Abstract
This study aimed to determine whether telomere length (TL) is a marker of cancer risk or genetic status amongst two cohorts of BRCA1 and BRCA2 mutation carriers and controls. The first group was a prospective set of 665 male BRCA1/2 mutation carriers and controls (mean age 53 years), all healthy at time of enrolment and blood donation, 21 of whom have developed prostate cancer whilst on study. The second group consisted of 283 female BRCA1/2 mutation carriers and controls (mean age 48 years), half of whom had been diagnosed with breast cancer prior to enrolment. TL was quantified by qPCR from DNA extracted from peripheral blood lymphocytes. Weighted and unweighted Cox regressions and linear regression analyses were used to assess whether TL was associated with BRCA1/2 mutation status or cancer risk. We found no evidence for association between developing cancer or being a BRCA1 or BRCA2 mutation carrier and telomere length. It is the first study investigating TL in a cohort of genetically predisposed males and although TL and BRCA status was previously studied in females our results don't support the previous finding of association between hereditary breast cancer and shorter TL.
Introduction
Telomeres are hundreds to thousands of nucleotide repeats (TTAGGG) located at eukaryotic chromosome ends [1], without which genetic material would be lost every time a cell divides. The capacity of a cell to replicate is limited by TL; when telomeres become critically short, cell cycle arrest, senescence and apoptosis is signalled [2]. If this process fails for some reason and cell division persists despite the telomeres being too short then chromosomal instability results with end-to-end fusion of chromosomes [3].
Many factors are known to be associated with reduced TL, a primary factor being age with peripheral blood lymphocyte telomeres shortening by, on average, 41 base pairs per year [4]. Various studies have shown other factors associated with TL including smoking [5], inflammatory processes, socio-economic status, diet [6], BMI [7], diabetes mellitus [8], coronary artery disease, ulcerative colitis [9], physical activity and psychological stress [10].
Conflicting results have come from studies investigating links between cancer risk and TL. A recent meta-analysis demonstrated an association between shorter TL and increased cancer risk in studies of bladder cancer, oesophageal cancer, gastric cancer, head and neck cancer, ovarian cancer and overall incident cancers [11]. There was inconsistency in studies looking at association between TL and risk of non-Hodgkins lymphoma, breast cancer, lung cancer and colorectal cancer; the single study in prostate cancer was negative. That study looked at leukocyte TL as a marker of prostate cancer risk within the participants of the Prostate, Lung, Colorectal and Ovarian (PLCO) screening trial [5]. In the main analysis prostate cancer cases and controls did not differ with respect to TL, although there was a trend to decreased risk of prostate cancer with shorter TL once adjusted for smoking status. Interestingly, in individuals with a family history of prostate cancer shorter telomeres seemed to be associated with increased prostate cancer risk, although again this did not reach significance. Published studies investigating breast cancer risk and TL have produced conflicting results, with two studies showing a significant association between longer telomeres and breast cancer risk [12], [13] but other studies showing no association, or association only in sub-groups [14]–[16]. A potential confounding factor is whether such studies are undertaken retrospectively or prospectively, with both chemotherapy and radiotherapy having the potential to affect peripheral blood lymphocyte TL [17], [18]. A recently published prospective study of over 47000 individuals followed-up for 20 years found an association between shorter telomere length and survival once diagnosed with cancer, but no association with cancer risk [19].
Both prostate and breast cancer have a heritable component to their aetiology [20]; much of the heritability of prostate cancer is thought to be due to the inheritance of multiple low penetrance susceptibility single nucleotide polymorphisms (SNPs), but inheritance of rarer BRCA1 or BRCA2 gene mutations is known to increase breast cancer risk and to a lesser extent prostate cancer risk. A recent paper demonstrated reduced TL in breast cancer patients who carried mutations in the BRCA1/2 genes compared with sporadic breast cancer cases [21]. The same group also demonstrated shorter TL in both sporadic and hereditary ovarian cancer compared with controls [22]; no similar investigation has been carried out in male BRCA1/2 mutation carriers with prostate cancer. Preclinical studies have suggested that BRCA2 has a role in telomere stabilisation [23]–[25]. Also some studies have shown that BRCA1 expression may have an effect on TL [26], [27], although the evidence is not as strong as for BRCA2. Both papers from Delgado-Martinez et al. included larger numbers of cases with mutations in BRCA1 than in BRCA2. In this study, we have analysed TL in two distinct cohorts; a retrospective female cohort of BRCA1/2 mutation carriers and their non-carrier relatives, a proportion of whom have a previous diagnosis of breast cancer, and a prospective male cohort of BRCA1/2 mutation carriers and controls, all cancer free at time of blood donation, a proportion of whom have developed prostate cancer during follow-up. Although BRCA1/2 mutations are predominantly associated with breast and ovarian cancer risk the risk of prostate cancer is increased in males, and TL may be useful for risk stratification. The association between genetic status and TL should not be affected by retrospective or prospective nature of the studies.
