Abstract
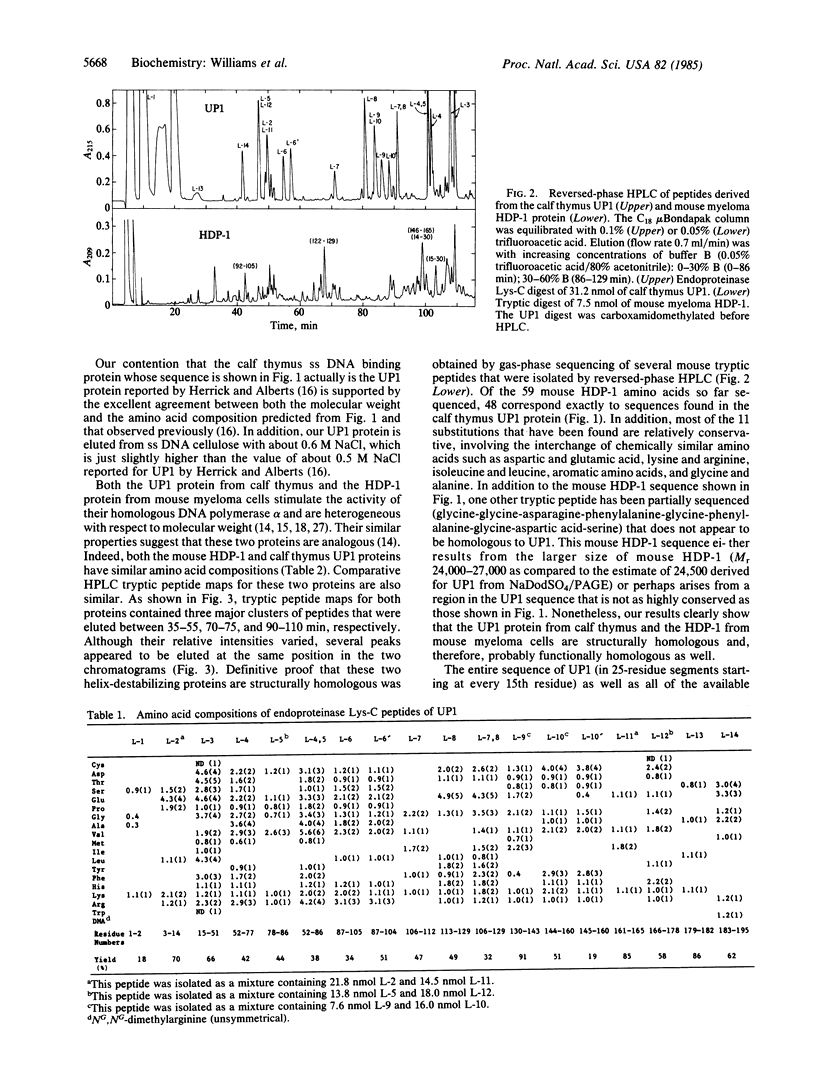
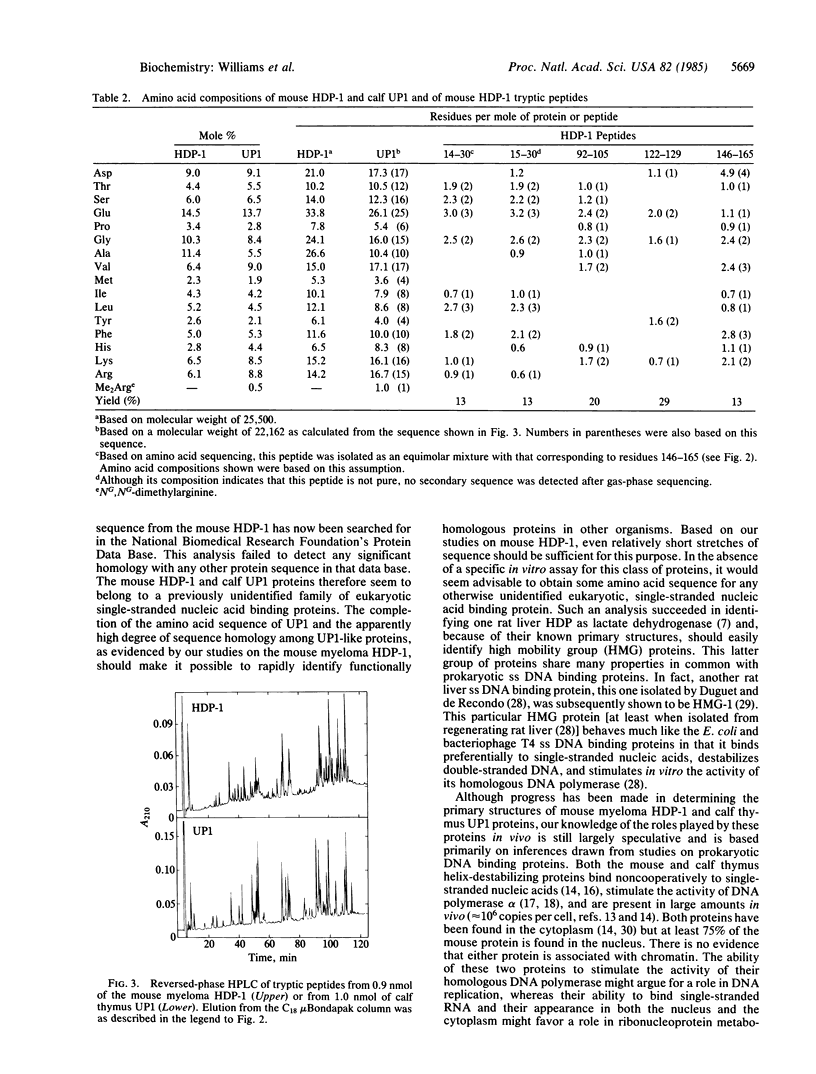
A complete amino acid sequence has been determined for the UP1 single-stranded DNA binding protein from calf thymus that was first described by G. Herrick and B. M. Alberts [(1976) J. Biol. Chem. 251, 2124-2132]. Peptides required to establish the UP1 sequence were isolated by reversed-phase HPLC of digests produced by endoproteinase Lys-C, trypsin, chymotrypsin, Staphylococcus aureus V8 protease, and cyanogen bromide cleavage of UP1. The purified peptides were coupled to aminopolystyrene prior to solid-phase sequencing. UP1 contains 195 amino acids and has a molecular weight of 22,162. UP1 has a blocked NH2 terminus and contains a single NG,NG-dimethylarginine residue near its COOH terminus. Gas-phase sequencing of tryptic peptides derived from an analogous protein from mouse myeloma cells [Planck, S. R. & Wilson, S. H. (1980) J. Biol. Chem. 255, 11547-11556] revealed that this mouse helix-destabilizing protein shares a high degree of sequence homology with UP1. Of the 59 amino acids in the mouse protein that have so far been found to be homologous with UP1, 48 correspond exactly to sequences found in UP1. Most of the 11 differences that have been found between the sequences of these two proteins are conservative in nature, involving primarily the interchange of chemically similar amino acids. One 9-residue mouse sequence that is not obviously homologous to UP1 may be a result of the larger size of the mouse myeloma protein as compared to UP1. Since none of the UP1 or mouse myeloma helix-destabilizing protein sequence appears to be homologous to that of any previously sequenced protein, we presume that these two proteins represent a distinct class of single-stranded nucleic acid binding proteins that probably play a role in metabolism of single-stranded RNA or DNA in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boffa L. C., Karn J., Vidali G., Allfrey V. G. Distribution of NG, NG,-dimethylarginine in nuclear protein fractions. Biochem Biophys Res Commun. 1977 Feb 7;74(3):969–976. doi: 10.1016/0006-291x(77)91613-8. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Sterner R., Vidali G., Allfrey V. G. Post-synthetic modifications of nuclear proteins. High mobility group proteins are methylated. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1322–1327. doi: 10.1016/0006-291x(79)92153-3. [DOI] [PubMed] [Google Scholar]
