Abstract
MicroRNAs (miRNAs) are a class of short endogenous non-coding small RNA molecules of about 18–22 nucleotides in length. Their main function is to downregulate gene expression in different manners like translational repression, mRNA cleavage and epigenetic modification. Computational predictions have raised the number of miRNAs in wheat significantly using an EST based approach. Hence, a combinatorial approach which is amalgamation of bioinformatics software and perl script was used to identify new miRNA to add to the growing database of wheat miRNA. Identification of miRNAs was initiated by mining the EST (Expressed Sequence Tags) database available at National Center for Biotechnology Information. In this investigation, 4677 mature microRNA sequences belonging to 50 miRNA families from different plant species were used to predict miRNA in wheat. A total of five abiotic stress-responsive new miRNAs were predicted and named Ta-miR5653, Ta-miR855, Ta-miR819k, Ta-miR3708 and Ta-miR5156. In addition, four previously identified miRNA, i.e., Ta-miR1122, miR1117, Ta-miR1134 and Ta-miR1133 were predicted in newly identified EST sequence and 14 potential target genes were subsequently predicted, most of which seems to encode ubiquitin carrier protein, serine/threonine protein kinase, 40S ribosomal protein, F-box/kelch-repeat protein, BTB/POZ domain-containing protein, transcription factors which are involved in growth, development, metabolism and stress response. Our result has increased the number of miRNAs in wheat, which should be useful for further investigation into the biological functions and evolution of miRNAs in wheat and other plant species.
Keywords: miRNA, computational prediction, wheat, target prediction, EST
Introduction
Wheat (Triticum aestivum L., AABBDD, 2 n = 42) is one of the most extensively grown crops throughout the world, providing protein content, as well as basic caloric value.1 Until recently, wheat was the last major crop for which no genome sequencing effort was underway. However, recent technological advances such as new-generation sequencing platforms now offer large scale programs that can deliver needed genomic resources for wheat. The International Wheat Genome Sequencing Consortium’s (IWGSC) project studies are already revealing valuable information about wheat genome structure.1-3
MicroRNAs (miRNAs) are a class of endogenous, small, noncoding, single-stranded RNAs that act as post-transcriptional regulators in eukaryotes.4 It has been estimated that miRNAs account for ~1% of predicted genes in higher eukaryotic genomes and up to 10–30% of genes may be regulated by miRNAs.5 miRNAs regulate expression of functional genes involved in plant development and other physiological processes.8 The maturation of miRNAs in plants involves several steps requiring key enzymes such as Dicer-like 1 enzyme (DCL1)and HASTY.8-10 Mature miRNAs are incorporated into RNA-induced silencing complex (RISC) which is induced by miRNAs to target mRNAs causing the cleavage or repression of target genes.11,12
Plant miRNAs negatively regulate the corresponding transcripts levels of their target genes and play important roles in plant growth including leaf morphology and polarity, organ development, cell differentiation and proliferation, cell death, signal transduction stress response, lateral root formation, hormone signaling, transition from juvenile to adult vegetative phase, vegetative to flowering phase, flowering time, floral organ identity and reproduction.9,13,14,20 Identification of miRNAs and their target genes therefore is an important step toward understanding the biological functions of miRNAs. Recently, computational approaches are used wildly as a rapid, accurate and affordable method to identify miRNAs. The computational approaches have been very effective in plants, where miRNA and its target mRNA have often nearly perfectly complementary.15 The earliest miRNAs from plant kingdom were discovered in Arabidopsis thaliana in 200216,17 and subsequent miRNAs have been identified in several plants by computational and experimental approaches.15,16 Conserved nature of mature miRNAs among different species and the unique secondary structure of pri-miRNAs16-20 facilitate miRNA prediction using bioinformatics approaches. Several miRNAs are regulated in response to diverse stress conditions, which suggests that miRNA-directed post-transcriptional regulation of their respective target genes is important to cope with the stress.3,6,7,21-24 Because miRNAs have emerged as vital components of post-transcriptional regulation of gene expression important for plant growth and development, as well as plant stress responses, identifying conserved miRNA homologs in as many plant species as possible is important. Computational approaches are successful in identifying conserved miRNAs in many plants and animals, but they require knowledge of the complete genome sequence, which is unavailable for most plant species including wheat. However, large genomic fragmented data in the form of genome survey sequences (GSSs), high-throughput genomics sequences (HTGSs) and non-redundant nucleotides (NRs), as well as expressed sequence tags (ESTs) are available for several plant species and can be used for identification of conserved miRNAs. GSS and HTGS of GeneBank represent only short stretches of genomic sequence but can still provide a broader sampling of unfinished genomes. The NR database contains finished genomic sequences and cDNAs. Previously Zhang et al.25 identified conserved miRNAs in plants using ESTs alone.
Large number of miRNA has been identified in many model crops but only few miRNA are reported in wheat till date which is very less compared with the other plant miRNAs. Steady significant increase in the wheat EST sequences in the database motivated us to predict additional miRNA in wheat. Computational approaches have been developed to identify miRNAs in wheat and their targets in publically available ESTs.26 Evidence suggesting that miRNAs play a role in plant stress responses arises from the discovery that miR398 targets genes with known roles in stress tolerance. In addition, the expression profiles of most miRNAs that are implicated in plant growth and development are significantly changed during stress. These later findings imply that attenuated plant growth and development under stress may be under the control of stress-responsive miRNAs. Here, in this study we examined all miRNAs deposited in the miRNA Registry Database publicly available at www.mirbase.org (Release 19, November 2012),27 to search against wheat EST sequences. We used newly identified miRNAs to predict their targets in wheat and found 14 target genes encoding transcription factors, enzymes implicated in metabolic processes and in stress responses. In this study, new miRNAs were mined for the purpose of understanding their roles in regulating growth, development, metabolism and other physiological processes in T. aestivum. By combining the EST expression with the computational approach, we found 5 new abiotic stress-responsive miRNAs in wheat not reported yet. These findings will be useful for tracing the evolution of small RNAs by examining their expression in common ancestors of the Arabidopsis-rice-wheat lineage.
