Abstract
Forkhead box E1 encodes the transcription factor FOXE1 (or TTF-2), which together with Homeobox protein NKX2-1, PAX8 and HHEX, are pivotal proteins required for thyroid gland formation, differentiation and function. Recently, genome-wide association studies have identified FOXE1 as a thyroid cancer (TC) susceptibility gene in populations of European descent. After that, a number of studies reported that the rs965513, rs1867277, and rs71369530 polymorphism in FOXE1 has been implicated in TC risk. However, the causal variants remain unknown. To derive a more precise estimation of the relationship, a meta-analysis of 9,828 TC cases and 109,995 controls from 14 case–control studies was performed. Overall, significant results were observed for rs965513 (OR = 1.71, 95% CI: 1.59–1.85, P<10−5), rs1867277 (OR = 1.64, 95% CI: 1.51–1.78, P<10−5) and rs71369530 (OR = 2.01, 95% CI: 1.66–2.44, P<10−5) polymorphism. In the subgroup analysis by ethnicity, we found that rs965513 polymorphism confer high risk for Caucasians with per-allele OR of 1.80 (95% CI: 1.69–1.92, P<10−5) compared to East Asians of 1.35 (95% CI: 1.09–1.67, P = 0.006). There was strong evidence of heterogeneity, which largely disappeared after stratification by ethnicity. In the subgroup analysis by sample size, and study design, significantly increased risks were found for the polymorphism. In conclusion, this meta-analysis demonstrated that common variations of FOXE1 are a risk factor associated with increased TC susceptibility.
Introduction
Thyroid cancer (TC) is the most common endocrine malignancy, and accounts for 1% of all neoplasias [1]. TC is classified histologically into four main groups: papillary (PTC), follicular (FTC), medullary (MTC) and undifferentiated thyroid carcinomas. Most of all thyroid tumors are PTC (80–85%) or FTC (10–15%) [2]. Although the etiology of this cancer is not well characterized, thyroid cancer is believed to be a complex disease, in which common genetic variants located in low penetrance genes may interact with each other and with the environment, determining individual susceptibility. Among the latter, ionizing radiation, especially exposure to fallout of radioactive iodine isotopes in childhood, strongly predisposes to TC [3]. The contribution of genetics to the risk of thyroid cancer is greater than to any other cancer, and the effect extends beyond the nuclear family [4], [5]. The identification and further assessment of the relevant genetic variations are important for understanding the potential mechanisms involved in thyroid carcinogenesis.
Recently, spectacular advance was made in identifying susceptible genes involved in TC through genome-wide association strategy (GWAS) among European descent [6], [7]. A number of recent studies have identified single nucleotide polymorphisms (SNPs) associated with TC risk on chromosomes 5q24, 8q24, 9q22, and 14q13 [6], [8]–[10]. Common genetic variant (rs965513) of FOXE1 at chromosome 9q22, has been identified as a new hotspot for thyroid cancer susceptibility by a recent GWA study [6], [7]. FOXE1 possesses a polymorphic polyalanine tract (rs71369530) just distal to its DNA-binding domain, with 11–22 alanine residues reported, although FOXE1 14Ala and FOXE1 16Ala account for greater than 98% of reported alleles [11]. Recently, Landa et al. [12] found strong evidence that one SNP located in the promoter region of the FOXE1 gene (rs1867277) was positively associated with sporadic thyroid cancer susceptibility. Over the past few years, these polymorphisms (rs965513, rs1867277, and rs71369530) in the FOXE1 region and thyroid cancer risk have been independently replicated by subsequent studies. As stated by McClellan and King, many if not most of the genetic polymorphisms that are reported to be associated with common disorders in GWA studies are factually spurious associations caused by subtle differences in ancestry between the populations being studied (population stratification) [13]. The interpretation of these studies has been further complicated by the use of different ethnic populations, insufficient power, small effect of the polymorphism on thyroid cancer risk and phenotypic heterogeneity. In addition, with the increased studies in recent years among East Asian populations, there is a need to reconcile these data. We therefore performed a meta-analysis of the published studies to clarify this inconsistency and to establish a comprehensive picture of the relationship between common variants on chromosome FOXE1 and thyroid cancer.