Materials and Methods
Ethics statement
IMPACT and RMH Carrier Clinic Set were approved by the National Health Service, Health Research Authority, National Research Ethics Service, London (reference numbers 05/MRE07/25 and 05/Q0801/7 respectively); all participants gave informed written consent.
Study cohorts
1. IMPACT set
TL was measured in blood DNA from male BRCA1 and BRCA2 mutation carriers and controls from the IMPACT study (Identification of Men with a genetic predisposition to ProstAte Cancer: Targeted Screening in BRCA1/2 mutation carriers and controls), an international study set up to evaluate PSA screening in male BRCA1 and BRCA2 carriers. To be eligible, men must be either a BRCA1 or BRCA2 mutation carrier or from a family harbouring the gene mutation but have tested negative themselves (controls). All men were aged between 40 and 69 years with no history of prostate cancer, and no previous biopsy for raised PSA. The recruits gave a blood sample for DNA extraction at enrolment and underwent annual PSA screening with PSA>3.0 ng/ml triggering a diagnostic prostate biopsy. A total of 240 BRCA1 and 207 BRCA2 male mutation carriers plus 218 controls were used in a prospective study of TL. As only four controls developed prostate cancer these were removed from the analyses i.e. the association between TL and prostate cancer risk was not tested in the non-carriers. Smoking status was divided into four categories (no, ex-smoker, yes currently, not known) with ‘no’ being the baseline category in the analyses.
2. Royal Marsden Hospital (RMH) Carrier Clinic set
BRCA1/2 mutation carriers and controls (non-carrier family members) were recruited to the RMH Carrier Clinic set, in which they donate blood samples for DNA extraction at enrolment. To be eligible, recruits must be of known BRCA1/2 mutation status and d over 18 years. Only samples from female recruits were used for the TL analysis. A total of 131 BRCA1 and 109 BRCA2 female mutation carriers plus 43 controls were used in a retrospective study of TL. One person was found to have mutations in both genes so was included in the analyses as a BRCA1 mutation carrier.
Telomere length quantification
Using quantitative real-time PCR, relative TL was measured in DNA commercially extracted from peripheral blood lymphocytes. 20 ng of DNA per sample was dried down in 384 plates. Each plate also contained serial dilutions of DNA giving 100ng DNA to 1ng DNA in duplicate to allow for efficiency calculations (efficiency = 10(−1/slope)) and three reference DNAs were added to each plate. All samples were tested in duplicate. The primer sequences quantifying TL were: 5′ CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT 3′ (TEL_F) and 5′ GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT 3′ (TEL_R) (Integrated DNA Technologies) [28].
The single copy gene used as a control (CON) was 36B4 (gene that encodes acidic ribosomal phosphoprotein) which had the advantage that it could be amplified under the same PCR conditions as the TEL, thus reducing the risk of inter-plate variation between the TEL and CON reactions. The 36B4 primer sequences were 5′ CAGCAAGTGGGAAGGTGTAATCC 3′ (36B4_F) and 5′ CCATTCTATCATCAACGGGTACAA 3′ (36B4_R) (Integrated DNA Technologies) [29]. 5 µl of 2× power SYBR Green PCR Master Mix (KapaBiosystems) and 4 µl H2O were added to each well; samples were left for two hours in the dark at 4°C for resuspension of DNA. The thermal cycling profile proceeded as follows: 10 minutes at 95°C followed by 35 cycles of 95°C for 15 seconds, 54°C for 2 minutes and 72°C for 15 seconds as used previously [21]. The qPCR amplification was performed using Applied Biosystems 7900HT real-time PCR system and the RQ Manager software version 1.2 was used to determine the relative TL.