Results
Identification of miRNAs using EST
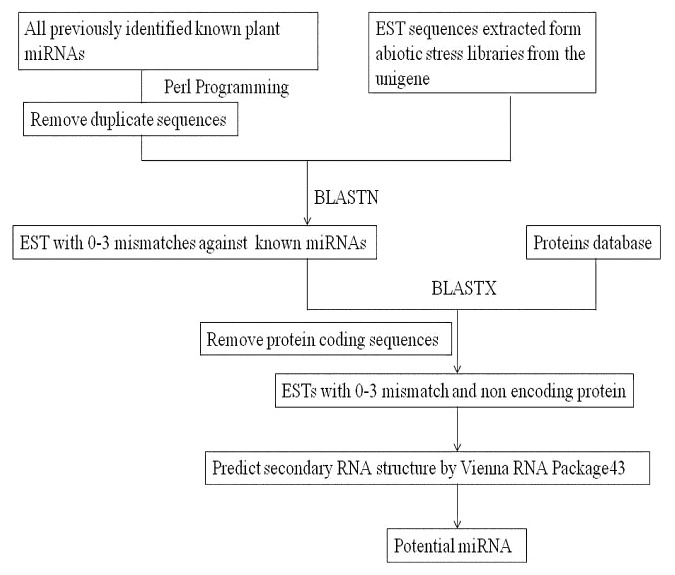
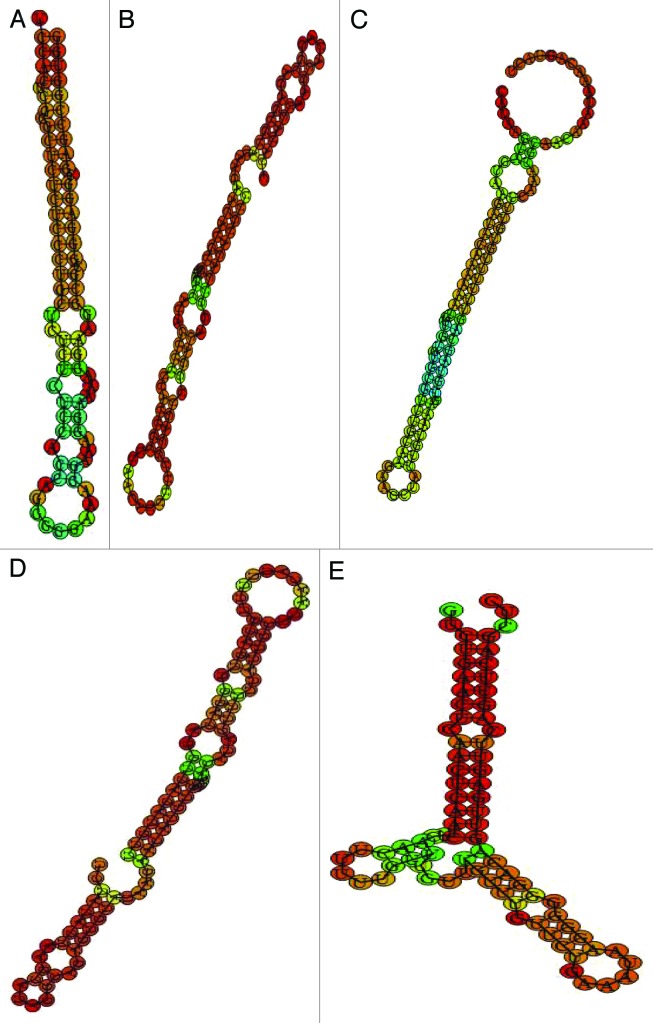
Plants are exposed to a wide array of environmental stresses leading to various functional and structural changes to cope up with these stresses. Molecular characterization of transcriptional and biochemical alterations are crucial to dissect the underlying regulatory mechanism of these abiotic stress. In order to identify new miRNAs in wheat we have to rely on wheat EST sequences, since the sequence information of wheat genome sequence is restricted.28 To discover new miRNAs in wheat, we exploited known mature miRNAs already submitted in miRBase database from various plant species including Arabidopsis, rice and maize (Fig. 1). Multiple sequence alignment was performed on this data set to remove previously reported wheat miRNA to avoid false-positive result. The other redundant miRNAs were omitted by perl script. As a result we got 4,677 non redundant miRNA data set which was made as query for BLAST program. The EST extracted from abiotic stress libraries of wheat were made as database and as a result we found 10 EST. The predicted EST was against set to various filters to make sure that they qualify plant miRNA annotation criteria.29 The precursor sequences were predicted with 250 ntd upstream and 250 downstream of the miRNA BLAST hit and used for the hairpin structure predictions. For ESTs with less than 400ntd we used the entire available sequence as a miRNA precursor sequence. These precursor sequences then BLASTXed, to remove the protein coding sequences and retained precursor sequences underwent hairpin structure prediction by Vienna RNA Package.30 The putative miRNA precursor was also BLASTed against RNA database to discard other RNAs such as tRNA, rRNA, snRNA and so on. As a result, 5 new miRNAs, (Table 1) were found. Furthermore, we provide computational evidence that these 5 newly identified miRNA were Arabidopsis (Ta-miR5653, Ta-miR855) rice (miR819k and miR5156), Picea abies (miR3708) homologs in wheat. To validate newly identified miRNAs, various calculated parameters were analyzed, for instance, the A+U content, the precursor length of miRNA, the minimal folding free energy index (MFEI) for each miRNA precursor. The precursors lengths was found to vary from 70 (nt) to 100 (nt) (Table 2). Previous report on miRNA identification also showed the similar length distribution of miRNAs and their precursor sequences.9,15,25,31 The analysis also showed that A+U contents in all identified miRNA precursors ranges from 30–70% with an average of 54.88% which is quite high and acceptable. At each arm of hairpin structures the identified miRNAs were uniformly distributed; only miR855 was located at the 5′ end of hairpin structures, with the left behind were located at the 3′ ends. The minimal folding free energy (MFE) has been considered as one of the significant feature described in earlier miRNA identification studied.32 All the newly identified wheat miRNA precursors have negative minimal folding free energies varying from −20.1–33.5 kcalmol−1. Lower the MFE value the higher the thermodynamically stable secondary structure of the miRNA and previous studies also concluded that MFE are highly related to precursor length.9 In this study MEFI value ranged from 0.64–0.83. miRNA precursor sequence has significantly higher MFEI value than other non-coding orcoding RNAs.33-35 All of above findings and analysis indicated that these five small RNAs were probably new miRNAs. The distributions of newly identified wheat miRNAs are similar to their counterparts in other plant predicted miRNA. All mature miRNAs precursors were found to fold into near hairpin-structures (Fig. 2). The statistics and characterized parameters of predicted T.aestivum precursor’s sequences such as mean, standard deviation are shown in Table 3.