Materials and Methods
Literature search strategy and selection criteria
Epidemiological genetic association studies published before the end of Nov. 2013 on thyroid cancer and polymorphism in the FOXE1 gene were sought by computer-based searches from databases including Pubmed, SCOPUS, ISI web of knowledge, Embase, Cochrane databases and CNKI (China National Knowledge Infrastructure) without language restriction. Search term combinations were keywords relating to the FOXE1 gene (e.g., “FOXE1”, “TTF-2”, “9q22”, “rs965513”, “rs1867277”, “rs71369530”, “polyalanine tracts”) in combination with words related to thyroid cancer (e.g., “thyroid cancer”, “thyroid carcinoma”, “thyroid tumor”). We replaced one term each time until all possible combination mode were searched to avoid any missing literature. The titles and abstracts of potential articles were screened to determine their relevance, and any clearly irrelevant studies were excluded. The full texts of the remaining articles were read to determine whether they contained information on the topic of interest. Furthermore, reference lists of primary studies and review articles were also reviewed by a manual search to identify additional relevant publications.
The included studies have to meet the following criteria: (1) evaluation of at least one of those three polymorphisms (rs965513, rs1867277, rs71369530) and thyroid cancer risks using case–control or cohort design, (2) original papers containing independent data, (3) identification of thyroid cancer patients was confirmed histologically or pathologically, (4) genotype distribution information or odds ratio (OR) with its 95% confidence interval (CI) and P-value, and (5) genotype distribution of control group must be consistent with Hardy–Weinberg equilibrium (HWE). The major reasons for exclusion of studies were (1) overlapping data, (2) case-only studies, and (3) review articles.
Data extraction
Information was carefully extracted from all eligible publications independently by the two authors according to the inclusion criteria listed above. For each included study, the following information was extracted from each report according to a fixed protocol: first author, publication year, definition and numbers of cases and controls, frequency of genotypes, age, sex, ethnicity, HWE status, source of control, histological subtypes and genotyping method. Review reports from the two were then compared to identify any inconsistency, and differences were resolved by further discussion among all authors. Studies with different ethnic groups were considered as individual studies for our analyses. Meanwhile, different case–control groups in one study were considered as independent studies. The instrument “Extended-quality score” was used to assess the quality of association studies [14]. In general, each article is scored on an extended-quality scale that designates them as “high,” “median,” or “poor” quality.
Statistical methods
Crude ORs with the corresponding 95% CIs were used to assess the strength of the association between the FOXE1 polymorphism and thyroid cancer risks. The per-allele OR of the risk allele was estimated. Then, we estimated the risks of the heterozygous and homozygous genotypes on TC compared with the wild-type homozygote [15]. Cochran's χ2 based Q-statistic and I2 test [16], [17] test was performed to assess possible heterogeneity in the combined studies. Generally, I2 values <25% correspond to no or little heterogeneity, values 25–50% correspond to moderate heterogeneity, and values >50% correspond to strong heterogeneity between studies. Random-effects and fixed-effect summary measures were calculated as inverse-variance-weighted average of the log odds ratio [18]. The results of random-effects summary were reported in the text because it takes into account the variation between studies. 95% CIs were constructed using Woolf's method [19]. Sources of heterogeneity were investigated by stratified meta-analyses based on ethnicity, sample size (No. cases ≥500 or, <500) and study design strategy (GWAS vs. candidate gene). Ethnic group was defined as East Asians (i.e., Chinese, Japanese, and Korean), and Caucasians (i.e. people of European origin). In addition, ethnicity, sample size, and study design (GWAS vs. candidate gene) was analyzed as covariates in meta-regression. The significance of the pooled OR was determined by Z test. Publication bias was assessed with the Egger test and Begg test [20], [21]. Sensitivity analysis was performed by removing each individual study in turn from the total and re-analyzing the remainder. The analysis was conducted using the STATA software version 10.0 (Stata Corporation, College Station, TX). All the P-values were for two-sided analysis and values of P<0.05 were considered statistically significant.
Results
Study Characteristics
The combined search yielded 96 references. Figure S1 shows the study selection process. Finally, a total of 14 eligible association studies with 9,828 TC cases and 109,995 controls were identified [6], [7], [11], [12], [22]–[31], with 4 studies genotyping more than one variant. There are 19 data sets from 12 studies with 8,602 cases and 102,846 controls concerning rs965513, and 7 data sets from 5 studies involving 2,017 cases and 13,281 controls concerning rs1867277. For the rs71369530 polymorphism, 5 data sets from 4 studies involved a total of 448 cases and 746 controls. Of the cases, 77% were Caucasian, and 23% were East Asian. Eleven studies were given high quality, and three studies were given median quality. No ‘poor quality’ study was found. The detailed characteristics of the studies included in this meta-analysis are shown in Table 1 (Figure S2).