The Ct value, defined as the number of PCR cycles taken for the amplified DNA to cross a predefined threshold, was used to calculate the telomere repeat copy number to single copy gene copy number ratio (T/S) using T/S = 2−ΔCt. The relative TL was determined by normalizing the ratio (T/S) of each sample to the calibrator DNA to standardise sample values across all reaction plates. For ease of data manipulation the natural log was taken of this value. Three internal controls DNA were run in each plate. For each run, PCR efficiency and internal controls values were monitored.
Statistical analysis
Age
Linear regression analysis was used in both sets to assess the association between age and TL. Correlation was assessed using Spearman's correlation.
Carrier status
Linear regression analyses were used to correlate TL with carrier status. The analyses were adjusted for age at enrolment and smoking status in the IMPACT set, and adjusted for age in the RMH Carrier Clinic set (smoking status was not available for the female set); analyses were carried out using a robust variance estimation to account for several individuals coming from the same family. The joint analysis of both BRCA1 and BRCA2 mutation carriers was stratified by gene.
Cancer Risk
Hazard ratios (HR) for prostate cancer according to TL in the IMPACT study were estimated using Cox regression, adjusting for smoking status and age at blood draw. Age at enrolment was used as the left-censor age.
Women in the RMH Carrier Clinic set were recruited on the basis of having a BRCA1/2 mutation or having a relative with such a mutation. A diagnosis of breast cancer, particularly at a young age, would very often be part of the criteria for genetic testing, and since women with breast cancer prior to recruitment were not excluded it is likely that cases of breast cancer would be overrepresented in the set. HRs from a standard Cox regression approach would be potentially biased, so we instead used a weighted cohort Cox regression analysis [30]. Individuals were weighted such that the observed breast cancer incidence rates in each age interval in the study samples were consistent with established external estimates of cancer risk among BRCA1 and BRCA2 mutation carriers in that age interval. As the study group was small and the majority of individuals (94%) were aged less than 60 years, weights were calculated using the post-1950 incidence rates. The analyses were carried out using a robust variance estimation to account for several individuals coming from the same family and were adjusted for age at blood draw. The joint analysis of both BRCA1 and BRCA2 mutation carriers was stratified by gene and the BRCA1 mutation incidence rates were used to calculate the weights.
Results
IMPACT set
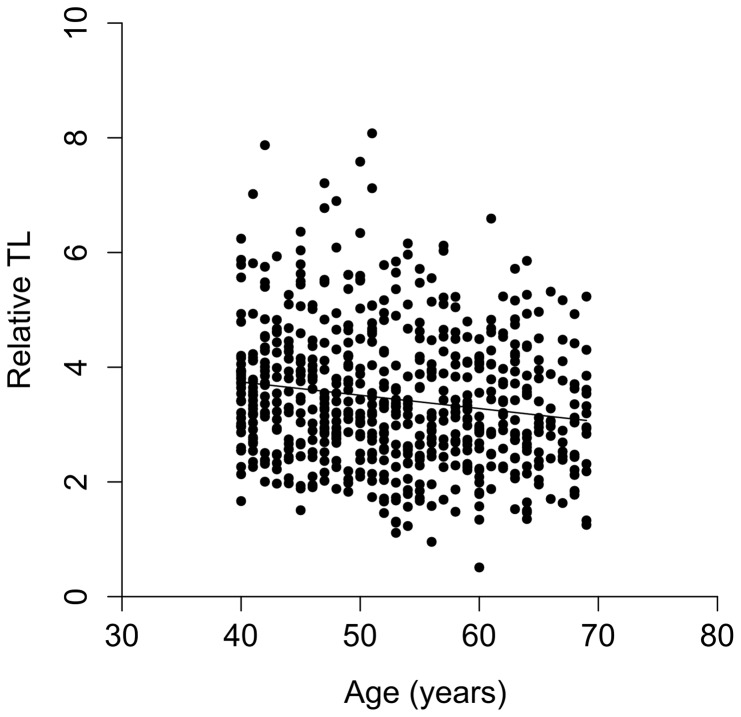
Participants' characteristics are summarised in Table 1. There was an approximately equal division of men between BRCA1 mutation carriers, BRCA2 mutation carriers and controls; 21 men had prostate cancer. The expected negative correlation between TL in and age was observed among those unaffected by cancer (r = −0.164, p<0.001), see Figure 1.