Figure 1. Schematic diagram explaining the comprehensive EST analysis of miRNA in wheat.
Table1. Sequence and location of new miRNAs identified in wheat.
| miRNAs | Sequence | Homologous | Location |
|---|---|---|---|
| miR5653 | GUUGAGUUGAGUUGAGUU | ath-miR5653 | 3′ |
| miR855 | AAAGCUAAGGAAAAGGAA | ath-miR855 | 5′ |
| miR819k | CCUGUAAAACUGCAAAAA | osa-miR819 | 3′ |
| miR3708 | CACACAACAUUUCUCGUA | pab- miR3708 | 3′ |
| miR5156 | CCUGUAAAACUGCAAAAA | osa-miR5156 | 3′ |
Table 2. New wheat miRNA families homologous to known miRNAs from other plant species.
| miRNAs | MFE(∆G,kcal/mol) | MFEI | LP (nt) | (G+C)% | (A+U)% | A% | C% | G% | U% | A/U ratio | C/G ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| miR5653 | 27.7 | 0.83 | 80 | 40 | 60 | 22.78 | 17.72 | 22.78 | 37.97 | 0.59 | 0.77 |
| miR855 | 33.5 | 0.64 | 79 | 65.8 | 34.2 | 21.51 | 30.37 | 35.44 | 13.92 | 1.54 | 0.85 |
| miR819k | 20.1 | 0.65 | 90 | 34.4 | 65.6 | 30 | 14.4 | 20 | 35.5 | 0.84 | 0.72 |
| miR3708 | 20.5 | 0.66 | 100 | 31 | 69 | 37 | 11 | 20 | 32 | 1.15 | 0.55 |
| miR5156 | 20.1 | 0.65 | 90 | 34.4 | 65.6 | 30 | 14.4 | 20 | 35.5 | 0.84 | 0.72 |
LP, length of pre-cursors; MFE, minimal folding free energy; MFEI, minimal folding free energy indexes.
Figure 2. The predicted secondary step-loop structures of newly identified wheat miRNAs. (A) miR855 (B) MiR819k (C) miR3708 (D) miR5156 (E) miR5653.
Table 3. Statistics and characterized parameters of predicted T.aestivum precursors.
| Parameters | Mean | Standard deviation | Minimal | Maximal |
|---|---|---|---|---|
| MFE(∆G, -kcal/mol) | 24.38 | 6.04 | 20.1 | 33.5 |
| MFEI | 0.69 | 0.08 | 0.65 | 0.83 |
| Precursor Length(nt) | 87.8 | 8.61 | 79 | 100 |
| (G + C)% | 41.12 | 14.17 | 31 | 65.8 |
| (A + U)% | 58.88 | 14.17 | 34.2 | 69 |
| A% | 28.25 | 6.29 | 21.51 | 37 |
| C% | 17.57 | 7.54 | 11 | 30.37 |
| G% | 23.64 | 6.70 | 20 | 35.44 |
| U% | 30.98 | 9.77 | 13.92 | 37.97 |
| A/U ratio | 0.99 | 0.37 | 0.59 | 1.15 |
| C/G ratio | 0.72 | 0.11 | 0.55 | 85 |
Target prediction of newly identified miRNAs functional annotation
Previous studied on miRNA target identification has shown that most plant miRNAs bind to the protein-coding region of their miRNA targets with complementarity and inhibit the translation mechanism.36 sRNA ToolKit was used to predict potential target of newly indentified miRNAs by searching against wheat mRNAs. Wheat miRNAs preferred to target the Ubiquitin carrier protein, serine/threonine protein kinase, transcriptional activator Myb, 40S ribosomal protein, F-box/kelch-repeat protein, BTB/POZ domain-containing protein involved in wheat development (Table 4). We observed that one miRNA family can have more than one targets. In contrast, miR5156 has one target gene. An additional target gene family was found to be involved during stress responses which greatly influence the wheat production. Identification and validation of miRNA targets is a landmark step to unravel the central role of miRNA in regulatory network of abiotic stress tolerance. EST based search in various databases played a vital role for the discovery of miRNA targets in plants based on the homology between miRNA and its target sequences.19 Our prediction of target genes for the 10 miRNAs (including new and previously reported) also supported that there could be more than one potential target for each miRNA (Table 4). In the functional annotation performed under gene ontology revealed that each miRNA has a specific target gene, for example miR855 are transcriptional activator and transporter activity, miR5653 and miR819k are involved in ubiquintin protein ligase, miR5156 and miR3708 are involved in translation and transcription, respectively. The pathway analysis of predicted target genes showed that miRNA5653 was associated with sulfur metabolism and signaling pathway, miRNA855 with transporters, miR819k with Chemokine signaling pathway and miR5156 with ribosome biogenesis pathway respectively. All the predicted targets share high homology with Arabidopsis, Oryza and Zea mays. Most of the predicted targets of newly identified miRNA may have potential role in plant growth and development.
