Abstract
A major problem in treating alcohol use disorders (AUDs) is the high rate of relapse due to stress and re-exposure to cues or an environment previously associated with alcohol use. Stressors can induce relapse to alcohol seeking in humans or reinstatement in rodents. Delta opioid peptide receptors (DOP-Rs) play a role in cue-induced reinstatement of ethanol-seeking, however their role in stress-induced reinstatement of ethanol-seeking is not known. The objective of this study was to determine the role of DOP-Rs in yohimbine-stress induced reinstatement of ethanol seeking. Male, Long-Evans rats were trained to self-administer 10% ethanol in daily 30 minute operant self administration sessions using a FR3 schedule of reinforcement, followed by extinction training. Once extinction criteria were met, we examined the effects of the DOP-R antagonist, SoRI-9409 (0-5 mg/kg, IP) on yohimbine (2 mg/kg, IP) stress-induced reinstatement. Additionally, DOP-R-stimulated [35S]GTPγS binding was measured in brain membranes and plasma levels of corticosterone (CORT) were determined. Pretreatment with SoRI-9409 decreased yohimbine stress-induced reinstatement of ethanol seeking but did not affect yohimbine-induced increases in plasma CORT levels. Additionally, yohimbine increased DOP-R-stimulated 35[S]GTPγS binding in brain membranes of ethanol-trained rats, an effect which was inhibited by SoRI-9409. This suggests that the DOP-R plays an important role in yohimbine stress-induced reinstatement of ethanol-seeking behavior and DOP-R antagonists may be promising candidates for further development as a treatment for AUDs.
Keywords: corticosterone, delta opioid receptor antagonist, ethanol-seeking, stress-induced reinstatement, SoRI-9409, yohimbine
Introduction
A major problem in treating Alcohol Use Disorders (AUDs) is the high rate of relapse, usually triggered by stressful events. An effective pharmacological treatment for AUDs would ideally prevent relapse of alcohol seeking. Naltrexone is a nonselective opioid antagonist that inhibits mu, delta, and kappa opioid receptors (MOP-R, DOP-R and KOP-R, respectively). Naltrexone reduces alcohol- and cue-induced reinstatement, but not foot-shock stress-induced reinstatement of ethanol-seeking in rodents (Ciccocioppo et al., 2002; Le et al., 1999; Liu and Weiss, 2002). This is supported by clinical studies that show naltrexone can reduce relapse in a subset of alcohol-dependent humans (O'Malley et al., 1996; Volpicelli et al., 1992). It has been shown that DOP-Rs rather than MOP-Rs are important for cue-induced reinstatement of ethanol seeking, as the DOP-R antagonist, naltrindole, reduces cue-induced reinstatement of ethanol seeking behavior in rodents more effectively than the MOP-R selective antagonists, naloxonazine or CTOP (Ciccocioppo et al., 2002; Marinelli et al., 2009). The DOP-R has been shown to play a role in the reinforcing effects of ethanol (Froehlich et al., 1991; Herz, 1997), as activation of DOP-Rs in the nucleus accumbens and ventral tegmental area leads to increased basal dopamine release (Borg and Taylor, 1997; Devine et al., 1993; Herz, 1997; Vetulani, 2001). However, the role of the DOP-R in stress-induced reinstatement of ethanol-seeking is currently not known.
Stress induces relapse to drug seeking in humans and induces reinstatement of drug seeking in rodents (Liu and Weiss, 2003; Shaham et al., 2000; Zironi et al., 2006). Although foot-shock has been the most commonly used method of stress-induced reinstatement of alcohol-seeking in rodents, the pharmacological stressor yohimbine has more recently been shown to be a viable alternative (Gass and Olive, 2007; Le et al., 2005; Marinelli et al., 2007). Blockade of α2-adrenoceptors by yohimbine causes a release of noradrenaline which stimulates the sympathetic nervous system (Bremner et al., 1996). Yohimbine activates c-fos and CRF mRNA in the same brain regions as that elicited after footshock-induced stress (Funk et al., 2006) and increases plasma levels of the stress hormone, corticosterone (CORT) (Marinelli et al., 2007). The brain regions activated by both foot-shock and yohimbine also express DOP-Rs and include the nucleus accumbens shell, basolateral and central amygdalar nuclei, and the bed nucleus of the stria terminalis (Borg and Taylor, 1997; Devine et al., 1993; Funk et al., 2006; Herz, 1997; Kang-Park et al., 2007). However, the role of the DOP-R in the stress response has been contradictory. Plasma CORT levels in mice with a triple knockout of MOP-R, DOP-R and KOP-R are not different compared to wild-type littermates following the forced swim test (Contet et al., 2006) suggesting opioid receptors are not involved in the hormonal stress response. However, previous studies have shown that rats housed in a stressful environment were more sensitive to the sedative effects of the DOP-R agonist, SNC80, compared to stimulant effects reported by SNC80 in rats that were not stressed (Pohorecky et al., 1999). DOP-R knockout mice have increased anxiety (Filliol et al., 2000), comparable to anxiogenic effects in rats administered the DOP-R antagonists, naltrindole or naltriben (Perrine et al., 2006; Saitoh et al., 2005). Conversely, anxiolytic activity is increased in rats administered the DOP-R agonist, SNC80 (Perrine et al., 2006; Saitoh et al., 2004). Plasma CORT levels were increased in rats administered naltrindole when exposed to the elevated plus maze and plasma CORT levels were not increased in rats co-administered naltrindole and SNC80 (Saitoh et al., 2005). In contrast, plasma CORT levels were increased in rats acutely administered the DOP-R agonists DPDPE and DADLE into the intracerebral ventricles (Gonzalvez et al., 1991; Iyengar et al., 1987).
The aim of our study was to determine the role of the DOP-R in yohimbine stress-induced reinstatement of ethanol seeking. We examined the effects of SoRI-9409, a potent DOP-R antagonist (Nielsen et al., 2008; Wells et al., 2001, Xu et al., 2001), on yohimbine stress-induced reinstatement. In comparison to naltrexone, SoRI-9409 has approximately 20-fold higher binding affinity at the DOP-R (Ki = 2.2 ± .2 nmol/L), approximately 20-fold reduced binding affinity for the MOP-R (Ki = 51 ± 8 nmol/L), with similar affinity at the KOP-R (Ananthan et al., 1999). Although previous behavioral studies initially reported SoRI-9409 to have partial agonist activity at the MOP-R (Wells et al., 2001), further in vitro studies have demonstrated SoRI-9409 has no agonist activity at MOP-R (or at DOP-R or KOP-R) but instead has weak antagonist activity at the MOP-R and KOP-R compared to potent antagonist activity at the DOP-R (Wells et al., 2001; Xu et al., 2001). We have previously shown that SoRI-9409 potently inhibits DOP-R-mediated [35S]GTPγS binding in brain membranes of high-ethanol consuming rats and that administration of SoRI-9409 to rats in a relatively low dose (5 mg/kg I.P.), a dose of which would selectively target the DOP-R, produces selective and long-lasting reductions in ethanol consumption (Nielsen et al., 2008). To investigate the contributions of the both the central nervous system and the peripheral stress pathway involved in modulation of the DOP-R on yohimbine stress-induced reinstatement we measured opioid receptor stimulated [35S]GTPγS binding in brain membranes and plasma levels of corticosterone (CORT) in ethanol-trained rats.
