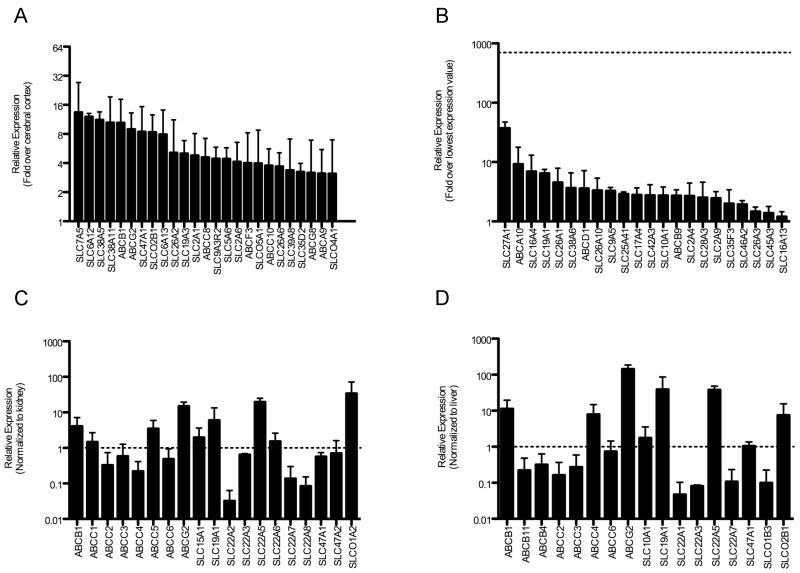
A custom RT-PCR OpenArray platform was used to profile expression of 359 SLC and 49 ABC transporters in validated BMV enriched and paired whole cerebral cortex samples from donors one and two (Table S1). We detected expression of 244 and 248 SLC genes in both BMV and cerebral cortex samples, respectively, while 42 ABC genes were expressed across all BMV and cerebral cortex samples. Out of all transporter genes detected, 225 SLC and 39 ABC genes were detected in both paired BMV- cerebral cortex samples, with 46 SLC and 13 ABC genes expressed at least 2-fold higher on average in BMVs relative to cerebral cortex (Table S2). The 25 most highly expressed transporter genes relative to cerebral cortex are shown in Figure 1A. Nineteen SLC and 3 ABC genes were uniquely detected in both BMV samples, and not detected in both cerebral cortex samples (Figure 1B). This analysis confirmed the presence of many transporters known to have enriched BBB expression (SLC7A5, SLCO2B1, ABCB1, and ABCG2), thereby validating our OpenArray expression results (see Supplemental Information and Figure S2). More importantly, transporters previously unknown to have enriched expression in human BMVs, including SLC26s, SLC19A3, SLC6A13, and SLC47A2 were identified (Figure 1A-B; Table S2).
Figure 1.
Transporter gene mRNA expression levels in BMVs. Relative expression of SLC and ABC genes was determined by OpenArray-based RT-PCR. (A) The top 25 genes with the most enriched mRNA expression in BMVs relative to paired cerebral cortex are depicted (see Table S2 for complete list). Only genes detected across both BMV and both cerebral cortex samples were included. (B) Genes detected in both BMV samples but not in both cerebral cortex samples. Expression levels were normalized to the lowest average expression value detected in BMV samples, and the dotted line represents the highest average expression level detected. BMV mRNA expression of drug transporters normalized to average (C) kidney and (D) liver expression levels. Average gene expression of SLC and ABC transporters with established roles in drug disposition across 59 kidney and 60 liver samples was determined by OpenArray-based RT-PCR. The standard deviation of gene expression in kidney and liver samples was up to 6 and 10% of housekeeping gene expression levels, respectively. Only genes detected across both BMV samples were included. Dotted lines represent the expression level in either kidney or liver. All values represent the mean ± SD (n=2: Donor #1 and #2).
