Abstract
Terrestrial plant pollen is classified into two categories based on its metabolic status: pollen with low-metabolism are termed “orthodox” and pollen with high-metabolism are termed “recalcitrant.” Nicotinamide adenine dinucleotide (NAD) is crucial for a number of metabolisms in all extant organisms. It has recently been shown that NAD homeostasis plays an important role in a broad range of developmental processes and responses to environment. Recently, a reverse genetic approach shed light on the significance of NAD biosynthesis on pollen fate. In orthodox Arabidopsis pollen, NAD+ that was accumulated in excess at dispersal dramatically decreased on rehydration. The lack of a key gene that is involved in NAD biosynthesis compromised the excess accumulation. Moreover, absence of the excess accumulation phenocopied the so-called recalcitrant pollen, as demonstrated by the germination inside anthers and the loss of desiccation tolerance. Upon rehydration, NAD+-consuming inhibitors impaired tube germination. Taken together, our results suggest that accumulation of NAD+ functions as a physiochemical molecular switch for suspended metabolism and that the decrease of NAD+ plays a very important role during transitions in metabolic states. Shifting of the redox state to an oxidizing environment may efficiently control the comprehensive metabolic network underlying the onset of pollen germination.
Keywords: nicotinamide adenine dinucleotide (NAD), NAD biosynthesis, NAD homeostasis, redox, pollen, germination
Terrestrial plants are equipped with mechanisms to maintain internal stability in the face of developmental and environmental changes. “Developmental arrest” is one of the strategies for adaptation to adverse environmental challenges.1 One exemplary phenomenon is programmed desiccation (i.e., drying without dying) that occurs during male gametophyte development in almost all extant gymnosperms and a large proportion of angiosperms; the resultant pollen with low-moisture and low-metabolism are termed “orthodox.”2 They remain viable for a few days or longer.3 Maintenance of orthodox pollen enables plants to avoid futile energy cycling, thereby allowing them to survive under air-dry conditions in nature until the pollen acquire water from the stigma, at which point they immediately begin germinating. Therefore, the metabolic status of pollen can play an important role in cell longevity. In contrast, in some angiosperms, the pollen does not undergo this desiccation process and contains relatively higher moisture levels as well as higher metabolism. Such pollen, termed “recalcitrant,” are metabolically active even after dispersal from the anther. Though the recalcitrant pollen can survive for a few hours only due to high susceptibility to desiccation damage, they can immediately elongate the pollen tube and execute subsequent fertilization (as observed in cleistogamous flowers). In other words, cross-pollination with the recalcitrant pollen can be limited to very proximate female sporophytes of chasmogamous flowers. Thus, a better understanding of the mechanisms underlying the physiological properties that allow classification of pollen into the categories “orthodox” and “recalcitrant,” based on the status of metabolism and desiccation is fairly significant in the evolution and establishment of reproductive isolation in plants.
The molecular mechanisms underlying the desiccation of orthodox pollen are thought to be analogous to those of seeds because seeds are another example of a reproductive structure demonstrating developmental arrest.4 For example, many studies have reported on intracellular physical properties,3,5 antioxidative scavengers6 and accumulation of protective molecules or compatible solutes.2,7-9 In contrast, no molecular genetic studies have been published that explain how the orthodox pollen maintain low metabolic rate without dying. As a result of failure in anther desiccation due to high humidity conditions, the raring-to-go (rtg) mutants in Arabidopsis demonstrate precocious germination inside anthers (as observed in some cleistogamous species).10 Therefore, the rtg pollen that resemble the recalcitrant pollen may provide an insight into the mechanisms of programmed desiccation and/or metabolic switching off. In addition to the rtg pollen, gift-wrapped pollen, polka dot pollen and emotionally fragile pollen are rtg-like mutants that have been isolated.10 However, the four mutants are non allelic; furthermore, the genes responsible for the rtg and rtg-like mutants remain unidentified and their underlying molecular mechanisms are largely unknown. Therefore, the physiochemical molecular switches of suspended metabolism need to be identified.
