Abstract
We have previously shown that live-attenuated rabies virus (RABV)-based vaccines infect and directly activate murine and human primary B cells in-vitro, which we propose can be exploited to help develop a single-dose RABV-based vaccine. Here we report on a novel approach to utilize the binding of Intracellular Adhesion Molecule-1 (ICAM-1) to its binding partner, Lymphocyte Function-associated Antigen-1 (LFA-1), on B cells to enhance B cell activation and RABV-specific antibody responses. We used a reverse genetics approach to clone, recover, and characterize a live-attenuated recombinant RABV-based vaccine expressing the murine Icam1 gene (rRABV-mICAM-1). We show that the murine ICAM-1 gene product is incorporated into virus particles, potentially exposing ICAM-1 to extracellular binding partners. While rRABV-mICAM-1 showed 10-100-fold decrease in viral titers on baby hamster kidney cells compared to the parental virus (rRABV), rRABV-mICAM-1 infected and activated primary murine B cells in-vitro more efficiently than rRABV, as indicated by significant upregulation of CD69, CD40, and MHCII on the surface of infected B cells. ICAM-1 expression on the virus surface was responsible for enhanced B cell infection since pre-treating rRABV-mICAM-1 with a neutralizing anti-ICAM-1 antibody reduced B cell infection to levels observed with rRABV alone. Furthermore, 100-fold less rRABV-mICAM-1 was needed to induce antibody titers in immunized mice equivalent to antibody titers observed in rRABV-immunized mice. Of note, only 103 focus forming units (ffu)/mouse of rRABV-mICAM-1 was needed to induce significant anti-RABV antibody titers as early as five days post-immunization. As both speed and potency of antibody responses are important in controlling human RABV infection in a post-exposure setting, these data show that expression of Icam1 from the RABV genome, which is then incorporated into the virus particle, is a promising strategy for the development of a single-dose RABV vaccine that requires only a minimum of virus.
Introduction
Rabies virus (RABV) causes a deadly zoonotic infection that targets and causes dysfunction within the central nervous system (CNS) of infected hosts. Upon manifestation of symptoms, rabies is nearly always fatal [1]. It is estimated that RABV is responsible for 55,000 human deaths per year worldwide, though this number may be much larger [2]. Most of the disease burden is located in the developing nations of Asia and Africa, where it is estimated that 3.3 billion people live at risk of RABV infection [2]. Of those infected, 40% are under 15-years-of-age [2]. Over 15 million people receive post-exposure prophylaxis (PEP) after exposure to a potentially infected animal [2]. If administered in a timely and appropriate manner, current PEP is nearly 100% successful in preventing human RABV infection. This, together with routine vaccination of domestic animals, has resulted in a dramatic reduction of human RABV infections in developed countries over the last 50–60 years [3].
Current, standard PEP for previously unvaccinated, immunocompetent individuals includes prompt wound cleaning and the administration of four to five doses of inactivated vaccine, and in the case of severe exposure, one dose of rabies immune globulin (RIG) [2], [4]. The efficacy of rabies PEP in developing countries where rabies is highly endemic is hindered by high costs and a lack of compliance, which emphasize the need for a single-dose RABV-based vaccine to combat this global public health threat [reviewed in [5], [6]. However, it does not appear that this single-dose vaccine will be based on currently available inactivated vaccines. A recent study by Strady et. al. showed that a minimum of three doses of the current inactivated RABV vaccine are required to reduce the percentage of non- or poor-responders [less than 0.5 international unit (IU)/ml] to just 3% in a pre-exposure setting [7]. In contrast, live attenuated virus vaccines tend to be much more immunogenic than their inactivated counterparts as shown in non-human primates immunized with a replication-deficient RABV-based vaccine compared to the commercially available Human Diploid Cell Vaccine (HDCV) [8].
To help delineate factors that contribute to the efficacy of live attenuated RABV-based vaccines in the context of B cell activation, immunity and protection, we recently showed that live, highly attenuated RABV-based vaccine vectors induce T cell-independent and extrafollicular T cell-dependent B cell responses that provide protection against pathogenic RABV challenge [9]. Importantly, vaccine-induced IgM helps to prevent the spread of a pathogenic RABV strain to the CNS and thereby contributing to protection within days of immunization [10]. We also showed that RABV-based vaccines efficiently infect naïve primary murine and human B cells ex-vivo, resulting in the significant upregulation of early markers of B cell activation and antigen presentation, including CD69, MHCII, and CD40 in murine B cells or HLA-DR and CD40 in human B cells compared to cells treated with an inactivated RABV-based vaccine [11]. Primary B cells infected with a live attenuated RABV expressing ovalbumin directly prime and stimulate naïve CD4+ OT-II T cells to proliferate and to secrete IL-2, demonstrating an important functional consequence of B cell infection and activation by live RABV-based vaccine vectors [11]. We propose that this direct B cell stimulation by live RABV-based vaccines is a potential mechanism underlying their induction of early, protective B cell responses, and that live RABV-based vaccines designed to infect and activate B cells represent a promising strategy to develop a single-dose post-exposure rabies vaccine where the generation of early protective antibody titers is critical.
To this end, in this report, we investigated the utility of incorporating ICAM-1 into a RABV-based vaccine to target B cells and thereby improve B cell infection, activation and anti-RABV humoral immunity. ICAM-1 (CD54) is a transmembrane protein of the immunoglobulin (Ig) superfamily of proteins [12] whose binding partners include LFA-1 (CD18/CD11a) and macrophage-1 antigen [(MAC-1); (CD18/CD11b)], both of which are integrin family members [13], as well as the clotting protein fibrinogen. ICAM-1 is present on various cell lineages including leukocytes, dendritic cells (DCs), follicular dendritic cells (FDCs), and endothelial cells [14]. LFA-1 is present on leukocytes, including B cells, DCs, and natural killer (NK) cells [15]. Traditionally, ICAM-1 has been known to participate in cell-cell adhesion, cell migration, and extravasation of immune cells into sites of inflammation. However, the function of ICAM-1 in immunity is much more complex. ICAM-1 has been implicated as an additional costimulatory molecule involved in T cell stimulation [16], [17]. Cooperative signaling through ICAM-1 interaction may be required for optimum response of CD4 T cells to MHCII-presented peptide antigen [18].