Materials and Methods
Subjects
Male, Long-Evans rats weighing 150-180g upon arrival (Harlan Indianapolis, IN), were individually housed in ventilated Plexiglas cages with a single bottle grommet at the front end of the cage. Rats were housed in a climate controlled room on a 12 hour light-dark cycle (lights on at 0700 hours). Operant training occurred Monday through Friday. Food and water were available ad libitum, except for short periods during initial training, as outlined below. All procedures were pre-approved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with NIH guidelines for the Humane Care and Use of Laboratory Animals.
Operant Self-Administration
Self-administration testing was conducted in standard operant conditioning chambers (Coulbourn Instruments, Allentown, PA). Details regarding the apparatus have been extensively described elsewhere (Richards et al., 2008; Steensland et al., 2007). Prior to beginning the operant self-administration training, rats were exposed to 10% ethanol as the only liquid source in their home cages for four days before a sucrose fading technique was initiated as previously described (Steensland et al., 2007). Following the fourth day of forced ethanol exposure, rats were placed in the operant chambers for a 14 hour overnight session on an FR1 schedule of reinforcement (0.1 ml reward after a single lever press). The start of the training session was signaled by the illumination of the house light and extension of the active lever. During this phase only the active lever was available for the rat to press, to facilitate learning. Rats were trained to respond for 10% sucrose in overnight sessions (1-3 nights) and continued on 10% sucrose until they reached the FR3 stage of training. Initial daily training consisted of 45 minute FR1 sessions and one-hour daily water access, with water access immediately following the training sessions. Once responding was established (2-4 days) rats were given free access to water in the home cage and continued on a 45 minute FR1 schedule for an additional three to four days. Subsequently, training sessions were reduced to 30 minutes and the work ratio was increased to an FR3 schedule of reinforcement (3 active lever presses required for 0.1 ml reward). A second, inactive lever was also introduced at this time. Upon pressing the inactive lever, no reinforcer, visual (light), or auditory stimuli were presented and the event was merely recorded as a measure of nonspecific behavioral activity. Following three sessions of FR3 training with 10% sucrose as the reinforcer, a modified sucrose fading technique was initiated (Samson, 1986). Ten percent ethanol was added to the 10% sucrose solution and over the next 12 sessions the sucrose concentration was gradually decreased (10%, 5%, 3%, 1.5% respectively) until rats responded on an FR3 schedule for 10% ethanol alone. Rats continued on the FR3 protocol for a minimum of 20 sessions until a stable level of pressing was established, defined as more than 50 active lever presses with less than 20% variation for 3 consecutive sessions. Any animals not reaching 50 presses per session were excluded from further study.
Extinction Training
Once rats were responding for 10% ethanol, they continued on FR3 30 minute-long sessions for a minimum of 4 weeks (>20 sessions) before extinction training was performed. In order to extinguish self-administration, rats continued with daily operant sessions under FR3 conditions, however, active lever pressing did not result in reward delivery (10% ethanol) despite light and tone cues still being presented. Extinction training continued until the rats responded with less than 10 active lever presses per session or less than 10% of their baseline pressing on the active lever for two consecutive sessions, which took approximately 5 weeks (22 sessions).
Effect of SoRI-9409 on Yohimbine-Induced Reinstatement of 10% Ethanol Seeking
For the reinstatement study, ethanol seeking behavior was extinguished in a group of rats (n=54) trained to self-administer 10% ethanol under FR3 conditions. The rats were assigned to one of four groups matched for their previous operant self-administration responding. We used the between subjects factor of SoRI-9409 dose (0, 1, 2.5, 5 mg/kg I.P.) and the within subjects factor of yohimbine dose (0, 2 mg/kg I.P.) to assess the effect of SoRI-9409 on yohimbine-induced reinstatement. Animals received two injections per test day. On test days, the rats were first injected with either SoRI-9409 or its vehicle and 30 minutes later with either yohimbine or vehicle. Rats were tested with vehicle and yohimbine consecutively over 2 test sessions, 7 days apart. Thirty minutes following the yohimbine or yohimbine-vehicle injection the animals were placed into the operant self-administration chambers for the 30 minute-long reinstatement test session. Reinstatement sessions were run under the same conditions as the extinction sessions; successful FR3 responses at the previously active lever resulted in light and tone cue presentation with no reward delivery. Regular 30 minute extinction sessions were run on the between-test days. Yohimbine dose was determined in accordance with previous studies (Ghitza et al., 2006; Le et al., 2005; Richards et al., 2009; Richards et al., 2008; Shepard et al., 2004).
Membrane Preparation
A separate group of rats (n=12) was trained to self-administer 10% ethanol and subsequently lever responding was extinguished. The animals were then divided into four groups (n=3 per group) matched for prior operant responding and given either SoRI-9409 (5 mg/kg I.P.) or vehicle, 30 min prior to a challenge with yohimbine (2 mg/kg I.P.) or vehicle (the groups were SoRI-9409-vehicle/yohimbine-vehicle, SoRI-9409/yohimbine-vehicle, SoRI-9409-vehicle/yohimbine, SoRI-9409/yohimbine). A separate group of naive (non-ethanol trained) rats (n=16) was also divided into four same treatment groups (n=4 per group). Thirty minutes following the yohimbine or yohimbine vehicle injection the animals were euthanized by rapid decapitation. Following decapitation, rat brains were removed and quickly frozen using liquid nitrogen and stored at -80°C until used. Rat brains were quickly thawed for dissection and the cerebral cortex, striatum and midbrain regions were removed. Rat brain regions were suspended in a homogenization buffer (50 mmol/L Tris-HCl, 1 mmol/L EDTA, 3 mmol/L MgCl2 pH 7.4; 1g brain tissue/20 mL buffer). The tissue from each brain region was individually homogenized on ice (900 rpm; 15 strokes), centrifuged (1000 × g, 10 min, 4°C followed by 20 000 × g, 15 min, 4°C), resuspended in HME assay buffer (pH 7.5; 100 mmol/L HEPES.NaOH, 10 mmol/L NaCl, 5 mmol/L MgCl2, 10 μg/mL saponin and one mammalian protease inhibitor tablet), before snap-frozen in liquid nitrogen and stored at -80°C until required.
[35S]GTPγS Binding Assay
Binding assays (n=3 for each group each in triplicate) were performed in 96-well plates on ice with each reaction containing [35S]GTPγS (50 pmol/L), cell membrane (10 μg protein), GDP (30 μmol/L), and SPA beads (0.5 mg) with assay buffer (as above) and the opioid ligands. Single drug dose-response curves (0.1-100 μmol/L) of [35S]GTPγS stimulated binding were performed with the DOP-R1 agonist, TAN67 (Kamei et al., 1995; Tseng et al., 1997), the DOP-R2 agonist, Deltorphin II (Mattia et al., 1991; Zaki et al., 1996), the MOP-R agonist, DAMGO and the KOP-R agonist, (-)U50488. Inhibition of TAN67-(10 μmol/L)-stimulated [35S]GTPγS binding was also performed in the presence of varying concentrations of SoRI-9409, naltrindole or BNTX (0.1 nmol/L - 100 μmol/L). Assay plates were shaken for 45 min at 25°C, and centrifuged (1500 rpm, 5 min, 25°C) before [35S]GTPγS stimulated binding was assessed using the NXT TOPCOUNTER™. [35S]GTPγS-stimulated binding is expressed as a percentage increase in basal [35S]GTPγS binding.