Table 1. Characteristics of the studies included in the meta-analysis.
| Study | Year | Polymorphism | Ethnicity | Cases | Controls | No. of cases/controls | Genotyping method | Quality |
| Gudmundsson [6] | 2009 | rs965513 | European, American | Histologically confirmed thyroid cancer | Cancer-free individuals | 962/38923 | SNP arrays, SNaPshot | High |
| Kallel [11] | 2010 | PolyAla | Spanish | Pathologically confirmed papillary thyroid cancer | General population | 170/218 | Sequencing | Median |
| Landa [12] | 2009 | rs1867277 | Spanish, Italian | Pathologically confirmed papillary thyroid carcinoma | General population | 984/1028 | GoldenGate | High |
| Takahashi [22] | 2010 | rs965513 | Belarusian, Russian | Histologically confirmed papillary thyroid carcinoma | General population | 660/1268 | SNP arrays, TaqMan | High |
| Matsuse [23] | 2011 | rs965513 | Japanese | Histologically confirmed papillary thyroid carcinoma | General population | 479/2764 | TaqMan | High |
| Denny [24] | 2011 | rs965513 | American | Pathologically confirmed thyroid cancer | Cancer-free individuals | 96/6274 | SNP arrays | High |
| Gudmundsson [7] | 2012 | rs965513 | European, American | Histologically confirmed thyroid cancer | Cancer-free individuals | 558/43108 | SNP arrays, SNaPshot | High |
| Tomaz [25] | 2012 | rs965513, rs1867277, PolyAla | Portuguese | Pathologically confirmed non-medullary thyroid cancer | General population | 140/130 | Sequencing | Median |
| Bullock [26] | 2012 | rs1867277, PolyAla | Australian, British | Pathologically confirmed papillary thyroid cancer | General population | 70/5733 | Sequencing, TaqMan | High |
| Jones [27] | 2012 | rs965513, rs1867277 | British | Histologically confirmed non-medullary thyroid cancer | General population | 753/6120 | Kaspar | High |
| Wang [28] | 2013 | rs965513 | Chinese | Histologically confirmed papillary thyroid cancer | Cancer-free individuals | 1348/1005 | SNaPshot | High |
| Liyanarachchi [29] | 2013 | rs965513 | Polish, American | Histologically confirmed papillary thyroid carcinoma | General population | 2494/2264 | SNaPshot, iPLEX | High |
| Köhler [30] | 2013 | rs965513 | Italian | Histologically confirmed papillary thyroid cancer | General population | 690/497 | SNP arrays | High |
| Damiola [31] | 2013 | rs965513, rs1867277, PolyAla | Byelorussian | Histologically confirmed papillary thyroid carcinoma | General population | 70/303 | HRM, TaqMan, PCR | High |
| Unpublished data | / | rs965513 | Chinese | Histologically confirmed papillary thyroid carcinoma | General population | 354/360 | iPLEX | Median |
Association of rs965513 polymorphism with thyroid cancer
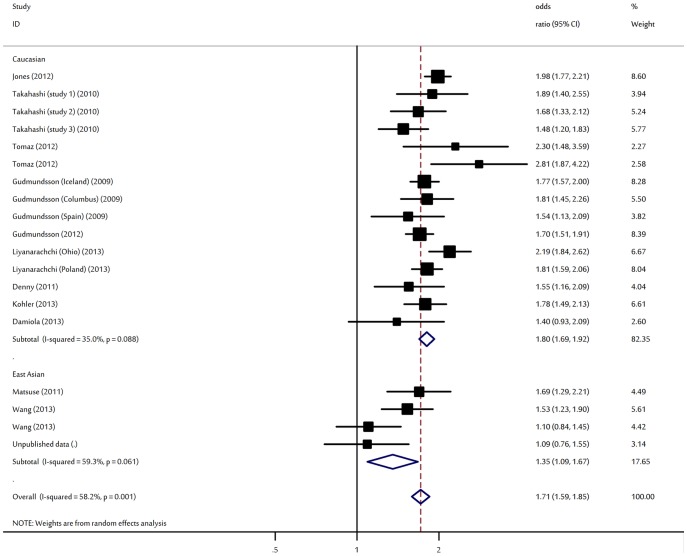
There was a wide variation in the A allele frequency of the rs965513 polymorphism among the controls across different ethnicities, ranging from 0.06 to 0.44 (Figure S2). For East Asian controls, the A allele frequency was 0.06 (95% CI: 0.05–0.08), which was lower than that in Caucasian controls (0.39; 95% CI: 0.34–0.44). For TC risk and the rs965513 polymorphism, our meta-analysis gave an overall OR of 1.71 (95% CI: 1.59–1.85, P<10−5; Figure 1) with statistically significant between-study heterogeneity (P = 0.001). Significantly increased TC risks were also found for those heterozygous (OR = 2.17, 95% CI: 1.86–2.57; P<10−5) and homozygous for the risk A allele (OR = 2.95, 95%CI: 2.29–3.94; P<10−5) when compared with the wild type genotype.