Table 1. Characteristics of IMPACT and RMH Carrier Clinic recruits.
| IMPACT | RMH Carrier Clinic set | |
| N | 665 | 283 |
| Mean age (range) | 53.0 (40–69) | 48.0 (20–78) |
| BRCA1 mutation carriers | 240 | 131 |
| BRCA2 mutation carriers | 207 | 109 |
| Controls | 214 | 43 |
| Affected by cancer (% of BRCA1/2 mutation carriers affected) | 21 (4.7%) | 124 (51.7%) |
| Mean age at cancer diagnosis (range) | 53.1 (41–68) | 42.7 (23–69) |
Figure 1. Correlation between relative TL of unaffected men in IMPACT set (n = 644) and age at blood-draw (r = −0.164, p<0.001).
We investigated if there was any association between BRCA mutation status and TL, analysing BRCA1 and BRCA2 mutation carriers separately and combined, and found no significant association, see Table 2. There was >90% power to detect a difference in TL of 10%.
Table 2. Association between BRCA1 or BRCA2 mutation status and mean relative TL in the IMPACT study, adjusting for age and smoking status, and in the RMH Carrier Clinic Set, adjusting for age.
| Cohort | Genetic status | n | Adjusted Mean rel TL (se) | P vs non-carriers |
| IMPACT set | BRCA1/2 Non-carrier | 214 | 1.17 (0.024) | - |
| BRCA1 Carriers | 240 | 1.16 (0.226) | 0.638 | |
| BRCA2 Carriers | 207 | 1.19 (0.024) | 0.768 | |
| All BRCA1&2 Carriers | 447 | 1.17 (0.017) | 0.900 | |
| RMH Carrier Clinic Set (all samples) | BRCA1/2 Non-carrier | 43 | 1.69 (0.082) | - |
| BRCA1 Carriers | 131 | 1.69 (0.041) | 0.969 | |
| BRCA2 Carriers | 110 | 1.65 (0.043) | 0.653 | |
| All BRCA1&2 Carriers | 241 | 1.67 (0.030) | 0.815 | |
| RMH Carrier Clinic Set (unaffected only) | BRCA1/2 Non-carrier | 43 | 1.72 (0.079) | - |
| BRCA1 Carriers | 65 | 1.70 (0.049) | 0.809 | |
| BRCA2 Carriers | 50 | 1.76 (0.049) | 0.746 | |
| All BRCA1&2 Carriers | 115 | 1.72 (0.037) | 0.987 |
Of the 240 BRCA1 mutation carriers, 5 developed prostate cancer, and of the 207 BRCA2 mutation carriers, 16 individuals were diagnosed. Statistical analysis showed no association between TL and prostate cancer risk. Hazard ratios (HR), 95% confidence intervals and P-values for the association of telomere lengths with prostate cancer risk are shown in Table 3.
Table 3. Hazard ratios for prostate cancer in the IMPACT study, (Cox regression analyses) and for breast cancer in the RMH study, (weighted retrospective Cox regression analyses), by BRCA1/2 mutation status.
| Cohort | Genetic status | Number with cancer | Number without cancer | HR | 95% CI | P |
| IMPACT set | BRCA1 mutation carriers | 5 | 235 | 1.00 | 0.085–11.7 | 0.999 |
| BRCA2 mutation carriers | 16 | 191 | 0.493 | 0.103–2.36 | 0.376 | |
| All mutation carriers | 21 | 426 | 0.658 | 0.188–2.30 | 0.512 | |
| RMH Carrier Clinic Set | BRCA1 mutation carriers | 66 | 65 | 1.063 | 0.448–2.53 | 0.889 |
| BRCA2 mutation carriers | 60 | 50 | 0.467 | 0.209–1.04 | 0.063 | |
| All mutation carriers | 126 | 115 | 0.782 | 0.449–1.36 | 0.384 |
RMH Carrier Clinic set
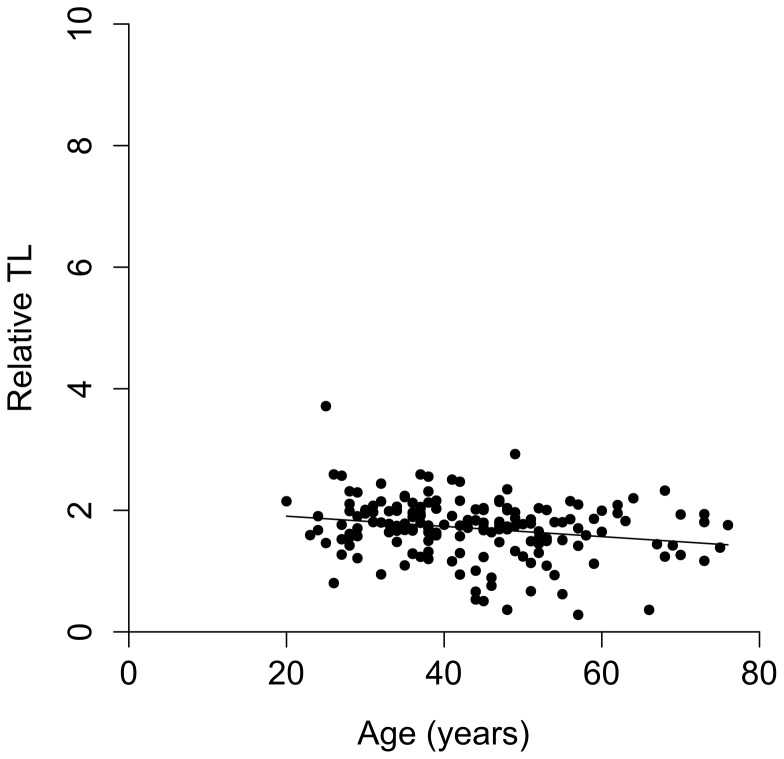