Corticosterone Measurements
Trunk blood was collected from rats (n=3) at the same time that rat brains were collected for each of the four groups described above. The blood was collected 30 minutes after the second injection (yohimbine or yohimbine-vehicle) and stored on ice in tubes containing EDTA (10% of total volume). The samples were centrifuged at 4°C for 12 minutes at 6000 rcf then frozen at -80°C until analysis. Triplicate serum corticosterone concentrations were determined for each sample by a commercially available ELISA kit according to the manufacturer's instructions (Assay Designs Inc., Ann Arbor, MI).
Drugs and Chemicals
Ethanol (190 proof) was purchased from Gold Shield Chemical Co (Hayward, CA). [35S]-Guanosine 5′-(γ-thio)triphosphate ([35S]-GTPγS) (250μCi; 9.25MBq) was supplied from Perkin-Elmer® (Boston, USA). SB 205607 dihydrobromide (TAN67) was purchased from Tocris (Ellisville, Missouri). [D-Ala2]-Deltorphin II, DAMGO [D-Ala2, N-Me-Phe4, Gly5-ol]-Enkephalin acetate salt, (−)-trans-(1S,2S)-U-50488 hydrochloride hydrate, naltrindole hydrochloride, 7-benzylidenenaltrexone maleate salt hydrate (BNTX), guanosine 5′-[γ-thio]triphosphate tetralithium salt (GTPγS) and guanosine 5′-diphosphate sodium salt (GDP), 2-hydroxy-ethylpiperazine-N-2-ethane sulphonic acid (HEPES), DL-dithiothreitol, tricine, dimethyl sulfoxide (DMSO), magnesium chloride (MgCl2) ethylenediaminetetraacetic acid (EDTA) and saponin were purchased from Sigma-Aldrich® (St. Louis, USA). Complete mini protease inhibitor cocktail tablets were purchased from Roche (Indianapolis, USA) and Wheatgerm Agglutinin SPA Beads were purchased from Amersham (Little Chalfont, England) Biosciences. SoRI-9409 (5′-(4-Chlorophenyl)-17-(cyclopropylmethyl)-6,7-didehydro-3,14-dihydroxy-4,5-alpha-epoxypyrido[2′,3′:6,7] morphinan) was synthesized at the Southern Research Institute (Birmingham, USA). SoRI-9409 was dissolved in 2% dimethyl sulfoxide (DMSO) in distilled water with a drop glacial acetic acid added to keep the drug in solution (pH 5.3) and delivered in a volume of 1 ml/kg. Yohimbine was dissolved in distilled water and administered at a dose of 2 mg/kg (I.P.) in a volume of 0.5 ml/kg, I.P. The 10% ethanol (v/v) solution was prepared using 95% ethyl alcohol (Gold Shield Chemical Co., Hayward, CA DSP-CA-151) and filtered tap water. In the sucrose fade experiments, 10%, 5%, 3% and 1.5% sucrose respectively were dissolved in 10% ethanol (w/v).
Statistics
The data from the reinstatement studies were analyzed by two way ANOVA using the between subjects factor of SoRI-9409 dose and within subjects factors of yohimbine dose. Active and inactive lever data were analyzed separately as there was no effect of either SoRI-9409 or yohimbine on inactive lever pressing and therefore no need for determination of any drug × lever interactions. All behavioral statistical analyses were performed using SigmaStat software and in all cases post-hoc analysis was determined using the Student Newman-Keuls test where statistical significance was P < 0.05. Data analysis for binding assays was performed using GraphPad Prism® (GraphPad, San Diego, CA). Data from in vitro functional binding assays were analyzed by non-linear regression using a sigmoidal curve with variable slope to determine EC50 and IC50 values with EC50 values compared using the Student's t test. Corticosterone was analyzed by two way analysis of variance (ANOVA).
Results
SoRI-9409 attenuates yohimbine-mediated stress-induced reinstatement of ethanol-seeking in rats
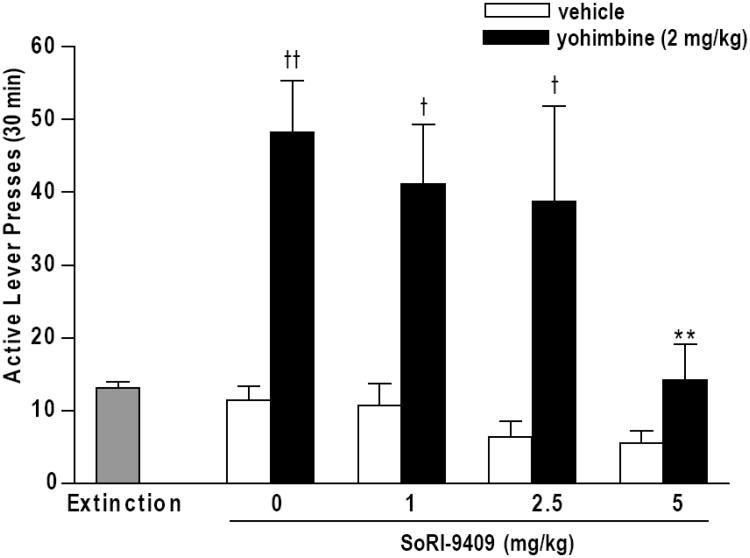
Rats were trained to respond for 10% ethanol and were maintained at a stable level (0.83 ± 0.04 g/kg ethanol intake, 173.1 ± 10.6 active lever presses, 1.8 ± 0.2 inactive lever presses, mean ± SEM for last ten sessions during maintenance) before extinction training was performed over five weeks (13.2 ± 1.2 active lever presses, 1.4 ± 0.4 inactive lever presses, mean + SEM for final three extinction sessions before testing). Yohimbine administration, in ethanol-extinguished rats previously trained to respond for 10% ethanol, induced significant reinstatement of ethanol-seeking. In rats administered SoRI-9409-vehicle prior to yohimbine (2 mg/kg, I.P.) active lever responding (48.0 ± 7.0 presses) was increased in comparison to SoRI-9409-vehicle/yohimbine-vehicle treated rats (10.9 ± 1.8 presses P < 0.001; Fig. 1). There was an overall effect of yohimbine in rats given SoRI-9409-vehicle or SoRI-9409 prior to yohimbine (F[1,100] = 39.43, P < 0.001). SoRI-9409 (5 mg/kg, I.P.) reduced yohimbine-induced reinstatement of ethanol-seeking (11.9 ± 4.4 active lever presses, P < 0.01) and there was an overall effect of SoRI-9409 on yohimbine-induced responding (F[3,100] = 4.64, P < 0.01; Fig. 1). SoRI-9409 treatment did not affect responding in rats which did not receive yohimbine (yohimbine-vehicle only) (F[3, 50] = 2.22, P > 0.05). Inactive lever responding was unaffected by rats given SoRI-9409-vehicle and yohimbine compared to SoRI-9409-vehicle/yohimbine-vehicle (P > 0.05) or by SoRI-9409 in both yohimbine-treated (F= [3, 50] = 2.15, P > 0.05) or yohimbine-vehicle-treated rats (F[3,50] = 1.78, P > 0.05; Table 1). No freezing or immobility behavior was observed in any of the treatment groups, consistent with our previous studies showing that SoRI-9409 does not produce anxiogenic or anxiolytic effects using the elevated plus maze test in rats (Nielsen et al., 2008).