Figure 1. Forest plot for association of FOXE1 rs965513 polymorphism and thyroid cancer risk.
In view of significant heterogeneity and to seek for its potential sources, we performed a panel of subgroup analyses on ethnicity and sample size. When studies were stratified for ethnicity, significant risks were found among Caucasians in all comparisons (A allele: OR = 1.80, 95% CI: 1.69–1.92, P<10−5; heterozygous: OR = 2.60, 95% CI: 2.06–3.02, P<10−5; homozygote: OR = 3.36, 95% CI: 2.75–4.51, P<10−5). Similar significant associations were also observed for East Asians with per-allele OR of 1.35 (95% CI: 1.09–1.67, P = 0.006). Analysis restricted to the 8 studies with at least 500 cases, which should be less prone to selective publication than smaller studies, yielded an OR of 1.75 (95% CI: 1.59–1.93, P<10−5). As for smaller studies, the summary OR of rs965513-A variant for TC was 1.67 (95% CI: 1.48–1.88, P<10−5). By considering study design subgroups, the OR was 1.75 (95% CI: 1.61–1.90, P<10−5) in GWAS compared to 1.70 (95% CI: 1.53–1.88, P<10−5) in candidate gene studies (Table 2). When subgroup analyses by histological types were considered (6, 833 cases and 47, 384 controls from 8 studies), the polymorphism had a significantly increased risk for papillary thyroid cancer with an OR of 1.65 (95% CI: 1.50–1.81, P<10−5; I2 = 59%, P heterogeneity = 0.003).
Table 2. Main results of overall and subgroups in the meta-analysis.
| Polymorphism (risk allele) | Total/Subgroup analysis | No. of data sets | No. of cases/controls | Risk allele | Heterozygous | Homozygous | ||||||||||||
| OR (95%CI) | P(Z) | P(Q)a | I2 (%) | P(Q)b | OR (95%CI) | P(Z) | P(Q)a | I2 (%) | P(Q)b | OR (95%CI) | P(Z) | P(Q)a | I2 (%) | P(Q)b | ||||
| rs965513 (A) | Total | 19 | 8602/102846 | 1.71 (1.59–1.85) | <10−5 | 0.001 | 58 | 2.17 (1.86–2.57) | <10−5 | <10−4 | 67 | 2.95 (2.29–3.94) | <10−5 | <10−4 | 77 | |||
| Ethnicity | <10−4 | <10−4 | <10−4 | |||||||||||||||
| Caucasian | 15 | 6521/98717 | 1.80 (1.69–1.92) | <10−5 | 0.09 | 35 | 2.60 (2.06–3.02) | <10−5 | 0.003 | 47 | 3.36 (2.75–4.51) | <10−5 | 0.0005 | 61 | ||||
| East Asian | 4 | 2181/4129 | 1.35 (1.09–1.67) | 0.006 | 0.06 | 59 | 1.68 (1.24–2.12) | <10−4 | 0.01 | 38 | 1.95 (1.51–3.21) | <10−4 | 0.003 | 55 | ||||
| Sample size | 0.09 | 0.05 | 0.05 | |||||||||||||||
| No. cases ≥500 | 8 | 6420/90190 | 1.75 (1.59–1.93) | <10−5 | 0.002 | 70 | 2.27 (1.93–2.72) | <10−5 | 0.007 | 43 | 3.16 (2.59–4.28) | <10−5 | <10−4 | 79 | ||||
| No. cases < 500 | 11 | 2182/12656 | 1.67 (1.48–1.88) | <10−5 | 0.06 | 43 | 1.97 (1.59–2.41) | <10−5 | 0.004 | 68 | 2.76 (2.00–3.65) | <10−5 | 0.001 | 75 | ||||
| Design strategy | 0.83 | 0.65 | 0.51 | |||||||||||||||
| GWAS | 5 | 1766/44587 | 1.75 (1.61–1.90) | <10−5 | 0.89 | 0 | 2.35 (1.97–2.84) | <10−5 | 0.77 | 0 | 3.03 (2.52–3.92) | <10−5 | 0.51 | 0 | ||||
| Candidate gene | 14 | 6836/58259 | 1.70 (1.53–1.88) | <10−5 | <10−4 | 69 | 2.00 (1.68–2.30) | <10−5 | <10−4 | 63 | 2.88 (2.21–4.07) | <10−5 | <10−4 | 78 | ||||
| rs1867277 (A) | Total (All Caucasian) | 7 | 2017/13281 | 1.64 (1.51–1.78) | <10−5 | 0.39 | 4 | 1.86 (1.57–2.23) | <10−5 | 0.26 | 9 | 2.63 (1.98–3.51) | <10−5 | 0.08 | 19 | |||
| Poly-Ala (16-Ala) | Total (All Caucasian) | 5 | 448/746 | 2.01 (1.66–2.44) | <10−5 | 0.29 | 20 | / | / | / | / | / | / | |||||
Cochran's chi-square Q statistic test used to assess the heterogeneity in subgroups.