Table 4. Major potential target genes for newly identified miRNAs in wheat.
| miRNA family | Targeted proteins | Targeted genes | Function anntotation | KEGG pathway |
|---|---|---|---|---|
| miR5653 | Wheat ubiquitin carrier protein | Ta.56283 | nucleotide binding, ubiquitin-protein ligase activity, ATP binding, ligase activity, acid-amino acid ligase activity | Sulfur relay system |
| serine/threonine protein kinase (PK4) | Ta.13351 | protein kinase activity, ATP binding, transferase activity, transferring phosphorus-containing groups, kinase activity | Signaling pathway | |
| miR855 | transcriptional activator Myb | Ta.56324 | transcription factor | No Hits found |
| transmembrane protein | Ta.26126 | transporter activity | transporters | |
| miR819k | BTB/POZ domain-containing protein | Ta.28720 | ubiquitin ligase complexes | No Hits found |
| Rho GTPase | Ta.38900 | activation protein | Chemokine signaling pathway | |
| miR5156 | 40S ribosomal protein | Ta.62246 | translation | Ribosome biogenesis in eukaryotes |
| miR3708 | F-box/kelch-repeat protein | Ta.34556 | regulation of transcription, DNA-dependent | No Hits found |
| miR1122 | TP53 regulating kinase |
Ta.58315 | regulation | No Hits found |
| Maf-like protein | Ta.43051 | attachment factor 1 | No Hits found | |
| single-strand DNA-binding protein | Ta.50966 | DNA binding | No Hits found | |
| miR1117 | receptor-like protein kinase | Ta.51694 | regulation pathway | No Hits found |
| miR1134 | - | - | no target found | - |
| miR1133 | FCA-like protein | TC368568 | nucleotide binding | No Hits found |
The miRNAs newly identified are shown in bold.
EST expression
To emphasize the mechanistic stage and/or tissue dependent roles of newly identified wheat miRNAs, we examined in silico expression patterns of miRNAs in different tissues using expressed sequence tags (ESTs) from GenBank database related to abiotic stress cDNA libraries expressed in different tissue types and developmental stages of T. aestivum. Newly identified miRNA from T. aestivum were detected in the seedling, sheath, leaf, root tips and root (Table 5). In this study, Ta-miR3708, Ta-miR819k-3p and Ta-miR5156 which were isolated from wheat drought stressed cDNA library, were found to be most abundant in leaf tissue, whereas miR3708 was detected in seedling. On the contrary, Ta-miR5653 and Ta-miR855 correspond to wheat cold-stressed and salt stressed libraries were found in seedling and sheath tissues. Ta-miR1134 and Ta-miR1117 belonging to drought stressed cDNA library were abundant in leaf tissue. Ta-miR1133 implies to wheat salt-stressed library and found abundantly in root. On the other hand, Ta-miR1122 belonging to cold-stressed and aluminum-stressed library was found in seedling and root tip. These newly detected miRNAs are potentially interesting, but require experimental verification by an independent technique. Using experimental approach to understand expression profile of identified miRNA in wheat will help to unravel a new dimension of regulatory network of miRNAs during abiotic stress.
Table 5. Identified miRNAs in wheat.