Figure 1. SoRI-9409 decreases yohimbine stress-induced reinstatement of ethanol-seeking.
SoRI-9409 (5 mg/kg, I.P.) reduces yohimbine-induced (2 mg/kg, I.P.) reinstatement of operant responding in the absence of ethanol in animals trained to self-administer 10% ethanol and subsequently extinguished. Rats were pretreated with SoRI-9409 (1, 2.5, 5 mg/kg, I.P.) or its vehicle 30 min before yohimbine (2 mg/kg, I.P.) or its vehicle. Yohimbine (or vehicle) was administered 30 min before the start of the reinstatement session. Data are presented as mean ± SEM active lever presses (30 min) (n=13-15 per group). Statistical analysis was performed by one-way ANOVA with Newman-Keuls post hoc testing. ** P < 0.01, compared to rats given SoRI-9409-vehicle and yohimbine, tt P < 0.001, t P < 0.01, compared to rats given SoRI-9409 and yohimbine-vehicle.
Table 1.
Inactive lever presses in groups of ethanol-trained rats given SoRI-9409 prior to yohimbine (or vehicle).
| Inactive lever presses | ||
|---|---|---|
|
| ||
| SoRI-9409 (mg/kg) | Yohimbine group | Vehicle group |
| Vehicle | 2.1 ± 0.7 | 1.1 ± 0.5 |
| 1 | 1.7 ± 0.5 | 0.8 ± 0.4 |
| 2.5 | 1.0 ± 0.4 | 0.3 ± 0.3 |
| 5 | 0.6 ± 0.4 | 0.2 ± 0.1 |
TAN67-mediated [35S]GTPγS binding is increased in the midbrain of yohimbine-treated rats trained to respond for 10% ethanol
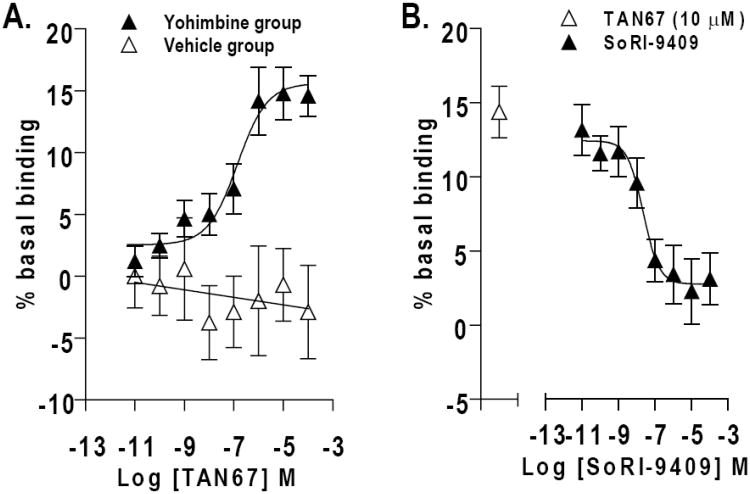
DOP-R-mediated [35S]GTPγS stimulation by the DOP-R1 agonist, TAN67, was high in midbrain membranes prepared from ethanol-trained rats administered SoRI-9409-vehicle prior to yohimbine (2 mg/kg I.P.; EC50 =140 ± 40 nmol/L) compared to reduced DOP-R1-mediated [35S]GTPγS stimulation in the midbrain membranes of SoRI-9409/yohimbine-treated rats and no DOP-R1-mediated [35S]GTPγS stimulation in midbrain membranes of SoRI-9409-vehicle/yohimbine-vehicle or SoRI-9409/yohimbine-vehicle-treated rats (Fig. 2A; Table 2). DOP-R-mediated [35S]GTPγS stimulation by the DOP-R1 agonist, TAN67, was absent (EC50 > 100 μM) in midbrain membranes prepared from naive (non-ethanol trained) rats of all treatment groups. DOP-R1 mediated [35S]GTPγS stimulation by TAN67 was low in membranes prepared from the cerebral cortex and absent in membranes prepared from the striatum in all treatment groups (Table 3). DOP-R2-mediated [35S]GTPγS stimulation by Deltorphin II was higher in midbrain membranes prepared from rats administered SoRI-9409-vehicle prior to yohimbine (2 mg/kg I.P.) compared to no DOP-R2-mediated [35S]GTPγS stimulation in midbrain membranes prepared from rats administered either SoRI-9409-vehicle or SoRI-9409 prior to yohimbine-vehicle but was not different to DOP-R2-mediated stimulation in midbrain membranes of SoRI-9409 prior to yohimbine (P > 0.05; Table 2). MOP-R- and KOP-R-mediated [35S]GTPγS stimulation with DAMGO and (-)U50488, respectively, was low in midbrain membranes of ethanol-trained rats and was not different between any of the treatment groups (Table 2).
Figure 2. Increased delta opioid receptor (DOP-R)-stimulated [35S]GTPγS binding in the midbrain of yohimbine-treated rats is inhibited by SoRI-9409.
(A) DOP-R-mediated [35S]GTPγS stimulation by the DOP-R agonist, TAN67, is higher in midbrain membranes prepared from rats administered SoRI-9409-vehicle prior to yohimbine compared to midbrain membranes prepared from rats given SoRI-9409-vehicle prior to yohimbine-vehicle. (B) SoRI-9409 inhibits TAN67-stimulated [35S]GTPγS DOP-R binding in midbrain membranes prepared from rats administered SoRI-9409-vehicle prior to yohimbine. Rats were pretreated with SoRI-9409 (5 mg/kg, I.P.) or its vehicle 30 min before yohimbine (2 mg/kg I.P.) or its vehicle. Yohimbine (or vehicle) was administered 30 min before rat brains were collected (n=3 per group, each in triplicate). The values are expressed as mean ± SEM percentage increase in basal [35S]GTPγS binding.
Table 2.
Opioid-mediated [35S]GTPγS-binding stimulation in rat midbrain membranes of ethanol-trained rats administered SoRI-9409 (5 mg/kg I.P.) or vehicle prior to yohimbine (2 mg/kg I.P.) or vehicle.
| Treatment Group | Mean (± SEM) EC50 (μmol/L) for Midbrain Opioid Stimulation | |||
|---|---|---|---|---|
|
| ||||
| TAN67 | Deltorphin II | DAMGO | (−)U50488 | |
| SoRI-9409-vehicle/Yohimbine-vehicle | > 100 | > 100 | 4.1 ± 0.2 | 1.4 ± 0.3 |
| SoRI-9409-vehicle/Yohimbine | 0.14 ± 0.04*** | 27 ± 2.9 | 3.7 ± 0.2 | 1.1 ± 0.4 |
| SoRI-9409/Yohimbine-vehicle | > 100 | > 100 | 4.0 ± 0.1 | 1.6 ± 0.5 |
| SoRI-9409/Yohimbine | 5.2 ± 0.3 | 22 ± 4.0 | 4.0 ± 0.3 | 1.7 ± 0.4 |
P < 0.001, compared to SoRI-9409/yohimbine group
Table 3.