Cochran's chi-square Q statistic test used to assess the heterogeneity between subgroups.
Significant heterogeneity was present among the 19 data sets (P<0.05). In meta-regression analysis, sample size (P = 0.68), and study design (P = 0.89), did not significantly explained such heterogeneity. By contrast, ethnicity (P = 0.001) was significantly correlated with the magnitude of the genetic effect.
Association of rs1867277 polymorphism with thyroid cancer
The A allele frequency in Caucasians was 0.40 (95% CI: 0.38–0.43). Using random effect model, the per-allele overall OR of the A variant for TC was 1.64 (95% CI: 1.51–1.78, P<10−5; Figure S3), with corresponding results for heterozygous and homozygous of 1.86 (95% CI: 1.57–2.23, P<10−5) and 2.63 (95% CI: 1.98–3.51, P<10−5), respectively.
Association of polyAla (rs71369530) polymorphism with thyroid cancer
The two most common alleles were the 14-alanine and the 16-alanine alleles, occurring with a frequency of 58.5% and 32.3% in Caucasian controls, respectively. Among the polyAla (rs71369530) alleles, 16-Ala was the alleles showing higher frequencies in cases than controls. The overall per-allele OR of the 16-Ala variant for total TC was 2.01 (95% CI: 1.66–2.44, P<10−5; Figure S4),
Sensitivity analyses and publication bias
Sensitivity analysis indicated that no single study influenced the pooled OR qualitatively, suggesting that the results of this meta-analysis are stable (Figure S5–S7). The shape of the funnel plots was symmetrical for these polymorphisms (Figure S8–S10). The statistical results still did not show publication bias in these studies for rs965513 (Egger's test, P = 0.21), rs1867277 (Egger's test, P = 0.92) and rs71369530 polymorphism (Egger's test, P = 0.19).
Discussion
GWAS have led to the identification of multiple new genetic variants associated with thyroid cancer risk. Most of these thyroid cancer GWAS and replication studies have been conducted in European populations [6], [7], [25]–[27], [30] and to a lesser extent in East Asians [23], [28]. Replication of initial GWAS findings is considered a gold standard for reporting genotype–phenotype associations. Besides, there are significant differences in allele frequencies and the prevalence of thyroid cancer among different ethnic populations. It is, therefore, important to quantitatively assess the effects of the GWAS-identified markers in different ethnic populations and to explore potential heterogeneity of published data. To the best of our knowledge, this is the first comprehensive meta-analysis examining the common variations on FOXE1 gene and its relationship to susceptibility for thyroid cancer. Its strength was based on the accumulation of published data giving great information to detect significant differences. In total, the meta-analysis involved 14 studies which provided 9,828 TC cases and 109,995 controls. Our results demonstrated that the 3 polymorphisms (rs965513, rs1867277, rs71369530) of FOXE1 is a risk factor for developing TC.
Genetic heterogeneity is inevitable in disease identification strategy [32]. As for rs965513 polymorphism, we identified ethnicity as a potential source of between-study heterogeneity by subgroup analysis and meta-regression. In the stratified analysis by ethnicity, we observed that association between rs965513 polymorphism and risk for TC in Caucasians (OR = 1.80) was stronger than that in East Asian populations (OR = 1.35). Here are several explanations to interpret above-mentioned phenomenon. Firstly, ethnic differences may attribute to these different results, since the C allele distributions of the rs965513 polymorphism varies between Caucasians, and East Asians, with a prevalence of ∼37%, and ∼11%, respectively [6], [28]. On the other hand, different populations usually have different linkage disequilibrium patterns. A polymorphism may be in close linkage with another nearby causal variant in one ethnic population but not in another [33]. Moreover, it is possible that variation at this locus has modest effects on TC, but environmental factors may predominate in its progress, and mask the effects of this variation. Specific environmental factors like ionizing radiation and deficiency in iodine intake that have been already well studied in recent decades [34]. Most of included studies did not consider those important environmental factors. It is still unknown whether the lifestyle characteristics of different populations influence the association between the polymorphisms and TC. The unconsidered factors mixed together may cover the role of the polymorphism in East Asian populations.