In addition to the expression and function of ICAM-1 on CD4 T cells to stabilize interactions with cells bearing MHCII-presented antigens, ICAM-1 may play important roles in viral immunology. Specifically, ICAM-1 is naturally incorporated into HIV-1 particles and enhances infection of LFA-1-expressing cells by stabilizing virus:cell interactions and supporting viral uptake [19]–[22], suggesting the incorporation of ICAM-1 into a virus that can target B cells, such as attenuated RABV strains [11], may help to enhance B cell infection and activation. In addition, Carrasco et. al. showed that upon BCR engagement, LFA-1 on the surface of B cells is recruited to the BCR synapse, where lipid bilayer-anchored ICAM-1 can bind to LFA-1 and lower the antigen threshold required for B cell activation [23]. This suggests vaccine strategies that exploit ICAM-1/LFA-1 binding interactions on B cells may promote effective B cell immunity with a minimal vaccine dose. Finally, Denning et. al. showed that B cells with higher levels of surface ICAM-1 expression, which might be the case for B cells infected with a recombinant RABV-based vaccine expressing ICAM-1, stimulate T cells to a greater magnitude than do B cells expressing low levels of surface ICAM-1 [24]. Together, an ICAM-1-based RABV vaccine holds the potential to influence B cells responses directly by aiding in B cell infection, B cell activation and B:T interactions, resulting in enhanced anti-RABV antibody responses.
In this report, we exploited the potential immune enhancing features of ICAM-1 expression in the context of RABV vaccination. We constructed, grew and characterized a live-attenuated rRABV-based vaccine expressing the murine Icam1 gene (rRABV-mICAM-1). When assessed in-vitro, rRABV-mICAM-1 showed greater infection and activation of naive primary mouse B cells compared to a parental rRABV-based vaccine strain. In addition, mice primed intramuscularly with one low-dose of rRABV-mICAM-1 showed higher, more rapid antibody responses in-vivo as early as five days post-immunization compared to mice primed with the same dose of rRABV. Taken together, these data indicate that rRABV-mICAM-1 is capable of significant infection and activation of B cells and is capable of inducing high, early antibody titers using only one, low-dose inoculation. As such, expression of Icam1 from the RABV genome is a promising strategy for the development of a single-dose RABV vaccine.
Materials and Methods
Ethics statement
All animal work was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Jefferson Medical College, Thomas Jefferson University. Work was completed in accordance with international standards [Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)] and in compliance with Public Health Service Policy on Humane Care and Use of Laboratory Animals, The Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH).
Recombinant RABV-based vaccine construction and recovery
rRABV is a recombinant RABV-based vaccine vector and is a molecular clone of the SAD-B19 vaccine strain of RABV [25], [26]. To construct rRABV expressing ICAM-1, the Icam1 gene of Mus musculus encoded in pBlueScript SK+ (ATCC) was amplified by polymerase chain reaction (PCR) with VENT polymerase [New England Biolabs (NEB)] using forward primer JPM24 (5′ – TTTCGTACGATTATGGCTTCAACCCGTGCCAAG – 3′) (BsiWi underlined) and reverse primer JPM25 (5′ – AAATCTAGATCAGGGAGGTGGGGCTTG – 3′) (XbaI underlined). The PCR product was digested with BsiW1 (NEB) and Xba1 (NEB) and ligated into the plasmid prRABV that had previously been digested with BsiW1 and Nhe1 (NEB), resulting in a plasmid named prRABV-ICAM-1. Infectious virus was recovered following standard methods to recover infectious RABV-based vaccines from cDNA [25], [27]. Virus was grown on baby hamster kidney cells and then purified by ultracentrifugation over a 20% sucrose cushion to remove soluble ICAM-1 (sICAM-1) contained in the supernatants of the produced virus.
Vaccine characterization
Western Immunoblotting and Silver Stain
BSR cells (a derivative of a baby hamster kidney cell line) were infected with rRABV or rRABV-mICAM-1 at a multiplicity of infection (MOI) of 2 focus-forming units (ffu). Uninfected cells served as negative controls. Cells were incubated at 37°C with 5% CO2 overnight. Cells were lysed with RIPA buffer (Sigma-Aldrich) and cellular debris pelleted out. Alternatively, sucrose-purified rRABV or rRABV-mICAM-1 were lysed with RIPA buffer. Proteins were separated via 10% SDS-PAGE and transferred to a polyvinylidene membrane. The membrane was blocked with 5% nonfat milk powder in 1X PBS for 1 hour at room temperature. The membrane was incubated with 0.1 µg/ml of polyclonal goat IgG murine ICAM-1 (R&D Systems, Inc.) in Western blot (WB) wash buffer (PBS, 0.05% Tween 20) at 4°C overnight. The membrane was washed three times with WB wash buffer and incubated with secondary antibody (donkey anti-goat IgG horseradish peroxidase; Jackson ImmunoResearch Laboratories) at 1∶50,000 in WB wash buffer for 1 hour at room temperature. The membrane was washed three times with WB wash buffer and twice with 1X PBS prior to performing a chemiluminescence assay (Thermo Fisher Scientific, Inc.) as instructed by the manufacturer. To help confirm incorporation of ICAM-1 into the RABV particle, virus particles were first purified over a 20% sucrose cushion and the purified virus was loaded onto a sucrose step gradient (20% to 40% in 5% increments) and then centrifuged for 2 hours at >48K g. Virus particles were isolated from the single band located at the 35–40% interface. Proteins were separated via 10% SDS-PAGE and then visualized by silver staining as described by the manufacturer (Pierce). The gel was scanned and then converted to grayscale for publication.
One- and Multi-Step Growth Curves
Growth kinetics of rRABV and rRABV-mICAM-1 were determined in parallel as previously described [28]. Briefly, BSR cells were seeded at 5.0×105cells/well in 6-well plates (Corning, Inc.) in Dulbecco's Modification of Eagle's Media (DMEM) containing 5% heat-inactivated FBS/1% Penicillin/Streptomycin and infected 24 hours later with rRABV or rRABV-mICAM-1 at a MOI of 5 or 0.01 for one-step or multi-cycle growth curves, respectively. After a two hour incubation, cells were extensively washed with 1X PBS, and supplemented with DMEM. Tissue culture supernatants (100 µl) were harvested at the indicated time points. Titers of infectious supernatants were determined using BSR cells in duplicate.
In-vitro infection, activation and flow cytometry analysis of primary murine splenocyte cultures
In-vitro infection
Single cell suspensions (106 cells/ml) from spleens of naïve female C57BL/6 mice aged 6–8 weeks were cultured in splenocyte medium (RPMI 1640 containing 10% FBS, 50 mM beta-mercaptoethanol, 100 IU/mL Penicillin/Streptomycin, and 100 mM HEPES). Cells were infected with rRABV, rRABV-mICAM-1, or media alone (mock-infected) at a MOI of 5 and incubated at 37°C with 5% CO2 for two days in the absence of additional mitogens to maintain the B cells and accessory splenocytes in a resting state similar to that in which they would exist in-vivo at the time of initial immunization [11].