The participants' characteristics of this cohort are summarised in Table 1; approximately half the subjects had been affected by cancer. There was a negative correlation between TL and age among those unaffected by cancer (r = −0.126) as shown in Figure 2, this does not quite reach significance (p = 0.078); however when a linear regression model was used to assess the impact of age on TL this was found to be significant (p = 0.001).
Figure 2. Correlation between relative TL in unaffected women in the RMH Carrier Clinic set (n = 159) and age at blood-draw (r = −0.126, p = 0.078).
Stratifying by cancer status, we found no significant association between BRCA mutation status and TL, either considering BRCA1 and BRCA2 mutation carriers as separate groups, or as a combined set, see Table 2, however the power to detect a 10% difference was only 42%.
TL was compared in BRCA1/2 mutation carriers with cancer and unaffected BRCA1/2 mutation carriers. Statistical analysis showed no association between TL and breast cancer risk. Hazard ratios (HR), 95% confidence levels and P-values for the association of telomere lengths with breast cancer risk are shown in Table 3.
Discussion
To our knowledge this is the first published study of TL in male BRCA1/2 mutation carriers and the largest published study in female BRCA1/2 mutation carriers. We found no association between TL and BRCA1/2 mutation status in either of the two cohorts. Martinez-Delgado et al [21] demonstrated shorter TL in a smaller set of affected BRCA1 mutation carriers (n = 45), BRCA2 mutation carriers (n = 48) and healthy controls (n = 276); there was no difference between affected individuals with a family history but no mutation in BRCA1/2 (n = 105) and the healthy control group. However, it is not clear whether the effect was due to the BRCA1/2 mutation status or their cancer status, or indeed previous cancer treatment, as both chemotherapy and radiotherapy have been shown to affect TL [17], [18]. In order to prevent the potential confounding effect of high cancer rates we performed our analyses of the association between genetic status and TL separately for the affected and unaffected individuals. However this reduced the numbers in the analysis, increasing the chance of a false negative finding. A recently opened study is recruiting unaffected BRCA1/2 mutation carriers to explore any relationship between TL (plus a variety of metabolic and lifestyle factors) and breast cancer risk, and will avoid the compounding factors such as treatment effects which may bias the retrospective studies [31].
In the male set it is important that the individuals underwent screening for prostate cancer, as men in the general population are not routinely screened and prostate cancer is a condition which can go undiagnosed for many years. BRCA1/2 mutation carriers are at increased risk of developing prostate cancer and therefore, despite the limitations of screening, an unscreened cohort would be more likely to have cases of undiagnosed cancer with potential for bias.