FOXE1 is important for both pituitary- and thyroid- gland formation [35], [36] and is at the center of a regulatory network of transcription factors and cofactors that initiate thyroid differentiation at the embryonic stage [37]. Furthermore, mutations of the FOXE1 gene cause human syndromes that are associated with thyroid agenesis, among other phenotypes [38]. FOXE1 is also necessary for the maintenance of the differentiated state of the thyroid, as it is involved in regulating the transcription of thyroid-specific genes, such as the thyroglobulin and thyroperoxidase genes. Furthermore, the expression of FOXE1 has been shown to be abnormal in thyroid tumors [39]. The 9q22.33 SNP rs965513 was first reported in a GWAS of TC in a European population, and has since been replicated in several later studies [6], [7], [28]. It has been suggested to tag a functional variation near the FOXE1 gene which contributes to an increased risk of developing thyroid cancer. Besides, the variant has also been associated with low serum concentrations of thyroid-stimulating hormone, and free thyroxin [7].
A significant association with TC was also found for the FOXE1 16-Ala and rs1867277 variant in the present meta-analysis. Carré et al. reported that FOXE1 with 16-Ala induced a stronger transactivation of the thyroglobulin promoter than the 14-Ala variant [40]. These results suggest a functional consequence for the presence of polyAla expansions (>14), but not for contractions (≤14). However, a recent study reported a modest transcriptional impairment of 16-Ala FOXE1, when compared with the function of the 14-Ala variant, on FOXE1 responsive promoters, which was not attributable to differences in DNA binding [26]. In the case of rs1867277, the sequence with the A allele was shown to increase the transcriptional activity of the FOXE1 gene promoter, by recruitment of leucine zipper upstream stimulatory factors 1 and 2 [12]. Thus, it is hypothesized that up-regulation of FOXE1 could have a role in the malignant behavior of thyroid cells.
In interpreting the results, some limitations of this meta-analysis should be addressed. Firstly, our results were based on unadjusted estimates, while a more precise analysis should be conducted if all individual raw data were available, which would allow for the adjustment by other co-variants including age, sex, cigarette consumption, and other lifestyle. Secondly, the vast majority of subjects in the study are of European descent, and statistical power for analyses in other ethnicities is limited. Because the sample size was considerably smaller for East Asians studies, the main conclusions from this manuscript are based on analyses among Caucasian populations. Future studies including larger numbers of East Asians or Africans are necessary to clarify the consistency of findings across ethnic groups. Thirdly, meta-analysis is a type of retrospective study, and the recall and selection bias might exist.
Despite these limitations, this meta-analysis suggests that the three common variations on FOXE1 (rs965513, rs1867277, rs71369530) was significantly associated with increased risk of TC, particularly in Caucasian population. As studies among other ethnic populations are currently limited, further studies including a wider spectrum of subjects to investigate the role of those variants in other populations will be needed. Besides, future studies are recommended to identify the possible gene-gene and gene-environmental interactions in this association.
Supporting Information
Study selection process.
(TIF)
Frequencies of the risk alleles of FOXE1 rs965513 among controls stratified by ethnicity. The “ ” represent outlier.
” represent outlier.
(TIF)
Forest plot for association of FOXE1 rs1867277 polymorphism and thyroid cancer risk.
(TIF)
Forest plot for association of FOXE1 polyAla variant (71369530) and thyroid cancer risk.
(TIF)
Result of sensitivity analyses for FOXE1 rs965513 polymorphism and thyroid cancer risk.
(TIF)
Result of sensitivity analyses for FOXE1 rs1867277 polymorphism and thyroid cancer risk.
(TIF)
Result of sensitivity analyses for FOXE1 rs71369530 polymorphism and thyroid cancer risk.
(TIF)
Begg's funnel plot for publication bias in studies on FOXE1 rs965513 polymorphism and thyroid cancer.
(TIF)
Begg's funnel plot for publication bias in studies on FOXE1 rs1867277 polymorphism and thyroid cancer.
(TIF)
Begg's funnel plot for publication bias in studies on FOXE1 rs71369530 polymorphism and thyroid cancer.
(TIF)
(DOC)
Funding Statement
This work was supported by National Natural Science Foundation of China (81272722). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.DeLellis RA (2004) Pathology and genetics of tumours of endocrine organs. Lyon: IARC Press.pp 320.