Immunostaining and Flow Cytometry
Infected or mock-infected cells were harvested two days post-infection (p.i.). Cells were washed with FACS buffer (PBS containing 2% heat-inactivated FBS) and stained with fluorophore-conjugated antibodies. Cellular surface markers stained included CD40, CD45R (B220), CD69, and MHCII (all from eBioscience). Cells were washed twice with FACS buffer and fixed in 2% paraformaldehyde at 4°C for 30 min. Cells were washed twice with FACS buffer and once with PermWash permeabilization buffer (BD Biosciences) for intracellular staining. Cells were incubated in PermWash containing FITC-conjugated RABV nucleoprotein (N)-specific antibody (Fujirebio Diagnostics, Inc.) and phycoerythrin-conjugated anti-murine ICAM-1 antibody (R&D Systems, Inc.) at 4°C for 15 min. Cells were washed twice with FACS buffer and resuspended in FACS buffer prior to flow cytometry analysis. Fluorescently-labeled cells were analyzed by FACScan [(BD LSRII; BD Biosciences); (Kimmel Cancer Center, Jefferson Flow Cytometry Facility, Jefferson Medical College, Thomas Jefferson University)] and FlowJo software (TreeStar, Inc.) [11], [29]. To determine the effects of blocking ICAM-1 function on the surface of virus particles, sucrose-purified rRABV-mICAM-1 virions were incubated with anti-mICAM-1 antibody (5 µg/ml; R&D Systems, Inc.) for 2 hours at 37°C with 5% CO2. Single cell suspensions of murine splenocytes were prepared and treated with virus:antibody mix for two days and then stained as described above for RABV N, B220 and MHCII. To compare two groups of data, we used an unpaired, two-tailed Student's t test. (* p<0.05; **, p<0.01; ***, p<0.001) (N = 3 completed in duplicate per group).
In-vivo immunogenicity studies
Immunization
Groups of 5 naïve C57BL/6 female mice aged 6–8 weeks (National Cancer Institute, National Institutes of Health, Bethesda, MD) were immunized intramuscularly (i.m.) in the hind legs with 103 ffu of rRABV or rRABV-mICAM-1. Additional groups of mice were immunized as just described except using a higher vaccine dose (105 ffu). PBS-immunized mice served as negative controls.
Sample collection and immune assays
Blood was collected from all mice by retro-orbital eye bleed on various days post-immunization. Sera were analyzed for anti-RABV G total IgG and IgM antibody titers by ELISA as previously described [30], [31]. Statistical difference in antibody titers by ELISA between two groups of data was determined using an unpaired, two-tailed t test and data is presented at the mean ± SEM. *p<0.05, **p = 0.01−0.001, ***p≤0.001. (N = 5 mice per group). Virus-neutralizing antibody titers were performed using the Modified Rapid Fluorescent Focus Inhibition Test (RFFIT) as previously described [8], [32]. Neutralization titers were normalized to IU/ml using the World Health Organization standard human anti-rabies immunoglobulin reference.
Results
Construction, Recovery and Characterization of a rRABV-based Vaccine Vector Expressing Murine Icam1 Gene (rRABV-mICAM-1)
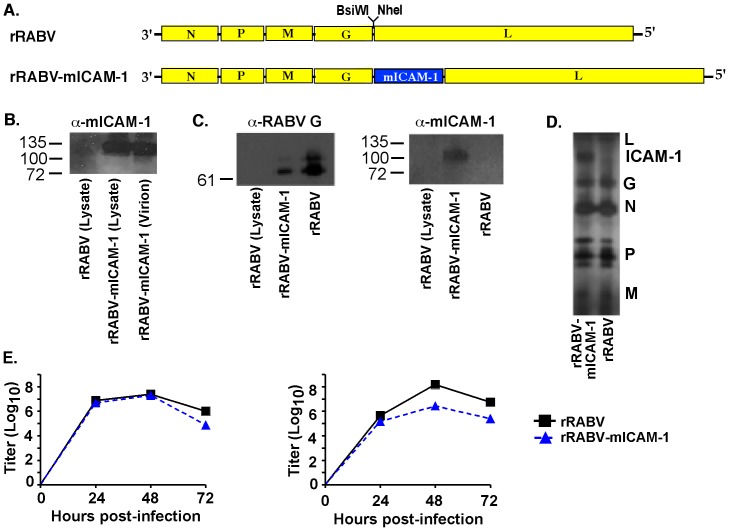
We previously showed that live RABV-based vaccines are highly immunogenic in mice and non-human primates [8], [31], [32]. We also showed that live RABV-based vaccines infect naïve primary murine and human B cells, resulting in B cell activation and the ability of B cells to prime naïve T cells to proliferate and secrete IL-2 [11]. To determine whether B cell infection and activation could be enhanced by expressing murine ICAM-1 from a rRABV-based vaccine in-vitro, and whether enhanced B cell infection and activation leads to improved anti-RABV antibody responses in-vivo, we constructed and recovered a recombinant, attenuated vaccine strain of RABV expressing the murine Icam1 gene (rRABV-mICAM-1, Figure 1A). Western blot analysis of lysed BSR cells, which do not express endogenous ICAM-1 or its binding partner LFA-1, infected with rRABV or rRABV-mICAM-1 confirmed expression of the Icam1 gene from rRABV-mICAM-1 but not rRABV or uninfected cells (Figure 1B). Western blot analysis of sucrose-purified and concentrated virus showed that the ICAM-1 protein was incorporated into the rRABV-mICAM-1 viral particle (Figure 1B). To help confirm that ICAM-1 was incorporated into the RABV particle, sucrose-purified rRABV or rRABV-mICAM-1 were isolated on a 5% step sucrose gradient and the single band collected. Western blot analysis detected ICAM-1 from the same density within the gradient as RABV (Figure 1C), indicating that ICAM-1 migrates with RABV in the sucrose gradient. To determine whether incorporation of ICAM-1 into the virus affects the content of viral proteins within the particle, we completed silver staining of the step gradient-purified particles. As shown in Figure 1D, similar levels of each viral protein were detected in rRABV or rRABV-mICAM-1 particles indicating incorporation of ICAM-1 does not affect the composition of viral proteins. While it was previously determined that the RABV G cytoplasmic domain is required for incorporation of a foreign protein into the RABV particle [33], this requirement is not absolute, as demonstrated here in the case of ICAM-1 expression and elsewhere [34]. One-step and multi-cycle growth curves were prepared to measure replication rates of rRABV-mICAM-1 compared to rRABV in BSR cells. Viral titers of supernatants harvested from cells infected with a high multiplicity of infection (MOI = 5; one-step growth curves) show that rRABV-mICAM-1 produces progeny virus at similar levels and with similar kinetics as does rRABV (Figure 1E, left panel). However, viral titers of supernatants harvested from cells infected with a low multiplicity of infection (MOI) (MOI = 0.01; multi-cycle growth curves) show that rRABV-mICAM-1 is slightly spread-deficient compared to rRABV, as indicated by 10–100-fold lower viral titers (Figure 1E, right panel). Since BSR cells are not expected to express a ligand for mICAM-1, differences in growth kinetics most likely arise from the insertion of a foreign gene and not due to ICAM-1 function. Together, an infectious live RABV-based vaccine vector that expresses the murine ICAM-1 protein from the viral genome, which is incorporated into viral particles, was cloned and recovered and shown to be somewhat deficient in its ability to spread in-vitro.