TAN67-mediated [35S]GTPγS-binding stimulation in rat cerebral cortex and striatal membranes of ethanol-trained rats administered SoRI-9409 (5 mg/kg I.P.) or vehicle prior to yohimbine (2 mg/kg I.P.) or vehicle.
| Treatment Group | Mean (± SEM) EC50 (μmol/L) for TAN67 Stimulation | |
|---|---|---|
|
| ||
| Cerebral Cortex | Striatum | |
| SoRI-9409-vehicle/Yohimbine-vehicle | 6.4 ± 0.9 | > 100 |
| SoRI-9409-vehicle/Yohimbine | 21 ± 3.9 | > 100 |
| SoRI-9409/Yohimbine-vehicle | 9.4 ± 0.6 | > 100 |
| SoRI-9409/Yohimbine | 12 ± 8.3 | > 100 |
SoRI-9409 inhibits TAN67-mediated [35S]GTPγS binding in the midbrain of yohimbine-treated rats trained to respond for 10% ethanol
TAN67-stimulated (10 μmol/L) [35S]GTPγS-binding was potently inhibited by SoRI-9409 (IC50 = 22 ± 3.5 nmol/L; Fig. 2B), BNTX (IC50 = 37 ± 4.8 nmol/L) and naltrindole (IC50 = 46 ± 3.8 nmol/L) in midbrain membranes prepared from ethanol-trained rats given SoRI-9409-vehicle prior to yohimbine (2 mg/kg I.P.).
SoRI-9409 does not alter yohimbine-induced increases in plasma corticosterone levels in ethanol-experienced rats
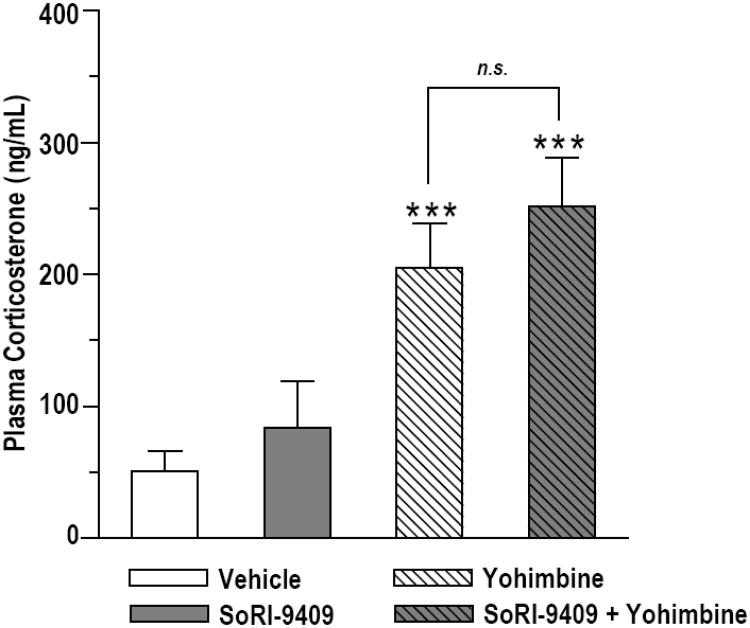
Plasma corticosterone (CORT) levels were increased in ethanol-trained rats given either SoRI-9409 (5 mg/kg I.P.) or SoRI-9409-vehicle prior to yohimbine (2 mg/kg I.P.) compared to rats given SoRI-9409-vehicle prior to yohimbine-vehicle (P < 0.01; Fig. 3). Plasma CORT levels of SoRI-9409/yohimbine treated rats were not different to plasma CORT levels of SoRI-9409-vehicle/yohimbine treated rats (P > 0.05). Similarly, plasma CORT levels of SoRI-9409/yohimbine-vehicle treated rats were not different to plasma CORT levels of SoRI-9409-vehicle/yohimbine-vehicle treated rats (P > 0.05). Statistical analysis of plasma CORT, using two way ANOVA, revealed a significant overall effect of yohimbine (F[1,24] = 5.25, P < 0.05), but no overall effect of SoRI-9409 treatment.
Figure 3. SoRI-9409 has no effect on yohimbine-induced increases in corticosterone levels in ethanol-seeking animals.
Plasma corticosterone (CORT) levels were increased in rats given either SoRI-9409 or SoRI-9409-vehicle prior to yohimbine (grey striped and white striped bars) compared to rats given SoRI-9409-vehicle prior to yohimbine-vehicle (white bar). Plasma CORT levels of SoRI-9409/yohimbine-vehicle treated rats (grey bar) were not different to SoRI-9409-vehicle/yohimbine-vehicle treated rats (white bar). Plasma CORT levels of SoRI-9409/yohimbine treated rats (grey striped bar) were not different to plasma CORT levels of SoRI-9409-vehicle/yohimbine treated rats (white striped bar). Rats were pretreated with SoRI-9409 (5 mg/kg I.P.) or its vehicle 30 min before yohimbine (2 mg/kg I.P.) or its vehicle. Yohimbine (or vehicle) was administered 30 min before trunk blood was collected. Data is presented as mean ± SEM (n=3 per group, each in triplicate). Statistical analysis was preformed by two-way ANOVA with Newman-Keuls post hoc testing. ** P < 0.01, compared to vehicle-vehicle group. n.s., not significant (P > 0.05).
Discussion
We show that yohimbine induces reinstatement of ethanol seeking and increases DOP-R activity in brain membranes and pretreatment with the DOP-R antagonist, SoRI-9409, reduces yohimbine stress-induced reinstatement of ethanol-seeking without affecting yohimbine-induced increases in plasma corticosterone (CORT) levels.
The DOP-R is located both at the plasma membrane and within the cytoplasmic compartment, often associated with dense-core vesicles in primary afferent neurons (Cahill et al., 2001; Commons, 2003; Scherrer et al., 2009). This contrasts with the MOP-R which is primarily expressed at the plasma membrane. It is well established that stressors, pain, psychostimulant and morphine administration change the distribution of DOP-R from the cytoplasmic compartment to the plasma membrane and increase the activity of the DOP-R (Ambrose-Lanci et al., 2008; Cahill et al., 2003; Cahill et al., 2001; Commons, 2003; Gendron et al., 2006). Our data suggests that yohimbine induces a translocation of the DOP-R to the surface and this may account for the increase in DOP-R activity measured in the brain membranes. It has been very difficult to directly demonstrate the redistribution of DOP-R using immunohistochemistry, as the DOP-R antibodies are not specific (Scherrer et al., 2009). Therefore we have measured the activity of the DOP-R using [35S]GTPγS binding that directly measures coupling to G proteins in membranes prepared from the brains of behaving animals. The most plausible explanation for the yohimbine-induced increase in DOP-R signaling is a redistribution of the DOP-R from the cytoplasmic compartment to the plasma membrane. However, we cannot rule out the possibility that DOP-R may more efficiently couple to G proteins following yohimbine administration.