- 2. Kondo T, Ezzat S, Asa SL (2006) Pathogenetic mechanisms in thyroid follicular cell neoplasia. Nat Rev Cancer 6: 292–306. [DOI] [PubMed] [Google Scholar]
- 3. Schneider AB, Sarne DH (2005) Long-term risks for thyroid cancer and other neoplasms after exposure to radiation. Nat Clin Pract Endocrinol Metab 1: 82–91. [DOI] [PubMed] [Google Scholar]
- 4. Hrafnkelsson J, Tulinius H, Jónasson JG, Sigvaldason H (2001) Familial non-medullary thyroid cancer in Iceland. J Med Genet 38: 189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amundadottir LT, Thorvaldsson S, Gudbjartsson DF, Sulem P, Kristjansson K, et al. (2004) Cancer as a complex phenotype: pattern of cancer distribution within and beyond the nuclear family. PLoS Med 1: e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Sigurdsson A, et al. (2009) Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat Genet 41: 460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Masson G, et al. (2012) Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat Genet 44: 319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jazdzewski K, Liyanarachchi S, Swierniak M, Pachucki J, Ringel MD, et al. (2009) Polymorphic mature microRNAs from passenger strand of pre-miR-146a contribute to thyroid cancer. Proc Natl Acad Sci U S A 106: 1502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruiz-Llorente S, Montero-Conde C, Milne RL, Moya CM, Cebrian A, et al. (2007) Association study of 69 genes in the ret pathway identifies low-penetrance loci in sporadic medullary thyroid carcinoma. Cancer Res 67: 9561–7. [DOI] [PubMed] [Google Scholar]
- 10. Wokolorczyk D, Gliniewicz B, Sikorski A, Zlowocka E, Masojc B, et al. (2008) A range of cancers is associated with the rs6983267 marker on chromosome 8. Cancer Res 68: 9982–6. [DOI] [PubMed] [Google Scholar]
- 11. Kallel R, Belguith-Maalej S, Akdi A, Mnif M, Charfeddine I, et al. (2010) Genetic Investigation of FOXE1 polyalanine tract in thyroid diseases: new insight on the role of FOXE1 in thyroid carcinoma. Cancer Biomarkers 8: 43–51. [DOI] [PubMed] [Google Scholar]
- 12. Landa I, Ruiz-Llorente S, Montero-Conde C, Inglada-Pérez L, Schiavi F, et al. (2009) The variant rs1867277 in FOXE1 gene confers thyroid cancer susceptibility through the recruitment of USF1/USF2 transcription factors. PLoS Genet 5: e1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McClellan J, King MC (2010) Genetic heterogeneity in human disease. Cell 141: 210–217. [DOI] [PubMed] [Google Scholar]
- 14. Li D, Collier DA, He L (2006) Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet 15: 1995–2002. [DOI] [PubMed] [Google Scholar]
- 15. Wu S, Cai J, Wang H, Zhang H, Yang W (2013) Association between 1p11-rs11249433 Polymorphism and Breast Cancer Susceptibility: Evidence from 15 Case-Control Studies. PLoS One 8: e72526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10: 101–129. [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 19. Woolf B (1955) On estimating the relation between blood group and disease. Ann Hum Genet 19: 251–253. [DOI] [PubMed] [Google Scholar]
- 20. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takahashi M, Saenko VA, Rogounovitch TI, Kawaguchi T, Drozd VM, et al. (2010) The FOXE1 locus is a major genetic determinant for radiation-related thyroid carcinoma in Chernobyl. Hum Mol Genet 19: 2516–23. [DOI] [PubMed] [Google Scholar]
- 23. Matsuse M, Takahashi M, Mitsutake N, Nishihara E, Hirokawa M, et al. (2011) The FOXE1 and NKX2-1 loci are associated with susceptibility to papillary thyroid carcinoma in the Japanese population. J Med Genet 48: 645–8. [DOI] [PubMed] [Google Scholar]
- 24. Denny JC, Crawford DC, Ritchie MD, Bielinski SJ, Basford MA, et al. (2011) Variants near FOXE1 are associated with hypothyroidism and other thyroid conditions: using electronic medical records for genome- and phenome-wide studies. Am J Hum Genet 89: 529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomaz RA, Sousa I, Silva JG, Santos C, Teixeira MR, et al. (2012) FOXE1 polymorphisms are associated with familial and sporadic nonmedullary thyroid cancer susceptibility. Clin Endocrinol (Oxf) 77: 926–33. [DOI] [PubMed] [Google Scholar]
- 26. Bullock M, Duncan EL, O'Neill C, Tacon L, Sywak M, et al. (2012) Association of FOXE1 polyalanine repeat region with papillary thyroid cancer. J Clin Endocrinol Metab 97: E1814–9. [DOI] [PubMed] [Google Scholar]