Figure 1. Construction, recovery and characterization of a recombinant RABV-based vaccine expressing the murine Icam1 gene (rRABV-mICAM-1).
A) At the top is a parental recombinant RABV-based vaccine containing an additional transcription stop-start signal flanked by two unique restriction sites between the G and the L genes. rRABV, which is a molecular clone of the SAD-B19 vaccine strain of RABV, was the target to introduce the gene encoding murine ICAM-1. B). BSR cells, which are not expected to express ICAM-1 or its binding partner LFA-1, were infected with rRABV or rRABV-mICAM-1 and lysed 48 hours later. Proteins were separated by SDS-PAGE and subjected to Western blotting with antibodies specific for ICAM-1. A protein of the expected size for ICAM-1 was detected from lysates of rRABV-mICAM-1-infected but not from rRABV-infected BSR cells. In parallel, sucrose-purified and concentrated virus was analyzed by western blot analysis and a protein of the expected size for ICAM-1 was detected in the virion particle. C) Sucrose-purified rRABV-mICAM-1 was re-purified using 5% sucrose step gradient and the single band was collected and analyzed by Western blot analysis to show ICAM-1 protein migrated within the sucrose gradient at the same density as rRABV. RABV and lysates from uninfected BSR cells served as controls for the Western blot analyses. D) Silver staining of separated proteins from sucrose step gradient-purified particles shows a protein at the expected size of ICAM-1 and that the incorporation of ICAM-1 into the virus particles does not affect viral protein compositions. E) BSR cells were infected with rRABV or rRABV-mICAM-1 at a MOI of 5 (one step growth; left) or .01 (multi-cycle growth curve; right). Aliquots of tissue culture supernatants were collected, and viral titers were determined in duplicate.
rRABV-mICAM-1 Infects Primary Murine B Cells In-vitro More Effectively Than Does rRABV
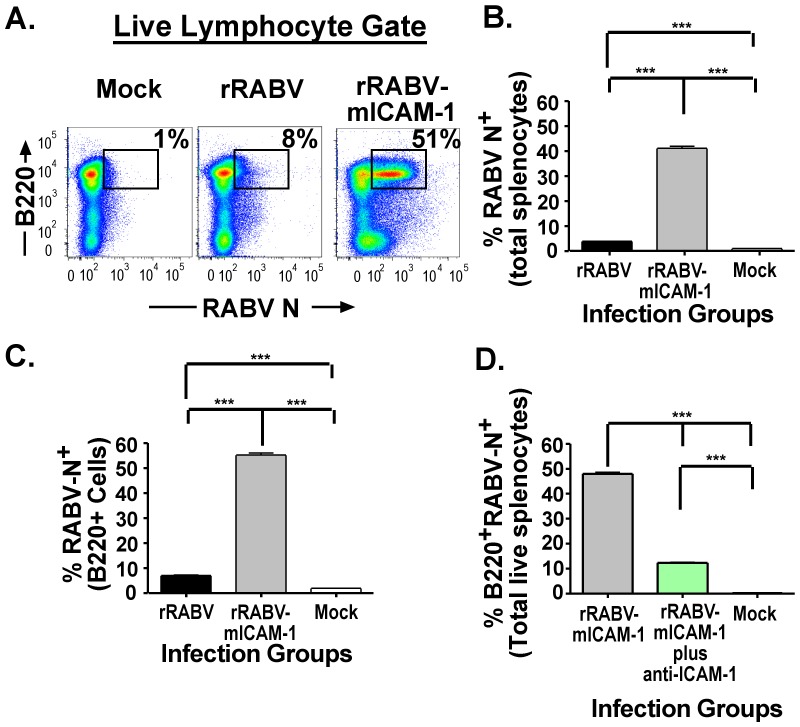
As noted previously, HIV-1 naturally incorporates ICAM-1 into HIV-1 particles, which enhances infectivity of LFA-1-expressing target cells [19]–[21]. Based on this finding, and our previous findings that live attenuated RABV vaccine strains infect and activate primary murine and human B cells in-vitro, we hypothesized that a recombinant RABV vaccine vector that expresses and incorporates ICAM-1 into the viral particle would promote infection of B cells, resulting in enhanced B cell activation in-vitro. Naïve primary murine splenocytes were infected at an MOI of 5 with rRABV or rRABV-mICAM-1, or treated with medium alone (mock infected) for two days in-vitro. No additional mitogens were added to the culture to avoid expressing activation molecules that could enhance sensitivity to RABV infection and activation [11]. Figure 2A shows a representative gating strategy of total live splenocytes stained for RABV N as a marker for infection and for cell-surface expression of B220 as a maker for B cells [9], [29]. Consistent with our previous data showing that live rRABV-based vaccines infect primary murine splenocytes [11], approximately 5% of the total splenocyte cells were infected with rRABV, which is statistically significant compared to mock-infected cells (<1%) (Figure 2B). However, almost 40% of the total splenocytes cells were infected with rRABV-mICAM-1 (Figure 2B). In addition, over 50% of the B cells in culture were infected with rRABV-mICAM-1 compared to about 8% of the B cells infected with rRABV, or 1% background levels in mock-infected cultures (Figure 2C). Of note, the enhanced infection of B cells with rRABV-mICAM-1 was largely due to ICAM-1 incorporation into the virus particle, since pretreatment of sucrose-purified rRABV-mICAM-1 with a neutralizing anti-ICAM-1 antibody significantly reduced infection of B cells in culture (Figure 1D). Together, these data indicate that expression of ICAM-1 from a rRABV-based vaccine enhances infection of primary murine splenocytes, with a propensity for infecting B cells.
Figure 2. rRABV-mICAM-1 infects primary murine splenocytes and B cells more efficiently than the parental virus, rRABV.