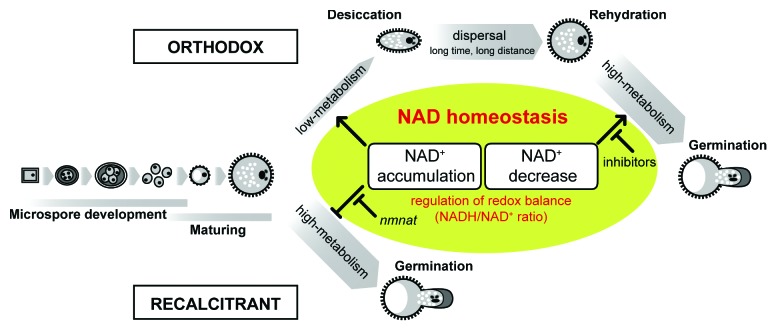
The involvement of pyridine nucleotides in a broad range of processes including bolting, senescence, and abiotic stress adaptation suggests that not only the nicotinamide adenine dinucleotide (NAD) pools but also the NAD metabolism regulator(s) may play important roles in these processes.11-13 We have used reverse genetics to identify the functional role and biological implications of NAD metabolism in Arabidopsis.11,14 The gametophytic promoter activity of nicotinate/nicotinamide mononucleotide adenyltransferase (NMNAT), a key gene of NAD biosynthesis, encouraged us to dissect the functional role of NAD metabolism during microspore development. We found that orthodox Arabidopsis pollen accumulates oxidized NAD (NAD+), resulting in a much lower redox state at dispersal. It was observed that hydration drastically decreased the NAD+ level and consequently increased the redox state.15 NAD+-consuming enzyme inhibitors efficiently retarded germination, and excess NAD+ loading impaired normal pollen tube growth. On the basis of these results, we speculate that NAD+ accumulation regulates pollen fate, and acts as a negative regulator of pollen germination (Fig. 1). Based on our hypothesis, nmnat pollen lacking NAD+ accumulation during microspore development enables them to germinate and the tube to elongate inside the anther under high-humidity conditions, thus mimicking rtg pollen and typical recalcitrant pollen.15 Therefore, NAD+ appears to participate directly in the molecular regulation of germination onset, although NAD+ accumulation is not essential for adequate pollen germination (Fig. 1). This mode of action of NAD+ was also supported by the fact that dispersed nmnat pollen was round, resembling hydrated pollen, the ectopic callose deposition and shortened pollen longevity observed under air-dry conditions are probably due to the loss of desiccation tolerance.15 The occurrence of germination inside the anther and the loss of desiccation tolerance are conclusive evidences to prove that the dispersed nmnat pollen remain metabolically active. A feasible mechanism, by which NAD+ accumulation downregulates pollen metabolism, could be the impairment of NADH-dependent redox reaction, which is essential for tube germination; for example, downregulation of mitochondrial ATP synthesis and reactive oxygen species (ROS) generation,16 by shifting the redox state to a more oxidizing environment. Taken together, we hypothesized that accumulation of NAD+ functions as a physiochemical molecular switch of suspended metabolism, and that decrease of NAD+ plays a crucial role during metabolic state transitions (Fig. 1).
Figure 1. Schematic representation of the role of NAD homeostasis on pollen germination.
Our results indicate that the NAD-associated redox homeostasis may be significant with regard to pollen desiccation susceptibility. Orthodox pollen may invest substrates (e.g., Asp) and energy (e.g., ATP) for NAD biosynthesis17 to control the redox balance during microspore development to acquire desiccation tolerance, thereby expanding its outcrossing distance for heterogamy in a fluctuating environment. Because orthodoxy and recalcitrance are not strict categories, dispersible distance and germination timing will vary depending on both the redox state at anthesis and the potency of NAD+ decrease at rehydration. It is noteworthy that nmnat is, at least, non-allelic to rtg and dissimilar to rtg-like, suggesting the existence of other factors controlling the metabolic switching off associated with the regulatory mechanisms of NAD biosynthesis or unknown downstream pathways. Screening of components that are involved in tuning the redox state suitable for pollen metabolism in response to rehydration is now underway at our laboratory.