- 27. Jones AM, Howarth KM, Martin L, Gorman M, Mihai R, et al. (2012) Thyroid cancer susceptibility polymorphisms: confirmation of loci on chromosomes 9q22 and 14q13, validation of a recessive 8q24 locus and failure to replicate a locus on 5q24. J Med Genet 49: 158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang YL, Feng SH, Guo SC, Wei WJ, Li DS, et al. (2013) Confirmation of papillary thyroid cancer susceptibility loci identified by genome-wide association studies of chromosomes 14q13, 9q22, 2q35 and 8p12 in a Chinese population. J Med Genet 50: 689–95. [DOI] [PubMed] [Google Scholar]
- 29.Liyanarachchi S, Wojcicka A, Li W, Czetwertynska M, Stachlewska E, et al.. (2013) Cumulative Risk Impact of Five Genetic Variants Associated with Papillary Thyroid Carcinoma. Thyroid. doi: 10.1089/thy.2013.0102 [DOI] [PMC free article] [PubMed]
- 30. Köhler A, Chen B, Gemignani F, Elisei R, Romei C, et al. (2013) Genome-wide association study on differentiated thyroid cancer. J Clin Endocrinol Metab 98: E1674–81. [DOI] [PubMed] [Google Scholar]
- 31.Damiola F, Byrnes G, Moissonnier M, Pertesi M, Deltour I, et al.. (2013) Contribution of ATM and FOXE1 (TTF2) to risk of papillary thyroid carcinoma in Belarusian children exposed to radiation. Int J Cancer doi: 10.1002/ijc.28483 [DOI] [PubMed]
- 32. Hemminki K, Lorenzo Bermejo J, Försti A (2006) The balance between heritable and environmental aetiology of human disease. Nat Rev Genet 7: 958–65. [DOI] [PubMed] [Google Scholar]
- 33. Zhou B, Song Z, Qian M, Li L, Gong J, et al. (2013) Functional Polymorphisms in the CYP2C19 Gene Contribute to Digestive System Cancer Risk: Evidence from 11,042 Subjects. PLoS One 8: e66865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williams D (2002) Cancer after nuclear fallout: lessons from the Chernobyl accident. Nat Rev Cancer 2: 543–549. [DOI] [PubMed] [Google Scholar]
- 35. Dathan N, Parlato R, Rosica A, De Felice M, Di Lauro R (2002) Distribution of the titf2/foxe1 gene product is consistent with an important role in the development of foregut endoderm, palate, and hair. Dev Dyn 224: 450–6. [DOI] [PubMed] [Google Scholar]
- 36. De Felice M, Ovitt C, Biffali E, Rodriguez-Mallon A, Arra C, et al. (1998) A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet 19: 395–8. [DOI] [PubMed] [Google Scholar]
- 37. Parlato R, Rosica A, Rodriguez-Mallon A, Affuso A, Postiglione MP, et al. (2004) An integrated regulatory network controlling survival and migration in thyroid organogenesis. Dev Biol 276: 464–75. [DOI] [PubMed] [Google Scholar]
- 38. Clifton-Bligh RJ, Wentworth JM, Heinz P, Crisp MS, John R, et al. (1998) Mutation of the gene encoding human TTF-2 associated with thyroid agenesis, cleft palate and choanal atresia. Nat Genet 19: 399–401. [DOI] [PubMed] [Google Scholar]
- 39. Sequeira MJ, Morgan JM, Fuhrer D, Wheeler MH, Jasani B, et al. Thyroid transcription factor-2 gene expression in benign and malignant thyroid lesions. Thyroid 11: 995–1001. [DOI] [PubMed] [Google Scholar]
- 40. Carré A, Castanet M, Sura-Trueba S, Szinnai G, Van Vliet G, et al. (2007) Polymorphic length of FOXE1 alanine stretch: evidence for genetic susceptibility to thyroid dysgenesis. Hum Genet 122: 467–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study selection process.
(TIF)
Frequencies of the risk alleles of FOXE1 rs965513 among controls stratified by ethnicity. The “ ” represent outlier.
” represent outlier.
(TIF)
Forest plot for association of FOXE1 rs1867277 polymorphism and thyroid cancer risk.
(TIF)
Forest plot for association of FOXE1 polyAla variant (71369530) and thyroid cancer risk.
(TIF)
Result of sensitivity analyses for FOXE1 rs965513 polymorphism and thyroid cancer risk.
(TIF)
Result of sensitivity analyses for FOXE1 rs1867277 polymorphism and thyroid cancer risk.
(TIF)
Result of sensitivity analyses for FOXE1 rs71369530 polymorphism and thyroid cancer risk.
(TIF)
Begg's funnel plot for publication bias in studies on FOXE1 rs965513 polymorphism and thyroid cancer.
(TIF)
Begg's funnel plot for publication bias in studies on FOXE1 rs1867277 polymorphism and thyroid cancer.
(TIF)
Begg's funnel plot for publication bias in studies on FOXE1 rs71369530 polymorphism and thyroid cancer.
(TIF)
(DOC)