| miRNA | EST | Length | cDNA library | Tissue |
|---|---|---|---|---|
| miR5653 | BQ161232 | 504 | Wheat cold-stressed | seedling |
| miR855 | BG313724 | 190 | Wheat salt-stressed | sheath |
| miR819k | BQ172254 | 481 | Chinese Spring wheat drought stressed | leaf |
| miR3708 | BE470984 | 250 | drought-stressed | seedling |
| miR5156 | BQ172254 | 481 | Chinese Spring wheat drought stressed | leaf |
| miR1122 | BQ483040, BU101273 | 669, 589 | Wheat cold-stressed, Chinese Spring aluminum-stressed | seedling, root tip |
| miR1117 | BQ172250 | 469 | Chinese Spring wheat drought stressed | leaf |
| miR1134 | BQ168491 | 472 | Wheat drought stressed | leaf |
| miR1133 | BQ744063 | 715 | Wheat salt-stressed | root |
Sequence alignment and phylogenetic analysis of the new miRNAs
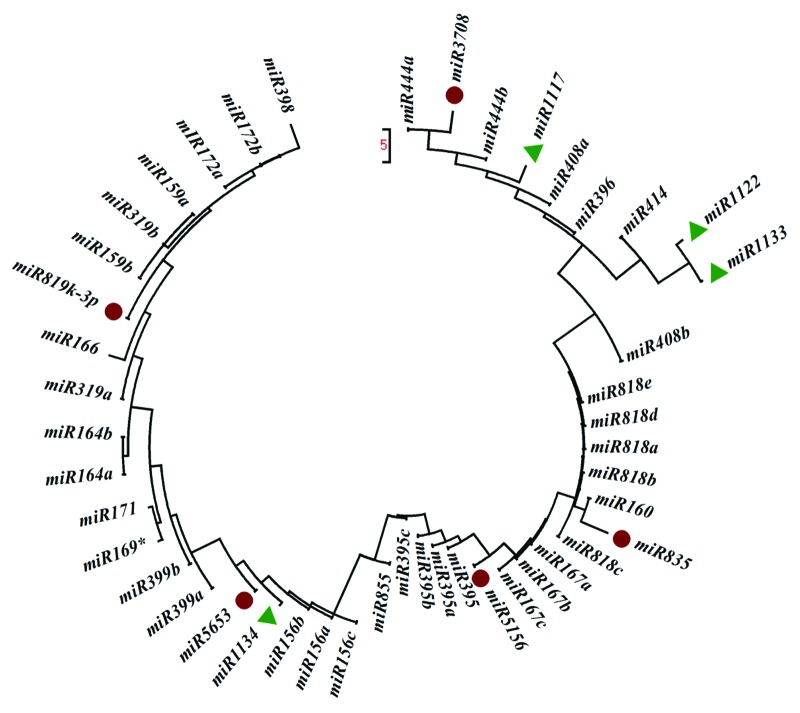
Primary and mature plant miRNAs are highly conserved among distantly related plant species.37 Comparison of the precursor sequences of the predicted miRNAs with other members in the same family showed that most members could be found to have a high degree of sequence similarity with others. The precursor sequence identity between miR819k-3p and miR5156 members was 100%, followed by that between miR3708 and miR5156, was over 46% (Table 6). Least identity was shown between miR855 and miR1122. Based on the pre-miRNA sequence comparisons, the evolutionary relationships of T.aestivum miRNAs with other members from the same families were analyzed using Mega 4. Phylogenetic analysis of identified miRNA along with previously identified miRNA in wheat revealed that the miR3708 and miR444a were closely related, mir5156 clustered with miR167 family i.e., 167a, 176 band 167c, while miR855 showing its relatedness with miR156 family with three class; 156a, 156 band 156c. MiR5653 showed evolutionary relatedness with miR1134 whereas miR819k-3p was related to miR159b, miR319B, miR172 and miR398 (Fig. 3).
Table 6. The ClustalW multiple sequence alignment of precursor sequences of miRNA.
| miR5653 | miR855 | miR819k | miR3708 | miR5156 | miR1122 | miR1117 | miR1134 | miR1133 | |
|---|---|---|---|---|---|---|---|---|---|
| miR5653 | - | 32.10 | 32.22 | 38 | 32.22 | 27.72 | 40.48 | 40.96 | 27.20 |
| miR855 | 32.10 | - | 27.47 | 26.00 | 27.47 | 23.76 | 37.35 | 33.73 | 23.33 |
| miR819k | 32.22 | 27.47 | - | 43.69 | 100 | 41.18 | 37.63 | 33.33 | 31.97 |
| miR3708 | 38.00 | 26.00 | 43.69 | - | 43.69 | 41.35 | 38.24 | 30.69 | 33.6 |
| miR5156 | 32.22 | 27.47 | 100 | 44.66 | - | 41.18 | 37.63 | 33.33 | 31.97 |
| miR1122 | 27.72 | 23.76 | 41.18 | 41.35 | 41.18 | - | 35.64 | 40.00 | 45.00 |
| miR1117 | 40.48 | 37.35 | 37.63 | 38.24 | 37.63 | 35.64 | - | 44.68 | 33.33 |
| miR1134 | 40.96 | 33.73 | 33.33 | 30.69 | 33.33 | 40.00 | 44.68 | - | 33.06 |
| miR1133 | 27.20 | 23.33 | 31.97 | 33.6 | 31.97 | 45.00 | 33.33 | 33.06 | - |
The miRNAs newly identified in wheat are shown in bold.
Figure 3. Phylogenetic analysis of precursor sequences of predicted miRNAs in different families. The red colored one represent newly identified miRNA family.
Discussion
The current literature suggests that plant genes are involved in response to abiotic stresses such as drought and heat which may be regulated at the post-transcriptional level by miRNAs. These plant miRNAs are involved in regulation of numerous cellular events under various stress responses.6 Computational identification of miRNA form wheat has been done earlier by using express sequence tag.28 Till now, only 270 known mature miRNA have been reported in wheat (https://pag.confex.com/pag/xxi/webprogram/Paper6335.html). This suggests that miRNA prediction and their validation in wheat requires more concerted efforts. In the present study, using wheat EST database, we have identified 5 new abiotic stress-responsive miRNAs along with their potential target genes (Table 4). These newly identified miRNAs belong to drought, cold and salt specific stress condition which is potentially interesting.