Naïve primary murine splenocytes were infected at an MOI of 5 with rRABV or rRABV-mICAM-1, or treated with media alone (mock-infected) for two days in-vitro. No additional mitogens were added to the culture to maintain the B cells and accessory splenocytes in a resting state similar to that in which they would exist in-vivo at the time of initial immunization. A) Representative gating strategy of total live splenocytes stained for intracellular RABV N as a marker for infection and for cell-surface expression of B220 as a maker for B cells. The percentage in the upper right quadrant is a representative example of the percentage of B220+ B cells infected with the rRABV-based vaccine. B) Percent RABV N+ cells in the total live lymphocyte gate. C) Percent RABV N+ cells in the B220+ cell population. D) Pretreatment of sucrose-purified rRABV-mICAM-1 with a neutralizing anti-ICAM-1 antibody significantly reduced infection of B cells in culture. To compare two groups of data, we used an unpaired, two-tailed Student's t test. (* p<0.05; **, p<0.01; ***, p<0.001)
Next, we wanted to evaluate the expression levels of ICAM-1 in primary murine splenocytes infected with rRABV and whether expression of ICAM-1 was increased in cultures infected with rRABV-mICAM-1. Figure 3A shows a representative gating strategy of total live splenocytes gated for ICAM-1 and for B220 expression. Figure 3B shows a representative strategy of B220+ B cells gated for ICAM-1 and RABV N. Expression of ICAM-1 was significantly increased in total mouse splenocyte cultures (Figure 3C) treated with rRABV compared to mock-infected cultures. This finding indicates that host-cell Icam1 gene expression is upregulated in murine splenocytes upon infection with attenuated RABV strains. ICAM-1 expression was significantly enhanced in splenocytes cultures treated with rRABV-mICAM-1 compared to rRABV-infected cultures (Figure 3C). Consistent with the pattern of ICAM-1 expression in total splenocytes infected with rRABV or rRABV-mICAM-1, the expression of ICAM-1 within the B cell component of the splenocyte culture was significantly increased in cultures treated with rRABV compared to mock-infected cells, which is statistically increased in B cells infected with rRABV-mICAM-1 (Figure 3D). To confirm that infection enhanced ICAM-1 expression, we analyzed total splenocytes (Figure 3E) or gated B220+ B cells (Figure 3F) for infection and ICAM-1 expression (i.e., RABV-N+ICAM-1+ or B220+RABV-N+ICAM-1+). While rRABV-infected total splenocytes or rRABV-infected B cells upregulated ICAM-1 expression compared to mock-infected cultures, rRABV-mICAM-1-infected cells showed almost 40% of the total splenocytes, or over 50% of the B cells, upregulated ICAM-1 expression. Together, these data indicate that attenuated RABV-based strains induce ICAM-1 expression in primary murine splenocytes, most notably in B220+ B cells, which is further enhanced by expressing ICAM-1 from rRABV-mICAM-1.
Figure 3. rRABV infection results in the upregulation of ICAM-1 expression, which is augmented by infection with rRABV-mICAM-1.
Total naïve primary murine splenocytes were infected as described in Figure 2 and then stained for B220, ICAM-1, and RABV-N for analysis by flow cytometry. A) Representative gating strategy of total live lymphocytes gated on ICAM-1 and RABV N staining. B) Representative gating strategy of B220+ B cells gated on RABV N and ICAM-1. C) Percent ICAM-1+ cells in the total live lymphocyte population. D) Percent ICAM-1+ cells in the B220+ cell population. E) Percent RABV N+ ICAM-1+ cells in the total live lymphocyte population. D) Percent RABV N+ ICAM-1+ cells in the B220+ cell population. To compare two groups of data, we used an unpaired, two-tailed Student's t test. (* p<0.05; **, p<0.01; ***, p<0.001)
rRABV-mICAM-1 Activates Primary Murine B cells In-vitro More Effectively Than Does rRABV
Next we wanted to evaluate whether the increased infection noted above in total splenocytes and B cells results in increased activation of primary murine B cells in-vitro. Naïve primary murine splenocytes cultures were infected with rRABV or rRABV-mICAM-1, or treated with medium alone (mock-infected). The cells were immunostained two days later for cell-surface expression of the activation markers, CD69, CD40 and MHCII. Figures 4A, 4C and 4E show representative gating strategies of B220+ B cells gated for CD69, CD40, or MHCII, respectively, and for RABV N expression. B220+ B cells infected with rRABV showed significant upregulation of the activation markers CD69 (Figure 4B), CD40 (Figure 4D) and MHCII (Figure 4F) compared to mock-infected cells. Moreover, the percentage of B220+ B cells infected with rRABV-mICAM-1 that express CD69 (Figure 4B, 11%), CD40 (Figure 4D, 43%) and MHCII (Figure 4F, 45%) statistically increased compared to B220+ B cells infected with rRABV. Of note, increased B cell activation in response to infection with rRABV-mICAM-1 was largely due to ICAM-1 incorporation into the virus particle, since pretreatment of purified rRABV-mICAM-1 with a neutralizing anti-ICAM-1 antibody significantly reduced MHCII expression in rRABV-mICAM-1-infected B cells (Figure 4G). Together, these data confirm our previous report showing that attenuated RABV-based strains activate naïve primary murine B cells, and that expressing ICAM-1 from rRABV-mICAM-1 enhances B cell activation in RABV-infected cells.
Figure 4. Infection with rRABV results in the upregulation of B cell activation markers, which are enhanced by infection with rRABV-mICAM-1.
The infected splenocyte cultures described in Figure 3 were analyzed for the activation of B cells, as measured by the upregulation of cell surface expression of CD69, CD40 or MHCII in RABV N+ B cells. Representative gating strategy of B220+ B cells from the total live population gated on CD69 (A), CD40 (C) or MHCII (E). The percentage of RABV N+CD69+ B cells (B), RABV N+CD40+ B cells (D) and RABV N+MHCII+ B cells (F) are indicated. G) Pretreatment of sucrose-purified rRABV-mICAM-1 with a neutralizing anti-ICAM-1 antibody significantly reduced the expression of MHCII on the infected B cells in culture. To compare two groups of data, we used an unpaired, two-tailed Student's t test. (* p<0.05; **, p<0.01; ***, p<0.001)
Kinetics Analyses Shows Enhanced Anti-RABV Antibody Responses in Mice Immunized with a Low Dose of rRABV-mICAM-1 Compared to Mice Immunized with rRABV
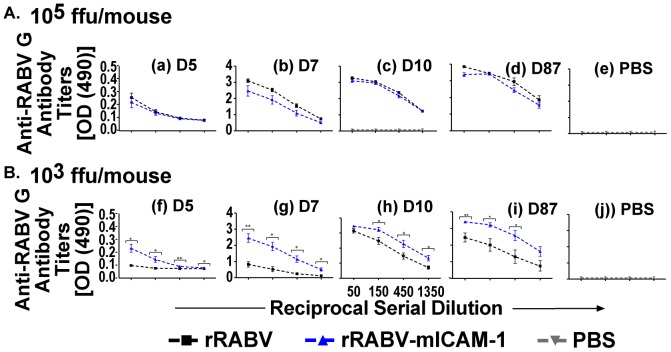
The above data indicate that rRABV-mICAM-1 infects and activates naïve primary murine B cells more effectively than does rRABV in-vitro, suggesting that rRABV-mICAM-1 might promote effective B cell responses in-vivo. Furthermore, ICAM-1 binding to LFA-1 on B cells was shown to lower the antigen threshold needed for B cell activation [23], suggesting that a low-dose of a RABV-based vaccine in which ICAM-1 is incorporated into the viral particle might induce potent anti-RABV B cell responses. To investigate anti-RABV antibody responses in-vivo, mice were immunized with a high dose (105 ffu/mouse) or a low dose (103 ffu/mouse) of rRABV-mICAM-1 or rRABV. PBS-immunized mice served as negative controls. As shown in Figure 5A, mice immunized with 105 ffu/mouse of rRABV-mICAM-1 showed similar anti-RABV G IgG antibody titers compared mice immunized with the same dose of rRABV at all time points tested (Figure 5A, a–e). However, mice immunized with only 103 ffu/mouse of rRABV-mICAM-1 showed significantly higher anti-RABV G antibody titers as early as five days post-immunization compared to mice similarly immunized with rRABV (Figure 5B, f–j), which were statistically higher at all time points tested thereafter, including 87 days post-immunization, which was the last time point tested. Of note, mice immunized with only 103 ffu/mouse with rRABV-mICAM-1 showed similar antibody kinetics compared to mice immunized with 105 ffu/mouse with rRABV-mICAM-1 or rRABV indicating that 100-fold less ICAM-1-expressing rRABV is needed to induce similar anti-RABV G antibody responses as the parental virus.