The previously unidentified transporters interact with a diverse assortment of nutrients and enhance our understanding of how the BBB supports CNS function. SLC26A1 and SLC26A2 transport sulfates, which are charged inorganic molecules required for normal cell function that cannot passively diffuse across cell membranes. Sulfates are used to synthesize sulfated proteoglycans in the brain that help regulate neuronal growth, plasticity, and regeneration (3). SLC19A3 transports thiamine, and mutations in this transporter cause a biotin-responsive basal ganglia disease with bilateral necrosis in the caudate nucleus and putamen (4). Though the mutated transporter is well recognized as being the cause of the disease, these studies establish that the transporter may also play a role in delivering its essential substrates to the CNS. SLC6A13 is a γ-aminobutyric acid (GABA) transporter that may help to regulate GABA signaling by modulating GABA levels in the brain interstitial fluid via the BBB. Finally, SLC47A2 has largely been thought to be a kidney specific transporter with a central role in renal secretion of organic cations (5), and may play a role in the entry or efflux of organic cations, including bioactive amines across the BBB.
Drug transporter expression in BMVs relative to human kidney and liver
A well-known function of the BBB is to regulate CNS exposure to circulating xenobiotics, such as drugs. To identify transporters that may support this critical BBB function, OpenArray drug transporter expression values in BMVs, and human kidney and liver samples were compared. ABCB1, ABCC5, ABCG2, SLC22A5, and SLCO1A2 were expressed at higher levels (> 2-fold) in BMVs than in the kidney, while ABCC1, ABCC3, ABCC6, SLC15A1, SLC19A1, SLC22A3, SLC22A6, SLC47A1, and SLC47A2 were expressed at similar levels (within 2-fold) between BMV and kidney samples (Figure 1C). ABCB1, ABCC4, ABCG2, SLC19A1, SLC22A5, and SLCO2B1 were expressed at higher levels in BMVs than in the liver, while ABCC6, SLC10A1, and SLC47A1 at similar levels between BMV and liver samples (Figure 1D). Even though some of these transporters are already known to interact with xenobiotics and/or to be highly expressed in the BBB (i.e., SLC22A5 and SLCO2B1), these results suggest that other drug transporters may also play a role in regulating xenobiotic penetration across the BBB. Moreover, two transporters important to BBB and hepatic drug disposition, ABCB1 and ABCG2, were expressed at approximately 10- and 150-fold higher mRNA levels, respectively in the BBB compared to the liver.
Transporter protein expression in BMVs
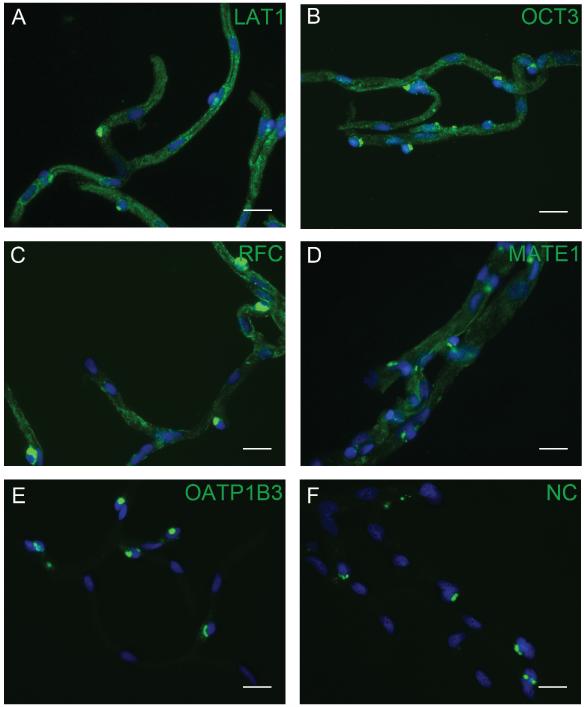
Protein expression of several transporters in isolated human BMVs was characterized by western blotting (Donor #1) and immunohistochemistry (IHC: Donor #1 and #4). LAT1 (SLC7A5) was included as a positive control, while OATP1B3 (SLCO1B3) was included as a negative control since its mRNA expression level was near the limit of detection. LAT1, OCT3 (SLC22A3), and the reduced folate carrier (RFC; SLC19A1) were detected in BMVs ≤10 μm in diameter and produced average signal intensities 4.5 - 5.5-fold higher than the no primary control (no primary antibody) (Figure 2A-C). MATE1 (SLC47A1) was primarily detected in BMVs >10 μm in diameter, yielding an average signal intensity approximately 3-fold greater than that of the no primary control (Figure 2D). OATP1B3 was not detected in BMVs, and had average signal intensities less than 2-fold above the no primary control (Figure 2E). Western blot analysis confirmed the IHC results, and verified antibody specificity in HEK-empty vector and - transporter transfected cells (Figure S3).