Abiotic stress in wheat is a major problem limiting wheat production. In the recent past, several attempts have been made to explore the active role of miRNAs to regulate various developmental stages under different abiotic stress condition. In this study, we found that Ta-miR855 target MYB transcription factor which primarily regulates leaf development and might also be involved in regulating genes of other organ development. This is in agreement with functionality of miR159.38 Various kinds of proteins such as ubiquitin carrier protein and Serine/threonine protein kinase were predicted to be the target of miR5653. Previous finding suggests that ubiquitin carrier protein play major role in regulating diverse cellular process such as control of cell cycle, activation of various transcription factors, recycling of abnormal proteins and metabolic regulation.40 Serine/threonine protein kinase has significant roles in controlling different signal transduction pathways leading to plant defense under both, biotic and abiotic stress.41 Earlier studied have documented that most of the miRNAs largely target transcription factors, metabolic transporters and signal transduction factors.14 Palatnik et al.39and Aukerman et al.,38 has confirmed the role of miRNA targets are involve in organ development, as floral organ identity, leaf morphogenesis, root development, various stress responses in model plant, Arabidopsis. Ta-miR5156 was identified to target 40S ribosomal proteins structural constituent of ribosome. Similarly, Ta-miR3708 was known to target F-box proteins which mediate hormone signaling in plants. F-box domain of F-box protein plays a connecting role for protein –protein interaction in a variety of processes, such as polyubiquitination, transcription elongation, centromere binding and translation repression.42 In wheat, Ta-miR819k was predicted to target Rho GTPase and BTB/POZ domain protein. Rho GTPases are central regulator various cellular functions in eukaryotes, such as organization of the cytoskeleton, stress-induced signal transduction, cell death, cell growth and differentiation43 while BTB/POZ domain protein mediates leaf morphogenesis in A. thaliana.44 Therefore, present findings of new miRNAs discovery suggests that apart from targeting genes of plant development, miRNAs are also involved in diverse biochemical and physiological processes leading to plant tolerance to abiotic stress. Unigene database provides expression pattern of miRNA in different tissues at different developmental stages (Table 5). The expression analysis of miRNAs revealed their significant role in growth and development of the respective tissues. miRNAs are highly conserved among various distinct plant species.37 Comparison of the identified precursor miRNA sequences with previously reported miRNA family revealed that most members seemed to have a high degree of sequence similarity with others (Table 6). The phylogenetic analysis suggested that different miRNAs might evolve at different rates not only within the same plant species, but also in different ones.
In-silico expression analysis of newly identified miRNAs from EST database suggests their differential regulatory role in different tissues under different abiotic stress conditions which might be involved in regulating numerous developmental stages of wheat. Using experimental approach to understand expression profile of identified miRNA in wheat will help to unravel a new dimension of regulatory network of miRNAs during abiotic stress.
Materials and Methods
Sequences and software
To search potential new miRNA in T.aestivum the sequences of previously known mature miRNA sequences from Arabidopsis, Brassica, Hordeum, Populus, Glycine, Saccharum, Sorghum, Vitis, Solanum, Oryza, Triticum and remaining from other plant species, were downloaded from the miRNA registry database (www.mirbase.org; release 19: November 2012). This data set contains contained, 6,220 mature miRNA sequences from 43 plants belonging to 50 miRNA families. The data set was screened with the help of in house perl script (www.perl.org) and the redundant miRNA sequences were removed. We retrieved 4,677 non-redundant miRNA sequences to be used as reference set. Wheat ESTs from abiotic stress-treated cDNA libraries were obtained from GenBank nucleotide database available at NCBI. This sequence information contained 3, 74, 608 ESTs (Till November 2012). Blast-2.2.25 was downloaded from NCBI and set up locally.
T.aestivum EST pre-processing
ESTs were cleaned to remove contaminating sequences. Vector sequences and other contaminations were identified by using VecScreen web server (www.ncbi.nlm.nih.gov/VecScreen/VecScreen.html). Poly-A/T tails have been completely trimmed by EST trimmer perl program. After pre-cleansing, EST sequences shorter than 50 bases were discarded. Furthermore, low complexity regions were masked by using Repeat Masker (www.repeatmasker.org).
Prediction of T. aestivum miRNAs
The sequences of previously known plant miRNAs were used as query sequences for BLASTN search (parameters for BLAST alignment was Expect: 0.01; Word Size; 11) against the wheat EST database.45,46 miRNA sequences matching at least 18 ntd and < 3 ntd mismatch with all known plant mature miRNA were selected for further analysis. Wherever available, precursor sequence of 250 nt base pair upstream and downstream to the BLAST hits were extracted and used for hairpin structure prediction. To predict real miRNA precursor, triplet-SVM classifier program,47 which is based on support vector machine was used. This software needs other packages namely RNAfold, LibSVM. The predicted precursor sequences were used against BLASTX program; protein coding precursor sequences were removed and non-coding were retained. BLASTN search was performed against Rfam 11.0 (rfam.sanger.ac.uk/) to distinguish between miRNA and other RNA families such as rRNA, snRNA, tRNA. The work was performed by in house script developed using ASP.NET technology48 and C# as scripting language for retrieving matching and non- matching sequences in BLAST result.
Prediction of secondary structure
The precursor sequences formed hairpin structure through Vienna RNA Package.30 Certain criteria mentioned below were chosen for the confirmation of miRNA homologs: (1) appropriate formation of stem-loop hairpin secondary structure; (2) presence of less than 3 nt substitutions in predicted mature miRNAs as compared with the known miRNAs; (3) miRNA sequences without any loop and break; and (4) MFE index (MFEI). The MFEI was calculated using the following equation:
| MEFI = [(MEF/length of the RNA sequence) × 100]/(G + C) % |
| Whereas MFE denotes the negative folding free energies (ΔG). |
Prediction of miRNA targets genes
The putative target sites of identified miRNAs were predicted using Plant Target Prediction Tool available on UEA sRNA ToolKit (srna-tools.cmp.uea.ac.uk/ plant/cgi-bin/srna-tools.cgi). miRNA binds to the targets with perfect or nearly-perfect complementarily and influence transcript regulation. Gaps and more than 4 mismatches between mature miRNAs and their potential target mRNA were not acceptable.