Figure 5. 100-fold less rRABV-mICAM-1 is needed to induce comparable immunity in mice compared to mice immunized with 105 ffu/mouse.
C57BL/6 mice were immunized i.m. with 105 (A) or 105 (B) ffu/mouse of rRABV and blood collected at the indicated time points. Four serial three-fold dilutions of sera were analyzed by ELISA to determine anti-RABV G antibody titers and presented as OD490 of the reciprocal serial dilution. For comparison, sera from PBS-immunized mice (panels e and j) were tested in parallel. Statistical difference in antibody titers by ELISA between two groups of data was determined using an unpaired, two-tailed t test and data is presented at the mean ± SEM. *p<0.05, **p = 0.01−0.001, ***p≤0.001. (N = 5 mice per group). (ffu = focus forming units; OD = optical density)
Low-dose rRABV-mICAM-1 Induces VNA Titers In-vivo More Rapidly and in Higher Quantities than does rRABV
VNAs directed against the single viral transmembrane glycoprotein are the primary correlate of protection against RABV infection. The World Health Organization suggests that a VNA titer greater than 0.5 international units (IU)/ml is indicative of a satisfactory immunization [5], [35]–[37]. Consistent with the antibody titers measured by ELISA in Figure 5, VNA titers were similar in mice immunized with 105 ffu/mouse with either rRABV or rRABV-mICAM-1 at all time points tested (data not shown). However, VNA titers in mice immunized with only 103 ffu/mouse of rRABV-mICAM-1 were twice the WHO limit indicative of a satisfactory immunization by five days post-immunization (Figure 6) while less than 0.1 IU/ml were detected in mice immunized with 103 ffu/mouse of rRABV at the same time point. At seven days post-immunization, over 3-fold higher VNA titers were detected in mice immunized with 103 ffu/mouse of rRABV-mICAM-1 compared to rRABV-immunized mice. Higher VNA titers were subsequently detected in rRABV-mICAM-1 immunized mice compared to rRABV-immunized mice at all time points thereafter. VNA titers in mice primed with 103 ffu or 105 ffu of rRABV-mICAM-1 showed similar profiles at all time points measured (data not shown), consistent with serum antibody titers shown in Figure 5.
Figure 6. Low-dose rRABV-mICAM-1 induces VNA titers in-vivo more rapidly and in higher quantities than does rRABV.
Sera from mice immunized with a single dose 103 ffu/mouse with rRABV-mICAM-1 or rRABV were pooled and then VNA titers measured by RFFIT and expressed as International Units (IU/ml). Data represents equal proportions of sera from five mice per group.
All together, we show that a recombinant RABV-based vaccine that expresses the murine Icam1 gene infects and activates naive primary murine B cells more effectively than the parental virus, rRABV. Furthermore, we show that rRABV-mICAM-1 induces significantly higher anti-RABV antibody responses in-vivo compared to mice immunized with rRABV alone. VNA titers indicative of a satisfactory immunization were detected as early as five days post-immunization with a single dose of vaccine containing only 103 ffu virus, suggesting expressing ICAM-1 from live RABV-based vaccines may be an excellent single-dose RABV-based vaccine to replace current multi-dose inactivated RABV-based vaccines.
Discussion
In this study, we demonstrated that a recombinant RABV-based vaccine expressing ICAM-1 (rRABV-mICAM-1), which is incorporated into the virus particle, infects and activates primary naïve murine B cells more effectively than the parental virus, rRABV. Furthermore, we showed that rRABV-mICAM-1 induces significantly higher anti-RABV antibody titers as early as five days post-infection with only a minimum of virus (i.e., 103 ffu/mouse). To the best of our knowledge, this is the first RABV-based vaccine strategy that exploits the immune enhancing properties of ICAM-1.
Interestingly, ICAM-1 is upregulated in total splenocytes, including B cells, infected with rRABV alone, indicating that attenuated RABV-based vaccines promote the expression of cell-derived ICAM-1. The function(s) of cell-derived ICAM-1 expression in response to live RABV vaccination is (are) not known and were not tested directly in this study. For attenuated RABV vaccine strains, the potential exists that the upregulation of ICAM-1 in infected cells influences a wide range of immune functions, including promoting immunological synapses in APC and CD4+ T cell interactions (i.e., the immunological synapse) [38], lowering the antigen threshold required for B cell activation thereby reducing the amount of antigen necessary to activate B cells [23], or by promoting T cell activation in B:T interactions [24]. It might be expected that virally encoded ICAM-1 would augment the beneficial effects of ICAM-1 expression on RABV-specific B cell immunity.
The incorporation of ICAM-1 into rRABV-mICAM-1 particles was not expected since the cytoplasmic domain (CD) of the RABV glycoprotein (G) is generally required for incorporation of a foreign protein into the virion particle [39]. Nonetheless, the requirement for the RABV CD appears not to be absolute as shown here and elsewhere [40]. The finding that ICAM-1 is incorporated into the rRABV-mICAM-1 particle may have implications for vaccine design since pre-treating rRABV-mICAM-1 particles with an ICAM-1 neutralizing antibody reduces B cell infection and activation to levels observed with rRABV alone. In addition, ICAM-1 is naturally incorporated into HIV-1 particles, which promotes virus infection by stabilizing virus adhesion to LFA-1 positive cells [21]. The potential exists that ICAM-1 expressed on the surface of the RABV particle enhances and/or prolongs virus:cell interactions, leading to greater virus uptake, infection and activation of B cells that constitutively express LFA-1. In addition, as noted above, ICAM-1 binding to LFA-1 on B cells reduces the antigen threshold needed for B cell activation. Indeed, we observed that rRABV-mICAM-1 induces B cells to produce equivalent antibody titers as 100-fold more of the parental virus, rRABV. Together, we show that attenuated RABV-based vaccines increase ICAM-1 expression in primary murine B cells, which can be augmented by encoding ICAM-1 into the virus genome.