- Bonne C., Sautiere P., Duguet M., de Recondo A. M. Identification of a single-stranded DNA binding protein from rat liver with high mobility group protein 1. J Biol Chem. 1982 Mar 25;257(6):2722–2725. [PubMed] [Google Scholar]
- Burke R. L., Alberts B. M., Hosoda J. Proteolytic removal of the COOH terminus of the T4 gene 32 helix-destabilizing protein alters the T4 in vitro replication complex. J Biol Chem. 1980 Dec 10;255(23):11484–11493. [PubMed] [Google Scholar]
- Chang F. N., Navickas I. J., Chang C. N., Dancis B. M. Methylation of ribosomal proteins in HeLa cells. Arch Biochem Biophys. 1976 Feb;172(2):627–633. doi: 10.1016/0003-9861(76)90117-x. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Merrill B. M., Williams K. R. F sex factor encodes a single-stranded DNA binding protein (SSB) with extensive sequence homology to Escherichia coli SSB. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5480–5484. doi: 10.1073/pnas.80.18.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M. E., Beyer A. L., Walker B., Lestourgeon W. M. Identification of NG, NG-dimethylarginine in a nuclear protein from the lower eukaryote physarum polycephalum homologous to the major proteins of mammalian 40S ribonucleoprotein particles. Biochem Biophys Res Commun. 1977 Jan 24;74(2):621–629. doi: 10.1016/0006-291x(77)90348-5. [DOI] [PubMed] [Google Scholar]
- Detera S. D., Becerra S. P., Swack J. A., Wilson S. H. Studies on the mechanism of DNA polymerase alpha. Nascent chain elongation, steady state kinetics, and the initiation phase of DNA synthesis. J Biol Chem. 1981 Jul 10;256(13):6933–6943. [PubMed] [Google Scholar]
- Duguet M., de Recondo A. M. A deoxyribonucleic acid unwinding protein isolated from regenerating rat liver. Physical and functional properties. J Biol Chem. 1978 Mar 10;253(5):1660–1666. [PubMed] [Google Scholar]
- Gardner W. D., Haselby J. A., Hoch S. O. Identification of a major serum DNA-binding protein as factor B of the alternative complement pathway. J Immunol. 1980 Jun;124(6):2800–2806. [PubMed] [Google Scholar]