Figure 2.
Protein expression of SLC transporters in BMVs. Immunofluorescence staining (green) determined the presence of LAT1 (A), OCT3 (B), RFC (C), and MATE1 (D), and the absence of OATP1B3 (E) in BMVs. OATP1B3 and LAT1 served as negative and positive controls, respectively. A control with primary antibody omitted is also shown in (F). Nuclei were stained with DAPI (blue), and scale bars are set to 20 μm in all images. Images are representative of the results observed for BMVs isolated from donors one and four.
These results complement and extend the seminal study of Uchida et al., which provided quantitative information on expression levels of 100 transporters in the human BBB (2). Our study confirms the BBB expression of LAT1 and RFC observed by Uchida et al. (2). However, we also detected OCT3 and MATE1 proteins, which were not detected by Uchida et al. (2). This discrepancy may be due to the different methodologies used to determine protein expression. In the previous study, protein expression was quantified using a liquid chromatography-tandem mass spectrometry method, and only proteins expressed above a pre-determined limit of quantification were reported. The immunoassays used to determine transporter protein expression in this study are sensitive enough to detect femtogram quantities of a target protein, but are not quantitative in nature and only determine the presence or absence of a particular protein. Future studies investigating OCT3 and MATE1 at the BBB will help confirm their role in supporting BBB function.
Conclusions
Three key findings emerge from this study. First, a large number of SLC transporters show enriched expression in the human BBB. Second, SLC transporters previously unknown to be expressed at the BBB were identified. Third, a comparative expression approach revealed that drug transporters with important roles in renal and hepatic drug disposition were expressed at similar or higher levels in the BBB. These findings suggest a broad role for SLC transporters in the BBB in terms of maintaining CNS homeostasis of vitamins, amino acids, neurotransmitters and various other essential nutrients. The finding that a broad array of xenobiotic transporters is expressed in the BBB suggests that these transporters may be targeted to achieve CNS delivery of pharmaceuticals for the treatment of neurodegenerative and other CNS diseases. As new SLC genes are identified and more transporter-targeted antibodies become available, future studies with additional samples will be important to confirm and expand upon these results, and further elucidate the SLC transporter expression profile of the human BBB.
Supplementary Material
Acknowledgments
This research was supported by the FDA’s Medical Countermeasures initiative. The authors acknowledge the following funding sources: The National Institutes of Health (GM61390 and Training Grant T32 GM007175), and The National Institute of General Medical Sciences (U01 GM092655).
Footnotes
Conflict of Interest
The authors declare no conflict of interests.
References
- (1).Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiology of disease. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- (2).Uchida Y, et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117:333–45. doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- (3).Kwok JC, Warren P, Fawcett JW. Chondroitin sulfate: a key molecule in the brain matrix. Int J Biochem Cell Biol. 2012;44:582–6. doi: 10.1016/j.biocel.2012.01.004. [DOI] [PubMed] [Google Scholar]
- (4).Zeng WQ, et al. Biotin-responsive basal ganglia disease maps to 2q36.3 and is due to mutations in SLC19A3. Am J Hum Genet. 2005;77:16–26. doi: 10.1086/431216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. Renal transporters in drug development. Annu Rev Pharmacol Toxicol. 2013;53:503–29. doi: 10.1146/annurev-pharmtox-011112-140317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.