We previously showed that RABV-based vaccines infect primary murine and human B cells, resulting in B cell activation and their ability to prime naïve mouse T cells to proliferate and to secrete IL-2. In the experiments described here, we wanted to determine if increased infection of primary splenocytes or B cells accompanies increased activation in response to infection with rRABV-mICAM-1. Three activation markers were used to study immune cell activation in the context of RABV infection. CD69 is the earliest expressed activation marker on the surface of B and T cells [41], [42] and provides insight into the speed by which rRABV activates primary murine B or T cells. CD40 is a costimulation/survival molecule constitutively expressed on B cells whose expression is upregulated upon B cell:CD4 T cell engagement and is required for optimal B and T cell activation and proliferation [43], [44]. MHCII molecules are loaded with peptide epitopes following internalization and processing of foreign antigen. MHCII-peptide complexes are then expressed on the surface of the B cell for recognition by CD4 T cells specific for the peptide. Engagement of MHCII-peptide complexes with the TCR is the first of two required signals for CD4 T cell activation (reviewed in [45]). Significantly increased expression of CD40 and MHCII on splenic B cells treated with rRABV-mICAM-1 is indicative of an activation state where B cells are capable of presenting antigen to CD4 T cells. In agreement with our previous data [11], we show here that the parental virus, rRABV, increases CD69, CD40 and MHCII expression on the surface of infected B cells. Furthermore, we show that expressing ICAM-1 from the RABV genome significantly increases the expression of these activation markers, suggesting ICAM-1 expression may enhance B cell-intrinsic priming of naive CD4+ T cells to proliferate and secrete IL-2 in the context of RABV vaccination. The influence of ICAM-1 expression on the ability of B cells to prime T cells was not directly studied here and remains to be experimentally defined. Nonetheless, the activated B cell phenotype suggests a potential role for virally encoded ICAM-1 to enhance B:T cell interactions.
The primary correlate of vaccine-induced protection against RABV is virus-neutralizing IgG antibodies (VNA). The WHO considers a VNA titer of 0.5 IU/ml to be indicative of a satisfactory immune response to RABV vaccination [5], [35]–[37]. VNA titers in mice primed with only 103 ffu of rRABV-mICAM-1 were 2-fold higher than the presumed level of a satisfactory immunization as early as five days post-prime inoculation, while only background levels of VNA titers were detected in mice immunized with an equivalent amount of rRABV. By day 7 post-immunization, almost a 4-fold increase in VNA titers were detected in mice immunized with 103 ffu/mouse of rRABV-mICAM-1 compared to mice similarly immunized with rRABV. This pattern continued for the duration of the experiment at all time points measured. These data indicate that one low-dose of rRABV-mICAM-1 is highly immunogenic, which is important from a vaccine standpoint. Since similar anti-RABV IgG antibody response were detected using 100-fold less virus (103 ffu vs. 105 ffu), this may allow for reduced manufacturing and preparation costs, helping to reduce the overall cost of RABV PEP.
The proof-of-principle studies described herein provide evidence that RABV-based vaccines can be enhanced by exploiting ICAM-1 expression. Nonetheless, due to safety concerns for the use of a live virus as a vaccine against RABV infection, it is not likely that a live replication-competent vaccine will replace currently used inactivated vaccines. We have previously shown that a replication-deficient RABV-based vaccine in which the matrix (M) gene is deleted (rRABV-ΔM) is both safe and immunogenic in mice and non-human primates [8]. Furthermore, rRABV-ΔM infects and activates primary murine B cells at levels similar as rRABV [11] and induces T cell-independent antibody responses rapidly (neutralizing IgM and IgG) [10]. Based on the preliminary data presented here in the context of replication-competent rRABV-based vaccines, future work will entail testing the potency rRABV-ΔM expressing ICAM-1. Furthermore, while B cells appear to be the major target for rRABV-mICAM-1 infection, rRABV-mICAM-1 appears to also target non-B cells more effectively than rRABV alone. Future work in the context of rRABV-ΔM expressing mICAM-1 will identify a role for these additional cell types in enhanced immunity and animal models of post-exposure protection. Together, we have identified a potential immune enhancing feature that may help increase the utility of highly efficacious and safe RABV-based vaccines, which warrant additional study.
Acknowledgments
We thank Andrew Glibicky and Matthew Farabaugh of the Kimmel Cancer Center Flow Cytometry Facility for assistance.
Funding Statement
This work was supported by NIH/NIAID grant R01AI079211 to JPM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.CDC website. Available: http://www.cdc.gov/rabies/location/usa/cost.html. Accessed 2014 Jan 6.
- 2.WHO (2013) WHO rabies fact sheet, no.99.
- 3.Lackay SN, Kuang Y, Fu ZF (2008) Rabies in small animals. Vet Clin North Am Small Anim Pract 38 : 851––61, ix. 10.1016/j.cvsm.2008.03.003; 10.1016/j.cvsm.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, et al. (2010) Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: Recommendations of the advisory committee on immunization practices. MMWR Recomm Rep 59: 1–9. [PubMed] [Google Scholar]
- 5. McGettigan JP (2010) Experimental rabies vaccines for humans. Expert Rev Vaccines 9: 1177–1186 10.1586/erv.10.105; 10.1586/erv.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ertl HC (2009) Novel vaccines to human rabies. PLoS Negl Trop Dis 3: e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strady C, Andreoletti L, Baumard S, Servettaz A, Jaussaud R, et al. (2009) Immunogenicity and booster efficacy of pre-exposure rabies vaccination. Trans R Soc Trop Med Hyg 103: 1159–1164 10.1016/j.trstmh.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 8. Cenna J, Hunter M, Tan GS, Papaneri AB, Ribka EP, et al. (2009) Replication-deficient rabies virus-based vaccines are safe and immunogenic in mice and nonhuman primates. Journal of Infectious Diseases 200: 1251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dorfmeier CL, Lytle AG, Dunkel AL, Gatt A, McGettigan JP (2012) Protective vaccine-induced CD4(+) T cell-independent B cell responses against rabies infection. J Virol 86: 11533–11540 10.1128/JVI.00615-12; 10.1128/JVI.00615-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorfmeier CL, Shen S, Tzvetkov EP, McGettigan JP (2013) Reinvestigating the role of IgM in rabies virus post-exposure vaccination. J Virol. 10.1128/JVI.00995-13. [DOI] [PMC free article] [PubMed]
- 11.Lytle AG, Norton EJ, Dorfmeier CL, Shen S, McGettigan JP (2013) B cell infection and activation by rabies virus-based vaccines. In Press. [DOI] [PMC free article] [PubMed]
- 12. Staunton DE, Marlin SD, Stratowa C, Dustin ML, Springer TA (1988) Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell 52: 925–933. [DOI] [PubMed] [Google Scholar]