- Gardner W. D., White P. J., Hoch S. O. Identification of a major human serum DNA-binding protein as beta 1H of the alternative pathway of complement activation. Biochem Biophys Res Commun. 1980 May 14;94(1):61–67. doi: 10.1016/s0006-291x(80)80187-2. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Williams K. R., Konigsberg W. H., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated neuronal phosphoprotein. I. Amino acid sequence around the phosphorylated threonine. J Biol Chem. 1984 Dec 10;259(23):14486–14490. [PubMed] [Google Scholar]
- Herrick G., Alberts B. Nucleic acid helix-coil transitions mediated by helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2133–2141. [PubMed] [Google Scholar]
- Herrick G., Alberts B. Purification and physical characterization of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2124–2132. [PubMed] [Google Scholar]
- Herrick G., Delius H., Alberts B. Single-stranded DNA structure and DNA polymerase activity in the presence of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2142–2146. [PubMed] [Google Scholar]
- Hotta Y., Stern H. A DNA-binding protein in meiotic cells of Lilium. Dev Biol. 1971 Sep;26(1):87–99. doi: 10.1016/0012-1606(71)90110-2. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Stern H. Meiotic protein in spermatocytes of mammals. Nat New Biol. 1971 Nov 17;234(46):83–86. doi: 10.1038/newbio234083a0. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Stern H. The effect of dephosphorylation on the properties of a helix-destabilizing protein from meiotic cells and its partial reversal by a protein kinase. Eur J Biochem. 1979 Mar 15;95(1):31–38. doi: 10.1111/j.1432-1033.1979.tb12936.x. [DOI] [PubMed] [Google Scholar]
- Kroll J., Larsen J. K., Loft H., Ezban M., Wallevik K., Faber M. DNA-binding proteins in Yoshida ascites tumor fluid. Biochim Biophys Acta. 1976 Jun 15;434(2):490–501. doi: 10.1016/0005-2795(76)90239-7. [DOI] [PubMed] [Google Scholar]
- Marvil D. K., Nowak L., Szer W. A single-stranded nucleic acid-binding protein from Artemia salina. I. Purification and characterization. J Biol Chem. 1980 Jul 10;255(13):6466–6472. [PubMed] [Google Scholar]
- Merrill B. M., Williams K. R., Chase J. W., Konigsberg W. H. Photochemical cross-linking of the Escherichia coli single-stranded DNA-binding protein to oligodeoxynucleotides. Identification of phenylalanine 60 as the site of cross-linking. J Biol Chem. 1984 Sep 10;259(17):10850–10856. [PubMed] [Google Scholar]