Within the IMPACT set there was no difference in TL in BRCA1/2 mutation carriers with prostate cancer versus cancer free BRCA1/2 mutation carriers. This finding is in keeping with Mirabello et al [5] who did not find a difference in TL in a retrospective study of men with prostate cancer versus healthy controls. However, they did find a trend towards shorter TL in those with a family history of prostate cancer, which, in combination with the fact that TL appeared to have an effect on breast cancer risk in female BRCA1/2 mutation carriers [21], had driven our investigation into association of TL with prostate cancer risk in male BRCA1/2 mutation carriers. It should be noted that in this study there were only 21 cases with cancer and mean follow-up time was 46 months. Within the IMPACT study, recruits undergo biopsy based on PSA screening with a PSA>3.0 ng/ml triggering biopsy. Given the known limitations of PSA screening [32], it may be that some recruits harbour occult prostate cancer, or some are yet to develop cancer and with further follow-up there is the possibility that a difference in TL may be appreciated.
In the retrospective female BRCA1/2 mutation carrier set, we found no significant difference in TL between those with breast cancer and cancer-free controls. There has been a discordance in previously published studies of TL in association with sporadic breast cancer, with some studies showing association and others not [14]. The only published study quantifying TL in individuals with breast cancer who carry BRCA1/2 mutations and individuals with sporadic breast cancer showed an association between shorter telomeres in those affected by hereditary cancer, but not in those affected by sporadic breast cancer. In this study, we compared BRCA1/2 mutation carriers with cancer and BRCA1/2 mutation carriers without cancer, predominantly to investigate the possibility of using TL as a method of risk stratification amongst BRCA1/2 mutation carriers. We found no evidence that TL could be useful in this way.
Supporting Information
The IMPACT study: Identification of Men with a Genetic Predisposition to Prostate Cancer: Targeted Screening in BRCA1 and BRCA2 carriers and controls. The IMPACT study collaborators.
(DOCX)
Acknowledgments
We would like to thank the patients who enrolled in these studies and their families; we would also like to thank the IMPACT study collaborators.
Funding Statement
The authors acknowledge funding from the Annabel Evans Memorial Fund, CRUK (grant numbers C5047/A13232 and C5047/A15007), NIHR support to the Biomedical Research Centre at The Institute of Cancer Research and Royal Marsden NHS Foundation Trust and the Ronald and Rita McAulay foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, et al. (1988) A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A 85: 6622–6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14: 501–513. [DOI] [PubMed] [Google Scholar]
- 3. Blackburn EH (1991) Structure and function of telomeres. Nature 350: 569–573. [DOI] [PubMed] [Google Scholar]
- 4. Vaziri H, Schachter F, Uchida I, Wei L, Zhu X, et al. (1993) Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet 52: 661–667. [PMC free article] [PubMed] [Google Scholar]
- 5. Mirabello L, Huang WY, Wong JY, Chatterjee N, Reding D, et al. (2009) The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell 8: 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiels PG, McGlynn LM, MacIntyre A, Johnson PC, Batty GD, et al. (2011) Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS One 6: e22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim S, Parks CG, DeRoo LA, Chen H, Taylor JA, et al. (2009) Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev 18: 816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murillo-Ortiz B, Albarran-Tamayo F, Arenas-Aranda D, Benitez-Bribiesca L, Malacara-Hernandez J, et al. (2012) Telomere length and type 2 diabetes in males, a premature aging syndrome. Aging Male 15: 54–58. [DOI] [PubMed] [Google Scholar]
- 9. Risques RA, Lai LA, Brentnall TA, Li L, Feng Z, et al. (2008) Ulcerative colitis is a disease of accelerated colon aging: evidence from telomere attrition and DNA damage. Gastroenterology 135: 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, et al. (2011) Life stress, emotional health, and mean telomere length in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. J Gerontol A Biol Sci Med Sci 66: 1152–1162. [DOI] [PubMed] [Google Scholar]
- 11. Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA (2011) The Association of Telomere Length and Cancer: a Meta-analysis. Cancer Epidemiol Biomarkers Prev 20: 1238–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gramatges MM, Telli ML, Balise R, Ford JM (2010) Longer relative telomere length in blood from women with sporadic and familial breast cancer compared with healthy controls. Cancer Epidemiol Biomarkers Prev 19: 605–613. [DOI] [PubMed] [Google Scholar]