Acknowledgments
We are grateful to Dr K. Kitazaki (CRIEPI, Japan) for helpful comments on the manuscript. This work was supported by grants to S.-n.H. from a Research Fellowship for Young Scientists of Japan Society for the Promotion of Science (JSPS).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23937
References
- 1.Footitt S, Cohn MA. Developmental arrest: from sea urchins to seeds. Seed Sci Res. 2001;11:3–16. doi: 10.1079/SSR200055. [DOI] [Google Scholar]
- 2.Pacini E, Guarnieri M, Nepi M. Pollen carbohydrates and water content during development, presentation, and dispersal: a short review. Protoplasma. 2006;228:73–7. doi: 10.1007/s00709-006-0169-z. [DOI] [PubMed] [Google Scholar]
- 3.Hong TD, Ellis RH, Buitink J, Walters C, Hoekstra FA, Crane J. A model of the effect of temperature and moisture on pollen longevity in air-dry storage environments. Ann Bot (Lond) 1999;83:167–73. doi: 10.1006/anbo.1998.0807. [DOI] [Google Scholar]
- 4.Franchi GG, Piotto B, Nepi M, Baskin CC, Baskin JM, Pacini E. Pollen and seed desiccation tolerance in relation to degree of developmental arrest, dispersal, and survival. J Exp Bot. 2011;62:5267–81. doi: 10.1093/jxb/err154. [DOI] [PubMed] [Google Scholar]
- 5.Buitink J, Walters-Vertucci C, Hoekstra FA, Leprince O. Calorimetric properties of dehydrating pollen (analysis of a desiccation-tolerant and an intolerant species) Plant Physiol. 1996;111:235–42. doi: 10.1104/pp.111.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank G, Pressman E, Ophir R, Althan L, Shaked R, Freedman M, et al. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J Exp Bot. 2009;60:3891–908. doi: 10.1093/jxb/erp234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang HH, Dandekar AM. Regulation of proline accumulation in Arabidopsis thaliana (L.) Heynh during development and in response to desiccation. Plant Cell Environ. 1995;18:1280. doi: 10.1111/j.1365-3040.1995.tb00187.x. [DOI] [Google Scholar]
- 8.Schwacke R, Grallath S, Breitkreuz KE, Stransky E, Stransky H, Frommer WB, et al. LeProT1, a transporter for proline, glycine betaine, and gamma-amino butyric acid in tomato pollen. Plant Cell. 1999;11:377–92. doi: 10.1105/tpc.11.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolkers WF, McCready S, Brandt WF, Lindsey GG, Hoekstra FA. Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochim Biophys Acta. 2001;1544:196–206. doi: 10.1016/S0167-4838(00)00220-X. [DOI] [PubMed] [Google Scholar]
- 10.Johnson SA, McCormick S. Pollen germinates precociously in the anthers of raring-to-go, an Arabidopsis gametophytic mutant. Plant Physiol. 2001;126:685–95. doi: 10.1104/pp.126.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashida SN, Itami T, Takahashi H, Takahara K, Nagano M, Kawai-Yamada M, et al. Nicotinate/nicotinamide mononucleotide adenyltransferase-mediated regulation of NAD biosynthesis protects guard cells from reactive oxygen species in ABA-mediated stomatal movement in Arabidopsis. J Exp Bot. 2010;61:3813–25. doi: 10.1093/jxb/erq190. [DOI] [PubMed] [Google Scholar]
- 12.Liu YJ, Nunes-Nesi A, Wallström SV, Lager I, Michalecka AM, Norberg FE, et al. A redox-mediated modulation of stem bolting in transgenic Nicotiana sylvestris differentially expressing the external mitochondrial NADPH dehydrogenase. Plant Physiol. 2009;150:1248–59. doi: 10.1104/pp.109.136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schippers JH, Nunes-Nesi A, Apetrei R, Hille J, Fernie AR, Dijkwel PP. The Arabidopsis onset of leaf death5 mutation of quinolinate synthase affects nicotinamide adenine dinucleotide biosynthesis and causes early ageing. Plant Cell. 2008;20:2909–25. doi: 10.1105/tpc.107.056341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashida SN, Takahashi H, Kawai-Yamada M, Uchimiya H. Arabidopsis thaliana nicotinate/nicotinamide mononucleotide adenyltransferase (AtNMNAT) is required for pollen tube growth. Plant J. 2007;49:694–703. doi: 10.1111/j.1365-313X.2006.02989.x. [DOI] [PubMed] [Google Scholar]
- 15.Hashida SN, Takahashi H, Takahara K, Kawai-Yamada M, Kitazaki K, Shoji K, et al. NAD+ accumulation during pollen maturation in Arabidopsis regulating onset of germination. Mol Plant. 2013;6:216–25. doi: 10.1093/mp/sss071. [DOI] [PubMed] [Google Scholar]
- 16.Wan C, Li S, Wen L, Kong J, Wang K, Zhu Y. Damage of oxidative stress on mitochondria during microspores development in Honglian CMS line of rice. Plant Cell Rep. 2007;26:373–82. doi: 10.1007/s00299-006-0234-2. [DOI] [PubMed] [Google Scholar]
- 17.Hashida SN, Takahashi H, Uchimiya H. The role of NAD biosynthesis in plant development and stress response. Ann Bot (Lond) 2009;103:819–24. doi: 10.1093/aob/mcp019. [DOI] [PMC free article] [PubMed] [Google Scholar]