EST expression and phylogenetic analysis of the identified miRNAs
The expression analysis of predicted miRNA was performed using unigene (www.ncbi.nlm.nih.gov/UniGene). The precursor sequences of the identified and the well known wheat miRNAs were aligned and phylogenetically analyzed to investigate their evolutionary relationships (www.clustal.org). Evolutionary distances were calculated neighbor-joining (NJ) method49 following 1,000 bootstrapped replicates. All the analyses were performed using the MEGA v4.0 software.50
Functional analysis of target genes
The functional annotation of predicted targets genes of 5 miRNAs were carried under the gene ontology system by AmiGO program (amigo.genontology.org) for consistent descriptions of biological process. The pathways and the network of molecular interaction of the predicted target genes were studied by KEGG (www.genome.jp/kegg).
Conclusions
In this paper with a bioinformatics approach, five new miRNAs were identified from the ESTs of abiotic stress treated libraries of T. aestivum. None of the predicted miRNAs showed identity with the previously reported miRNAs in wheat and these are addition into wheat miRNA data set. In addition, five new ESTs identified as a miRNA and 14 potential targets of them were predicted, which appear to be related to the development, growth, metabolism and other physiological processes under stress response. Identification of new miRNAs and their target genes will provide the future path leading to the understanding of the core regulatory interactions during abiotic stress in wheat. Researcher can further varify theses predicted miRNA experimentally by high throughput sequencing of small RNA libraries.
Acknowledgment
Authors thankfully acknowledge the financial support for Indian Council of Agricultural (ICAR), Ministry of Agriculture, Government of India, New Delhi for Grant-in-Aid project No. DWR/RP/10-5.3 and grateful to anonymous reviewers for their helpful suggestions.
Glossary
Abbreviations:
- GSS
genomic survey sequences
- EST
expressed sequenced tag
- MFEI
minimal folding free energy index
- DCL1
Dicer-like 1 enzyme
- RISC
RNA-induced silencing complex
- GSSs
genome survey sequences
- HTGSs
high-throughput genomics sequences
- NRs
non-redundant nucleotides
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23932
References
- 1.Gill BS, Appels R, Botha-Oberholster AM, Buell CR, Bennetzen JL, Chalhoub B, et al. A workshop report on wheat genome sequencing: International Genome Research on Wheat Consortium. Genetics. 2004;168:1087–96. doi: 10.1534/genetics.104.034769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wicker T, Mayer KF, Gundlach H, Martis M, Steuernagel B, Scholz U, et al. Frequent gene movement and pseudogene evolution is common to the large and complex genomes of wheat, barley, and their relatives. Plant Cell. 2011;23:1706–18. doi: 10.1105/tpc.111.086629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vitulo N, Albiero A, Forcato C, Campagna D, Dal Pero F, Bagnaresi P, et al. First survey of the wheat chromosome 5A composition through a next generation sequencing approach. PLoS ONE. 2011;6:e26421. doi: 10.1371/journal.pone.0026421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL. Regulation of phosphate homeostasis by MicroRNA in Arabidopsis. Plant Cell. 2006;18:412–21. doi: 10.1105/tpc.105.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- 7.Papp I, Mette MF, Aufsatz W, Daxinger L, Schauer SE, Ray A, et al. Evidence for nuclear processing of plant micro RNA and short interfering RNA precursors. Plant Physiol. 2003;132:1382–90. doi: 10.1104/pp.103.021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, Poethig RS. Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci USA. 2005;102:3691–6. doi: 10.1073/pnas.0405570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R, Hu Z, Zhang H. Identification of microRNAs in wild soybean (Glycine soja) J Integr Plant Biol. 2009;51:1071–9. doi: 10.1111/j.1744-7909.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 10.Sunkar R, Chinnusamy V, Zhu J, Zhu JK. Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci. 2007;12:301–9. doi: 10.1016/j.tplants.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136:669–87. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 12.Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP. Prediction of plant microRNA targets. Cell. 2002;110:513–20. doi: 10.1016/S0092-8674(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang XJ, Reyes JL, Chua NH, Gaasterland T. Prediction and identification of Arabidopsis thaliana microRNAs and their mRNA targets. Genome Biol. 2004;5:R65. doi: 10.1186/gb-2004-5-9-r65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnet E, Wuyts J, Rouzé P, Van de Peer Y. Detection of 91 potential conserved plant microRNAs in Arabidopsis thaliana and Oryza sativa identifies important target genes. Proc Natl Acad Sci USA. 2004;101:11511–6. doi: 10.1073/pnas.0404025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang BH, Pan XP, Wang QL, Cobb GP, Anderson TA. Identification and characterization of new plant microRNAs using EST analysis. Cell Res. 2005;15:336–60. doi: 10.1038/sj.cr.7290302. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, Pan X, Cannon CH, Cobb GP, Anderson TA. Conservation and divergence of plant microRNA genes. Plant J. 2006;46:243–59. doi: 10.1111/j.1365-313X.2006.02697.x. a. [DOI] [PubMed] [Google Scholar]
- 17.Qiu CX, Xie FL, Zhu YY, Guo K, Huang SQ, Nie L, et al. Computational identification of microRNAs and their targets in Gossypium hirsutum expressed sequence tags. Gene. 2007;395:49–61. doi: 10.1016/j.gene.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 18.Yao Y, Guo G, Ni Z, Sunkar R, Du J, Zhu JK, et al. Cloning and characterization of microRNAs from wheat (Triticum aestivum L.) Genome Biol. 2007;8:R96. doi: 10.1186/gb-2007-8-6-r96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin M, Wang Y, Yao Y, Xie C, Peng H, Ni Z, et al. Diverse set of microRNAs are responsive to powdery mildew infection and heat stress in wheat (Triticum aestivum L.) BMC Plant Biol. 2010;10:123. doi: 10.1186/1471-2229-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallory AC, Vaucheret H. Functions of microRNAs and related small RNAs in plants. Nat Genet. 2006;38(Suppl):S31–6. doi: 10.1038/ng1791. [DOI] [PubMed] [Google Scholar]