- Moise H., Hosoda J. T4 gene 32 protein model for control of activity at replication fork. Nature. 1976 Feb 12;259(5543):455–458. doi: 10.1038/259455a0. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Kim S. Enzymatic methylation of protein fractions from calf thymus nuclei. Biochem Biophys Res Commun. 1967 Oct 11;29(1):14–20. doi: 10.1016/0006-291x(67)90533-5. [DOI] [PubMed] [Google Scholar]
- Perucho M., Salas J., Salas M. L. Identification of the mammalian DNA-binding protein P8 as glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1977 Dec;81(3):557–562. doi: 10.1111/j.1432-1033.1977.tb11982.x. [DOI] [PubMed] [Google Scholar]
- Planck S. R., Wilson S. H. Studies on the structure of mouse helix-destabilizing protein-1. DNA binding and controlled proteolysis with trypsin. J Biol Chem. 1980 Dec 10;255(23):11547–11556. [PubMed] [Google Scholar]
- Riva S., Clivio A., Valentini O., Cobianchi F. DNA binding proteins from calf thymus with an enhanced ability to stimulate DNA polymerase alpha in vitro. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1053–1062. doi: 10.1016/0006-291x(80)90059-5. [DOI] [PubMed] [Google Scholar]
- Siddiqui A. A., Hughes A. E., Davies R. J., Hill J. A. The isolation and identification of alpha 1-antichymotrypsin as a DNA-binding protein from human serum. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1737–1742. doi: 10.1016/s0006-291x(80)80099-4. [DOI] [PubMed] [Google Scholar]
- Tsai R. L., Green H. Study of intracellular collagen precursors using DNA-cellulose chromatography. Nat New Biol. 1972 Jun 7;237(75):171–173. doi: 10.1038/newbio237171a0. [DOI] [PubMed] [Google Scholar]
- Valentini O., Biamonti G., Mastromei G., Riva S. Structural and functional heterogeneity of single-stranded DNA-binding proteins from calf thymus. Biochim Biophys Acta. 1984 Jun 16;782(2):147–155. doi: 10.1016/0167-4781(84)90018-6. [DOI] [PubMed] [Google Scholar]
- Williams K. R., Spicer E. K., LoPresti M. B., Guggenheimer R. A., Chase J. W. Limited proteolysis studies on the Escherichia coli single-stranded DNA binding protein. Evidence for a functionally homologous domain in both the Escherichia coli and T4 DNA binding proteins. J Biol Chem. 1983 Mar 10;258(5):3346–3355. [PubMed] [Google Scholar]