It has been shown before that the endogenous ligands for DOP-Rs, the enkephalins, regulate stress responses (Gibson et al., 1979; Hashimoto et al., 1987; Pechnick, 1993; Szekely, 1990). Enkephalins are expressed in interneurons in the hypothalamic paraventricular nucleus (PVN) of the hypothalamus, a region of the limbic system involved in regulation of the hormonal stress responses and a primary controller of glucocorticoid release in the HPA axis (Bilkei-Gorzo et al., 2008; Drolet et al., 2001; Herman et al., 2002; Williams and Dockray, 1983). Acute stress including restraint, morphine withdrawal, formalin-induced pain, immobilization, hypertonic saline injection and colchicine injection increase enkephalin gene expression within the PVN neurons (Ceccatelli and Orazzo, 1993; Dumont et al., 2000; Lightman and Young, 1987, 1989; Palkovits, 2000; Young and Lightman, 1992). Furthermore, enkephalins are co-localized with corticotrophin releasing factor (CRF) and enkephalins increase the release of corticotrophin releasing factor (CRF) (Buckingham, 1982; Buckingham and Cooper, 1984). Yohimbine up-regulates CRF expression in the basolateral and central amygdalar nuclei (Funk et al., 2006) and the CRF antagonist, antalarmin, reduces yohimbine-induced reinstatement of ethanol-seeking (Marinelli et al., 2007). As DOP-Rs are reported to regulate ethanol actions in the PVN and the central and basolateral amygdala (Barson et al.,; Bie et al., 2009; Hyytia and Kiianmaa, 2001; Kang-Park et al., 2007), this suggests that DOP-Rs within the CNS may modulate yohimbine stress-induced reinstatement. Whether DOP-Rs alter CRF release in the amygdala remains to be investigated.
Although DOP-R agonists can reduce anxiety and DOP-R antagonists can increase both anxiety and CORT levels (Perrine et al., 2006; Saitoh et al., 2004; Saitoh et al., 2005), SoRI-9409 does not produce anxiogenic or anxiolytic effects using the elevated plus maze test in rats (Nielsen et al., 2008) and does not change plasma CORT levels or lever responding when administered alone. Furthermore, when SoRI-9409 was administered prior to yohimbine, there were no changes in CORT levels compared to rats given vehicle with yohimbine and there were also no competing behaviors observed in any rats such as freezing or immobility. It therefore seems unlikely that treatment with SoRI-9409 prevents any physiological compensatory response aimed at controlling anxiety exacerbation and thus potentiating the anxiogenic effects of yohimbine. These results therefore indicate that SoRI-9409 does not produce any effects on anxiety which could account for the reduction in yohimbine-mediated responding for ethanol.
In our experiments, TAN67- and Deltorphin II-mediated DOP-R activity were increased in membranes of yohimbine-treated ethanol-extinguished rats compared to vehicle-treated rats. The increase in DOP-R-mediated [35S]GTPγS stimulation with yohimbine-treatment in ethanol-trained, but not in naive (non ethanol-trained) rats suggests that a history of ethanol self-administration plays an important role in the regulation of DOP-R signaling. In contrast to DOR activity, there were no changes in DAMGO-mediated MOP-R or (-)U50488-mediated KOP-R activity in ethanol-trained rats. This indicates the DOP-R plays a greater role than MOP-R or KOP-R in yohimbine stress-induced reinstatement of ethanol-seeking supporting previous work showing DOP-Rs rather than MOP-Rs are important for cue-induced reinstatement of ethanol-seeking (Ciccocioppo et al., 2002; Marinelli et al., 2009). These findings may also suggest that the non-selective opioid antagonist, naltrexone, reduces cue-induced reinstatement via the DOP-R rather than the MOP-R. However, the lack of reductions in foot-shock stress-induced reinstatement of ethanol-seeking by naltrexone (Le et al., 1999; Liu and Weiss, 2002) may be attributed to doses of naltrexone (0.2-2 mg/kg) which would predominantly block MOP-Rs over DOP-Rs. The effects of naltrexone on yohimbine stress-induced reinstatement of ethanol-seeking would be worthy of investigation.
It is not known whether different subtypes of DOP-R are involved in the stress response. One DOP-R gene has been cloned (Evans et al., 1992; Kieffer et al., 1992), however two subtypes of DOP-R have been pharmacologically identified in vivo: the DOP-R1 and DOP-R2 (Mattia et al., 1991; Zaki et al., 1996). Unlike in vitro studies using cell lines either transfected with or endogenously expressing the DOP-R (Parkhill and Bidlack, 2002; Toll et al., 1997), studies in brain membranes have reported the existence of two distinct subtypes of DOP-R (Buzas et al., 1994; Negri et al., 1991). The higher activity of TAN67-mediated DOP-R1 activity (> 700-fold) than deltorphin II-mediated DOP-R2 activity (≥4-fold) in yohimbine-treated rats compared to vehicle-treated rats (Table 2) indicates that DOP-R1 plays a greater role than DOP-R2 in stress-induced reinstatement. Pretreatment with SoRI-9409 in yohimbine-treated rats reduced TAN67-mediated DOP-R1 activity by 37-fold but did not change DOP-R2, MOP-R or KOP-R activity. Although TAN67-mediated DOP-R1 activity in yohimbine-treated rats was not completely reversed by SoRI-9409 (5 mg/kg) to low levels under basal conditions, a higher dose of SoRI-9409 would be predicted to completely reduce DOP-R activity. The potent inhibition of TAN67-stimulated DOP-R1 activity in yohimbine-treated rat membranes by both SoRI-9409 and BNTX, a DOP-R1 antagonist (Sofuoglu et al., 1993), suggests that SoRI-9409 reduces yohimbine induced ethanol-seeking via the DOP-R1. Taken together with our previous studies showing that SoRI-9409 effectively reduces ethanol consumption in rats and potently reduces TAN67-mediated DOP-R1 activity in brain membranes of high-ethanol consuming rats (Nielsen et al., 2008), DOP-R1 inhibition appears to play a role in both reducing voluntary ethanol intake and reinstatement of ethanol-seeking in rats. However, the reduction in DOP-R1 but not DOP-R2 activity by SoRI-9409 may not to be due to higher selectivity of SoRI-9409 for DOP-R1 over DOP-R2 since previous studies have shown that SoRI-9409 inhibits both deltorphin II-mediated DOP-R2 analgesia and DPDPE-mediated DOP-R1 analgesia in mice (Wells et al., 2001). It therefore appears that the subtypes of the DOP-R have different roles in different behaviors and species. This is demonstrated further in studies reporting opposing effects of DOP-R1 and DOP-R2 on voluntary ethanol intake such that inhibition of DOP-R2, but not DOP-R1, reduces ethanol intake in mice (van Rijn and Whistler, 2009). However, the potent increase in DOP-R1 over DOP-R2 activity with yohimbine treatment and the potent reduction of DOP-R1 but not DOP-R2 activity by SoRI-9409 suggest that inhibition of DOP-R1, but not DOP-R2, is important for reductions in yohimbine stress-induced reinstatement of ethanol-seeking in rats. Further studies to determine whether yohimbine-mediated increases in ethanol self-administration (Le et al., 2005) and reinstatement of ethanol-seeking are altered by DOP-R subtype-selective ligands would be worthy of further investigation. Whether the reductions in cue-induced reinstatement of ethanol-seeking by the dual DOP-R1 and DOP-R2 antagonist, naltrindole (Ciccocioppo et al., 2002; Marinelli et al., 2009), are mediated primarily by one or both of the DOP-R subtypes is unknown.