- 21.Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol. 2005;15:2038–43. doi: 10.1016/j.cub.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Lu S, Sun YH, Shi R, Clark C, Li L, Chiang VL. Novel and mechanical stress-responsive MicroRNAs in Populus trichocarpa that are absent from Arabidopsis. Plant Cell. 2005;17:2186–203. doi: 10.1105/tpc.105.033456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahlgren N, Howell MD, Kasschau KD, Chapman EJ, Sullivan CM, Cumbie JS, et al. High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS ONE. 2007;2:e219. doi: 10.1371/journal.pone.0000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sunkar R, Kapoor A, Zhu JK. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell. 2006;18:2051–65. doi: 10.1105/tpc.106.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang BH, Pan XP, Anderson TA. Identification of 188 conserved maize microRNAs and their targets. FEBS Lett. 2006;580:3753–62. doi: 10.1016/j.febslet.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 26.Lucas SJ, Budak H. Sorting the wheat from the chaff: identifying miRNAs in genomic survey sequences of Triticum aestivum chromosome 1AL. PLoS ONE. 2012;7:e40859. doi: 10.1371/journal.pone.0040859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34(Database issue):D140–4. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han Y, Luan F, Zhu H, Shao Y, Chen A, Lu C, et al. Computational identification of microRNAs and their targets in wheat (Triticum aestivum L.) Sci China C Life Sci. 2009;52:1091–100. doi: 10.1007/s11427-009-0144-y. [DOI] [PubMed] [Google Scholar]
- 29.Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, et al. A uniform system for microRNA annotation. RNA. 2003;9:277–9. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofacker IL. Vienna RNA secondary structure server. Nucleic Acids Res. 2003;31:3429–31. doi: 10.1093/nar/gkg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabu GR, Mandal AK. Computational identification of miRNAs and their target genes from expressed sequence tags of tea (Camellia sinensis) Genomics Proteomics Bioinformatics. 2010;8:113–21. doi: 10.1016/S1672-0229(10)60012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Zhang J, Li F, Gu J, He T, Zhang X, et al. MicroRNA identification based on sequence and structure alignment. Bioinformatics. 2005;21:3610–4. doi: 10.1093/bioinformatics/bti562. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, Wang Q, Wang K, Pan X, Liu F, Guo T, et al. Identification of cotton microRNAs and their targets. Gene. 2007;397:26–37. doi: 10.1016/j.gene.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Pani A, Mahapatra RK, Behera N, Naik PK. Computational identification of sweet wormwood (Artemisia annua) microRNA and their mRNA targets. Genomics Proteomics Bioinformatics. 2011;9:200–10. doi: 10.1016/S1672-0229(11)60023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhardwaj J, Mohammad H, Yadav SK. Computational identification of microRNAs and their targets from the expressed sequence tags of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) J Struct Funct Genomics. 2010;11:233–40. doi: 10.1007/s10969-010-9098-3. [DOI] [PubMed] [Google Scholar]
- 36.Zhao B, Liang R, Ge L, Li W, Xiao H, Lin H, et al. Identification of drought-induced microRNAs in rice. Biochem Biophys Res Commun. 2007;354:585–90. doi: 10.1016/j.bbrc.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Zhang B, Pan X, Cannon CH, Cobb GP, Anderson TA. Conservation and divergence of plant microRNA genes. Plant J. 2006;46:243–59. doi: 10.1111/j.1365-313X.2006.02697.x. [DOI] [PubMed] [Google Scholar]
- 38.Aukerman MJ, Sakai H. Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell. 2003;15:2730–41. doi: 10.1105/tpc.016238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, et al. Control of leaf morphogenesis by microRNAs. Nature. 2003;425:257–63. doi: 10.1038/nature01958. [DOI] [PubMed] [Google Scholar]
- 40.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 41.Hardie DG. PLANT PROTEIN SERINE/THREONINE KINASES: Classification and Functions. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:97–131. doi: 10.1146/annurev.arplant.50.1.97. [DOI] [PubMed] [Google Scholar]
- 42.Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–9. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 43.Winge P, Brembu T, Kristensen R, Bones AM. Genetic structure and evolution of RAC-GTPases in Arabidopsis thaliana. Genetics. 2000;156:1959–71. doi: 10.1093/genetics/156.4.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ha CM, Jun JH, Nam HG, Fletcher JC. BLADE-ON-PETIOLE1 encodes a BTB/POZ domain protein required for leaf morphogenesis in Arabidopsis thaliana. Plant Cell Physiol. 2004;45:1361–70. doi: 10.1093/pcp/pch201. [DOI] [PubMed] [Google Scholar]
- 45.Jin W, Li N, Zhang B, Wu F, Li W, Guo A, et al. Identification and verification of microRNA in wheat (Triticum aestivum) J Plant Res. 2008;121:351–5. doi: 10.1007/s10265-007-0139-3. [DOI] [PubMed] [Google Scholar]
- 46.Arazi T, Talmor-Neiman M, Stav R, Riese M, Huijser P, Baulcombe DC. Cloning and characterization of micro-RNAs from moss. Plant J. 2005;43:837–48. doi: 10.1111/j.1365-313X.2005.02499.x. [DOI] [PubMed] [Google Scholar]
- 47.Xue C, Li F, He T, Liu GP, Li Y, Zhang X. Classification of real and pseudo microRNA precursors using local structure-sequence features and support vector machine. BMC Bioinformatics. 2005;6:310. doi: 10.1186/1471-2105-6-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walther S. ASP.NET 3.5: Unleashed: Sams Publishing, 2007, pp. 337-657. [Google Scholar]
- 49.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 50.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]