- 13. Svenson U, Nordfjall K, Stegmayr B, Manjer J, Nilsson P, et al. (2008) Breast cancer survival is associated with telomere length in peripheral blood cells. Cancer Res 68: 3618–3623. [DOI] [PubMed] [Google Scholar]
- 14. Pooley KA, Sandhu MS, Tyrer J, Shah M, Driver KE, et al. (2010) Telomere length in prospective and retrospective cancer case-control studies. Cancer Res 70: 3170–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Vivo I, Prescott J, Wong JY, Kraft P, Hankinson SE, et al. (2009) A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 18: 1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen J, Terry MB, Gurvich I, Liao Y, Senie RT, et al. (2007) Short telomere length and breast cancer risk: a study in sister sets. Cancer Res 67: 5538–5544. [DOI] [PubMed] [Google Scholar]
- 17.Maeda T, Nakamura K, Atsumi K, Hirakawa M, Ueda Y, et al.. (2012) Radiation-associated changes in the length of telomeres in peripheral leukocytes from inpatients with cancer. Int J Radiat Biol. [DOI] [PubMed]
- 18.Diker-Cohen T, Uziel O, Szyper-Kravitz M, Shapira H, Natur A, et al.. (2013) The effect of chemotherapy on telomere dynamics: clinical results and possible mechanisms. Leuk Lymphoma. [DOI] [PubMed]
- 19. Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, et al. (2013) Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst 105: 459–468. [DOI] [PubMed] [Google Scholar]
- 20. Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, et al. (2000) Environmental and heritable factors in the causation of cancer – analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343: 78–85. [DOI] [PubMed] [Google Scholar]
- 21. Martinez-Delgado B, Yanowsky K, Inglada-Perez L, Domingo S, Urioste M, et al. (2011) Genetic anticipation is associated with telomere shortening in hereditary breast cancer. PLoS Genet 7: e1002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinez-Delgado B, Yanowsky K, Inglada-Perez L, de la Hoya M, Caldes T, et al. (2012) Shorter telomere length is associated with increased ovarian cancer risk in both familial and sporadic cases. J Med Genet 49: 341–344. [DOI] [PubMed] [Google Scholar]
- 23. Min J, Choi ES, Hwang K, Kim J, Sampath S, et al. (2012) The breast cancer susceptibility gene BRCA2 is required for the maintenance of telomere homeostasis. J Biol Chem 287: 5091–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Badie S, Escandell JM, Bouwman P, Carlos AR, Thanasoula M, et al. (2010) BRCA2 acts as a RAD51 loader to facilitate telomere replication and capping. Nat Struct Mol Biol 17: 1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bodvarsdottir SK, Steinarsdottir M, Bjarnason H, Eyfjord JE (2012) Dysfunctional telomeres in human BRCA2 mutated breast tumors and cell lines. Mutat Res 729: 90–99. [DOI] [PubMed] [Google Scholar]
- 26. Hu H, Zhang Y, Zou M, Yang S, Liang XQ (2010) Expression of TRF1, TRF2, TIN2, TERT, KU70, and BRCA1 proteins is associated with telomere shortening and may contribute to multistage carcinogenesis of gastric cancer. J Cancer Res Clin Oncol 136: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 27. Cabuy E, Newton C, Slijepcevic P (2008) BRCA1 knock-down causes telomere dysfunction in mammary epithelial cells. Cytogenet Genome Res 122: 336–342. [DOI] [PubMed] [Google Scholar]
- 28. McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I (2007) Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 16: 815–819. [DOI] [PubMed] [Google Scholar]
- 29. Codd V, Mangino M, van der Harst P, Braund PS, Kaiser M, et al. (2010) Common variants near TERC are associated with mean telomere length. Nat Genet 42: 197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Antoniou AC, Goldgar DE, Andrieu N, Chang-Claude J, Brohet R, et al. (2005) A weighted cohort approach for analysing factors modifying disease risks in carriers of high-risk susceptibility genes. Genet Epidemiol 29: 1–11. [DOI] [PubMed] [Google Scholar]
- 31. Guinan EM, Hussey J, McGarrigle SA, Healy LA, O'Sullivan JN, et al. (2013) A prospective investigation of predictive and modifiable risk factors for breast cancer in unaffected BRCA1 and BRCA2 gene carriers. BMC Cancer 13: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Killick E, Bancroft E, Kote-Jarai Z, Eeles R (2012) Beyond Prostate-specific Antigen – Future Biomarkers for the Early Detection and Management of Prostate Cancer. Clin Oncol (R Coll Radiol). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The IMPACT study: Identification of Men with a Genetic Predisposition to Prostate Cancer: Targeted Screening in BRCA1 and BRCA2 carriers and controls. The IMPACT study collaborators.
(DOCX)