- 13. Sanchez-Madrid F, Nagy JA, Robbins E, Simon P, Springer TA (1983) A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: The lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/mac-1), and the p150,95 molecule. J Exp Med 158: 1785–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA (1986) Induction by IL 1 and interferon-gamma: Tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol 137: 245–254. [PubMed] [Google Scholar]
- 15. Kurzinger K, Reynolds T, Germain RN, Davignon D, Martz E, et al. (1981) A novel lymphocyte function-associated antigen (LFA-1): Cellular distribution, quantitative expression, and structure. J Immunol 127: 596–602. [PubMed] [Google Scholar]
- 16. Gaglia JL, Greenfield EA, Mattoo A, Sharpe AH, Freeman GJ, et al. (2000) Intercellular adhesion molecule 1 is critical for activation of CD28-deficient T cells. J Immunol 165: 6091–6098. [DOI] [PubMed] [Google Scholar]
- 17. Shinde S, Wu Y, Guo Y, Niu Q, Xu J, et al. (1996) CD40L is important for induction of, but not response to, costimulatory activity. ICAM-1 as the second costimulatory molecule rapidly up-regulated by CD40L. J Immunol 157: 2764–2768. [PubMed] [Google Scholar]
- 18. Dubey C, Croft M, Swain SL (1995) Costimulatory requirements of naive CD4+ T cells. ICAM-1 or B7-1 can costimulate naive CD4 T cell activation but both are required for optimum response. J Immunol 155: 45–57. [PubMed] [Google Scholar]
- 19. Cantin R, Methot S, Tremblay MJ (2005) Plunder and stowaways: Incorporation of cellular proteins by enveloped viruses. J Virol 79: 6577–6587 10.1128/JVI.79.11.6577-6587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tardif MR, Tremblay MJ (2003) Presence of host ICAM-1 in human immunodeficiency virus type 1 virions increases productive infection of CD4+ T lymphocytes by favoring cytosolic delivery of viral material. J Virol 77: 12299–12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kondo N, Melikyan GB (2012) Intercellular adhesion molecule 1 promotes HIV-1 attachment but not fusion to target cells. PLoS One 7: e44827 10.1371/journal.pone.0044827; 10.1371/journal.pone.0044827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fortin JF, Cantin R, Lamontagne G, Tremblay M (1997) Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol 71: 3588–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD (2004) LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity 20: 589–599. [DOI] [PubMed] [Google Scholar]
- 24. Dennig D, Lacerda J, Yan Y, Gasparetto C, O'Reilly RJ (1994) ICAM-1 (CD54) expression on B lymphocytes is associated with their costimulatory function and can be increased by coactivation with IL-1 and IL-7. Cell Immunol 156: 414–423 10.1006/cimm.1994.1186. [DOI] [PubMed] [Google Scholar]
- 25. Schnell MJ, Foley HD, Siler CA, McGettigan JP, Dietzschold B, et al. (2000) Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc Natl Acad Sci U S A 97: 3544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conzelmann KK, Cox JH, Schneider LG, Thiel HJ (1990) Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175: 485–99. [DOI] [PubMed] [Google Scholar]
- 27. McGettigan JP, Pomerantz RJ, Siler CA, McKenna PM, Foley HD, et al. (2003) Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 gag have greatly reduced pathogenicity but are highly immunogenic. Journal of Virology 77: 237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGettigan JP, Naper K, Orenstein J, Koser M, McKenna PM, et al. (2003) Functional human immunodeficiency virus type 1 (HIV-1) gag-pol or HIV-1 gag-pol and env expressed from a single rhabdovirus-based vaccine vector genome. Journal of Virology 77: 10889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dorfmeier CL, Tzvetkov EP, Gatt A, McGettigan JP (2013) Investigating the role for IL-21 in rabies virus vaccine-induced immunity. PLoS Negl Trop Dis 7: e2129 10.1371/journal.pntd.0002129; 10.1371/journal.pntd.0002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorfmeier CL, Lytle AG, Dunkel AL, Gatt A, McGettigan JP (2012) Protective vaccine-induced CD4+ T cell-independent B cell responses against rabies infection. J Virol. 10.1128/JVI.00615-12. [DOI] [PMC free article] [PubMed]
- 31. Dorfmeier CL, Tzvetkov EP, Gatt A, McGettigan JP (2013) Investigating the role for IL-21 in rabies virus vaccine-induced immunity. PLoS Negl Trop Dis 3: e2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cenna J, Tan GS, Papaneri AB, Dietzschold B, Schnell MJ, et al. (2008) Immune modulating effect by a phosphoprotein-deleted rabies virus vaccine vector expressing two copies of the rabies virus glycoprotein gene. Vaccine 26: 6405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mebatsion T, Conzelmann KK (1996) Specific infection of CD4+ target cells by recombinant rabies virus pseudotypes carrying the HIV-1 envelope spike protein. Proc Natl Acad Sci U S A 93: 11366–11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siler CA, McGettigan JP, Dietzschold B, Herrine SK, Dubuisson J, et al. (2002) Live and killed rhabdovirus-based vectors as potential hepatitis C vaccines. Virology 292: 24–34. [DOI] [PubMed] [Google Scholar]
- 35. Moore SM, Hanlon CA (2010) Rabies-specific antibodies: Measuring surrogates of protection against a fatal disease. PLoS Negl Trop Dis 4: e595 10.1371/journal.pntd.0000595; 10.1371/journal.pntd.0000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Plotkin SA (2008) Vaccines: Correlates of vaccine-induced immunity. Clin Infect Dis 47: 401–409 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 37.WHO. (1997) WHO recommendations on rabies post-exposure treatment and the correct technique of intradermal immunization against rabies.
- 38. Bromley SK, Iaboni A, Davis SJ, Whitty A, Green JM, et al. (2001) The immunological synapse and CD28–CD80 interactions. Nat Immunol 2: 1159–1166 10.1038/ni737. [DOI] [PubMed] [Google Scholar]
- 39. Mebatsion T, Finke S, Weiland F, Conzelmann KK (1997) A CXCR4/CD4 pseudotype rhabdovirus that selectively infects HIV-1 envelope protein-expressing cells. Cell 90: 841–847. [DOI] [PubMed] [Google Scholar]
- 40. Blaney JE, Wirblich C, Papaneri AB, Johnson RF, Myers CJ, et al. (2011) Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and ebola viruses. J Virol 85: 10605–10616 10.1128/JVI.00558-11; 10.1128/JVI.00558-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Testi R, D'Ambrosio D, De Maria R, Santoni A (1994) The CD69 receptor: A multipurpose cell-surface trigger for hematopoietic cells. Immunol Today 15: 479–483 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 42. Ziegler SF, Ramsdell F, Alderson MR (1994) The activation antigen CD69. Stem Cells 12: 456–465 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]
- 43. Grewal IS, Flavell RA (1998) CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 16: 111–135 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 44. Grewal IS, Foellmer HG, Grewal KD, Xu J, Hardardottir F, et al. (1996) Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science 273: 1864–1867. [DOI] [PubMed] [Google Scholar]
- 45. Neefjes J, Jongsma ML, Paul P, Bakke O (2011) Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol 11: 823–836 10.1038/nri3084; 10.1038/nri3084 [DOI] [PubMed] [Google Scholar]