These results show yohimbine increases DOP-R activity in the brain and that inhibiting the DOP-R reduces yohimbine-stress induced reinstatement of ethanol-seeking. We hypothesize that yohimbine induces a stress-like response that leads to activation of DOP-Rs. This suggests that the DOP-R may be a promising target for the treatment and prevention of relapse to alcohol seeking. As the DOP-R antagonist, SoRI-9409, has now been shown to effectively reduce stress-induced reinstatement of ethanol-seeking and voluntary ethanol consumption (Nielsen et al., 2008), this compound appears to be a promising candidate for further development for the treatment of alcohol use disorders.
Acknowledgments
We thank Joan Holgate, Hilary Garcia and Isabel Kanholm for technical assistance. This work was supported by funding from the State of California for Medical Research through UCSF to SEB, NIH Grant 1R01AA017924–01 to SEB and Department of Defense Grant W81XWH-06–1-0240 to SEB SEB and CKN were supported in part by the Sidney Baer Trust and the Essel Foundation as National Alliance for Research on Schizophrenia and Depression Young Investigators. The experiments contained herein comply with the current laws of the United States. All procedures were pre-approved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with NIH guidelines for the Humane Care and Use of Laboratory Animals.
Footnotes
Author contributions: CKN, JAS and SEB designed the research. JAS and JJB performed the behavioral experiments. CKN performed the binding experiments. RL prepared membranes for the binding experiments. JJB performed the ELISA experiments. CKN, JAS and JJB performed data analysis. CKN and SEB drafted the manuscript. SA synthesized and supplied the test compound. All authors critically reviewed content and approved final version for publication.
References
- Ambrose-Lanci LM, Peiris NB, Unterwald EM, Van Bockstaele EJ. Cocaine withdrawal-induced trafficking of delta-opioid receptors in rat nucleus accumbens. Brain Res. 2008;1210:92–102. doi: 10.1016/j.brainres.2008.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthan S, Kezar HS, 3rd, Carter RL, Saini SK, Rice KC, Wells JL, Davis P, Xu H, Dersch CM, Bilsky EJ, Porreca F, Rothman RB. Synthesis, opioid receptor binding, and biological activities of naltrexone-derived pyrido- and pyrimidomorphinans. J Med Chem. 1999;42:3527–38. doi: 10.1021/jm990039i. [DOI] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Rada P, Leibowitz SF, Hoebel BG. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcohol Clin Exp Res. 34:214–22. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie B, Zhu W, Pan ZZ. Rewarding morphine-induced synaptic function of delta-opioid receptors on central glutamate synapses. J Pharmacol Exp Ther. 2009;329:290–6. doi: 10.1124/jpet.108.148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmuller D, Zimmer A. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology. 2008;33:425–36. doi: 10.1016/j.psyneuen.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Borg PJ, Taylor DA. Involvement of mu- and delta-opioid receptors in the effects of systemic and locally perfused morphine on extracellular levels of dopamine, DOPAC and HVA in the nucleus accumbens of the halothane-anaesthetized rat. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:582–8. doi: 10.1007/pl00004987. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Buckingham JC. Secretion of corticotrophin and its hypothalamic releasing factor in response to morphine and opioid peptides. Neuroendocrinology. 1982;35:111–6. doi: 10.1159/000123364. [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinology. 1984;38:411–7. doi: 10.1159/000123927. [DOI] [PubMed] [Google Scholar]
- Buzas B, Izenwasser S, Portoghese PS, Cox BM. Evidence for delta opioid receptor subtypes regulating adenylyl cyclase activity in rat brain. Life Sci. 1994;54:PL101–6. doi: 10.1016/0024-3205(94)00412-9. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccatelli S, Orazzo C. Effect of different types of stressors on peptide messenger ribonucleic acids in the hypothalamic paraventricular nucleus. Acta Endocrinol (Copenh) 1993;128:485–92. doi: 10.1530/acta.0.1280485. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–9. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Commons KG. Translocation of presynaptic delta opioid receptors in the ventrolateral periaqueductal gray after swim stress. J Comp Neurol. 2003;464:197–207. doi: 10.1002/cne.10788. [DOI] [PubMed] [Google Scholar]
- Contet C, Gaveriaux-Ruff C, Matifas A, Caradec C, Champy MF, Kieffer BL. Dissociation of analgesic and hormonal responses to forced swim stress using opioid receptor knockout mice. Neuropsychopharmacology. 2006;31:1733–44. doi: 10.1038/sj.npp.1300934. [DOI] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266:1236–46. [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:729–41. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Kinkead R, Trottier JF, Gosselin I, Drolet G. Effect of chronic psychogenic stress exposure on enkephalin neuronal activity and expression in the rat hypothalamic paraventricular nucleus. J Neurochem. 2000;75:2200–11. doi: 10.1046/j.1471-4159.2000.0752200.x. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–5. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Zweifel M, Harts J, Lumeng L, Li TK. Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology (Berl) 1991;103:467–72. doi: 10.1007/BF02244246. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–43. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Reinstatement of ethanol-seeking behavior following intravenous self-administration in wistar rats. Alcohol Clin Exp Res. 2007;31:1441–5. doi: 10.1111/j.1530-0277.2007.00480.x. [DOI] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O'Donnell D, Vincent JP, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26:953–62. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–96. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A, Ginsburg M, Hall M, Hart SL. The effect of intracerebroventricular administration of methionine-enkephalin on the stress-induced secretion of corticosterone in mice. Br J Pharmacol. 1979;66:164–6. doi: 10.1111/j.1476-5381.1979.tb13660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvez ML, Milanes MV, Vargas ML. Effects of acute and chronic administration of mu- and delta-opioid agonists on the hypothalamic-pituitary-adrenocortical (HPA) axis in the rat. Eur J Pharmacol. 1991;200:155–8. doi: 10.1016/0014-2999(91)90678-j. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Suemaru S, Ono N, Hattori T, Inoue H, Takao T, Sugawara M, Kageyama J, Ota Z. Dual effects of (D-Ala2,Met5)-enkephalinamide on CRF and ACTH secretion. Peptides. 1987;8:113–8. doi: 10.1016/0196-9781(87)90173-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16:381–5. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Kim HS, Wood PL. Mu-, delta-, kappa- and epsilon-opioid receptor modulation of the hypothalamic-pituitary-adrenocortical (HPA) axis: subchronic tolerance studies of endogenous opioid peptides. Brain Res. 1987;435:220–6. doi: 10.1016/0006-8993(87)91604-0. [DOI] [PubMed] [Google Scholar]
- Kamei J, Saitoh A, Ohsawa M, Suzuki T, Misawa M, Nagase H, Kasuya Y. Antinociceptive effects of the selective non-peptidic delta-opioid receptor agonist TAN-67 in diabetic mice. Eur J Pharmacol. 1995;276:131–5. doi: 10.1016/0014-2999(95)00026-h. [DOI] [PubMed] [Google Scholar]
- Kang-Park MH, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. Presynaptic delta opioid receptors regulate ethanol actions in central amygdala. J Pharmacol Exp Ther. 2007;320:917–25. doi: 10.1124/jpet.106.112722. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci U S A. 1992;89:12048–52. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–73. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–44. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Young WS., 3rd Changes in hypothalamic preproenkephalin A mRNA following stress and opiate withdrawal. Nature. 1987;328:643–5. doi: 10.1038/328643a0. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Young WS., 3rd Influence of steroids on the hypothalamic corticotropin-releasing factor and preproenkephalin mRNA responses to stress. Proc Natl Acad Sci U S A. 1989;86:4306–10. doi: 10.1073/pnas.86.11.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–61. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse. Psychopharmacology (Berl) 2003;168:184–91. doi: 10.1007/s00213-002-1267-z. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Harding S, Li Z, Juzytsch W, Le AD. Roles of opioid receptor subtypes in mediating alcohol-seeking induced by discrete cues and context. Eur J Neurosci. 2009;30:671–8. doi: 10.1111/j.1460-9568.2009.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–55. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Mattia A, Vanderah T, Mosberg HI, Porreca F. Lack of antinociceptive cross-tolerance between [D-Pen2, D-Pen5]enkephalin and [D-Ala2]deltorphin II in mice: evidence for delta receptor subtypes. J Pharmacol Exp Ther. 1991;258:583–7. [PubMed] [Google Scholar]
- Negri L, Potenza RL, Corsi R, Melchiorri P. Evidence for two subtypes of delta opioid receptors in rat brain. Eur J Pharmacol. 1991;196:335–6. doi: 10.1016/0014-2999(91)90450-5. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Pierson HB, Li R, Saini SK, Ananthan S, Bartlett SE. A novel delta opioid receptor antagonist, SoRI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats. Biol Psychiatry. 2008;64:974–81. doi: 10.1016/j.biopsych.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley SS, Jaffe AJ, Chang G, Rode S, Schottenfeld R, Meyer RE, Rounsaville B. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Arch Gen Psychiatry. 1996;53:217–24. doi: 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Stress-induced expression of co-localized neuropeptides in hypothalamic and amygdaloid neurons. Eur J Pharmacol. 2000;405:161–6. doi: 10.1016/s0014-2999(00)00549-5. [DOI] [PubMed] [Google Scholar]
- Parkhill AL, Bidlack JM. Several delta-opioid receptor ligands display no subtype selectivity to the human delta-opioid receptor. Eur J Pharmacol. 2002;451:257–64. doi: 10.1016/s0014-2999(02)02241-0. [DOI] [PubMed] [Google Scholar]
- Pechnick RN. Effects of opioids on the hypothalamo-pituitary-adrenal axis. Annu Rev Pharmacol Toxicol. 1993;33:353–82. doi: 10.1146/annurev.pa.33.040193.002033. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol. 2006;147:864–72. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky LA, Skiandos A, Zhang X, Rice KC, Benjamin D. Effect of chronic social stress on delta-opioid receptor function in the rat. J Pharmacol Exp Ther. 1999;290:196–206. [PubMed] [Google Scholar]
- Richards JK, Simms JA, Bartlett SE. Conditioned cues and yohimbine induce reinstatement of beer and near-beer seeking in Long-Evans rats. Addict Biol. 2009;14:144–51. doi: 10.1111/j.1369-1600.2008.00139.x. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–17. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–80. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yoshikawa Y, Onodera K, Kamei J. Role of delta-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology (Berl) 2005;182:327–34. doi: 10.1007/s00213-005-0112-6. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–42. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–59. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–9. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Portoghese PS, Takemori AE. 7-Benzylidenenaltrexone (BNTX): a selective delta 1 opioid receptor antagonist in the mouse spinal cord. Life Sci. 1993;52:769–75. doi: 10.1016/0024-3205(93)90240-4. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–23. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely JI. Opioid peptides and stress. Crit Rev Neurobiol. 1990;6:1–12. [PubMed] [Google Scholar]
- Toll L, Polgar WE, Auh JS. Characterization of the delta-opioid receptor found in SH-SY5Y neuroblastoma cells. Eur J Pharmacol. 1997;323:261–7. doi: 10.1016/s0014-2999(97)00031-9. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Narita M, Mizoguchi H, Kawai K, Mizusuna A, Kamei J, Suzuki T, Nagase H. Delta-1 opioid receptor-mediated antinociceptive properties of a nonpeptidic delta opioid receptor agonist, (-)TAN-67, in the mouse spinal cord. J Pharmacol Exp Ther. 1997;280:600–5. [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL. The delta(1) opioid receptor is a heterodimer that opposes the actions of the delta(2) receptor on alcohol intake. Biol Psychiatry. 2009;66:777–84. doi: 10.1016/j.biopsych.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetulani J. Drug addiction. Part II. Neurobiology of addiction. Pol J Pharmacol. 2001;53:303–17. [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Wells JL, Bartlett JL, Ananthan S, Bilsky EJ. In vivo pharmacological characterization of SoRI 9409, a nonpeptidic opioid mu-agonist/delta-antagonist that produces limited antinociceptive tolerance and attenuates morphine physical dependence. J Pharmacol Exp Ther. 2001;297:597–605. [PubMed] [Google Scholar]
- Williams RG, Dockray GJ. Distribution of enkephalin-related peptides in rat brain: immunohistochemical studies using antisera to met-enkephalin and met-enkephalin Arg6Phe7. Neuroscience. 1983;9:563–86. doi: 10.1016/0306-4522(83)90175-6. [DOI] [PubMed] [Google Scholar]
- Xu H, Lu YF, Rice KC, Ananthan S, Rothman RB. SoRI 9409, a non-peptide opioid mu receptor agonist/delta receptor antagonist, fails to stimulate [35S]-GTP-gamma-S binding at cloned opioid receptors. Brain Res Bull. 2001;55:507–11. doi: 10.1016/s0361-9230(01)00550-0. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Lightman SL. Chronic stress elevates enkephalin expression in the rat paraventricular and supraoptic nuclei. Brain Res Mol Brain Res. 1992;13:111–7. doi: 10.1016/0169-328x(92)90050-l. [DOI] [PubMed] [Google Scholar]
- Zaki PA, Bilsky EJ, Vanderah TW, Lai J, Evans CJ, Porreca F. Opioid receptor types and subtypes: the delta receptor as a model. Annu Rev Pharmacol Toxicol. 1996;36:379–401. doi: 10.1146/annurev.pa.36.040196.002115. [DOI] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH. Context is a trigger for relapse to alcohol. Behav Brain Res. 2006;167:150–5. doi: 10.1016/j.bbr.2005.09.007. [DOI] [PubMed] [Google Scholar]