Abstract
A full-length cDNA clone for human myoglobin has been isolated from a human skeletal muscle cDNA library. The clone as isolated has a cDNA insert approximately one kilobase long and has 5' and 3' untranslated regions of approximately 80 and 530 base pairs, respectively. The sequence of the translated region corresponds exactly to that predicted for human myoglobin. The cDNA was expressed in high yield in Escherichia coli as a fusion protein consisting of the first 31 amino acids of the phage lambda cII gene, the tetrapeptide Ile-Glu-Gly-Arg, and the myoglobin sequence by following the approach of Nagai and Thogersen [Nagai, K. & Thogersen, M. C. (1984) Nature (London) 309, 810-812]. The fusion product was isolated, reconstituted with heme, cleaved with trypsin, and purified to generate a protein whose properties are indistinguishable from those for authentic human myoglobin. Myoglobin can be readily prepared on a gram scale by using these methods.
Full text
PDF
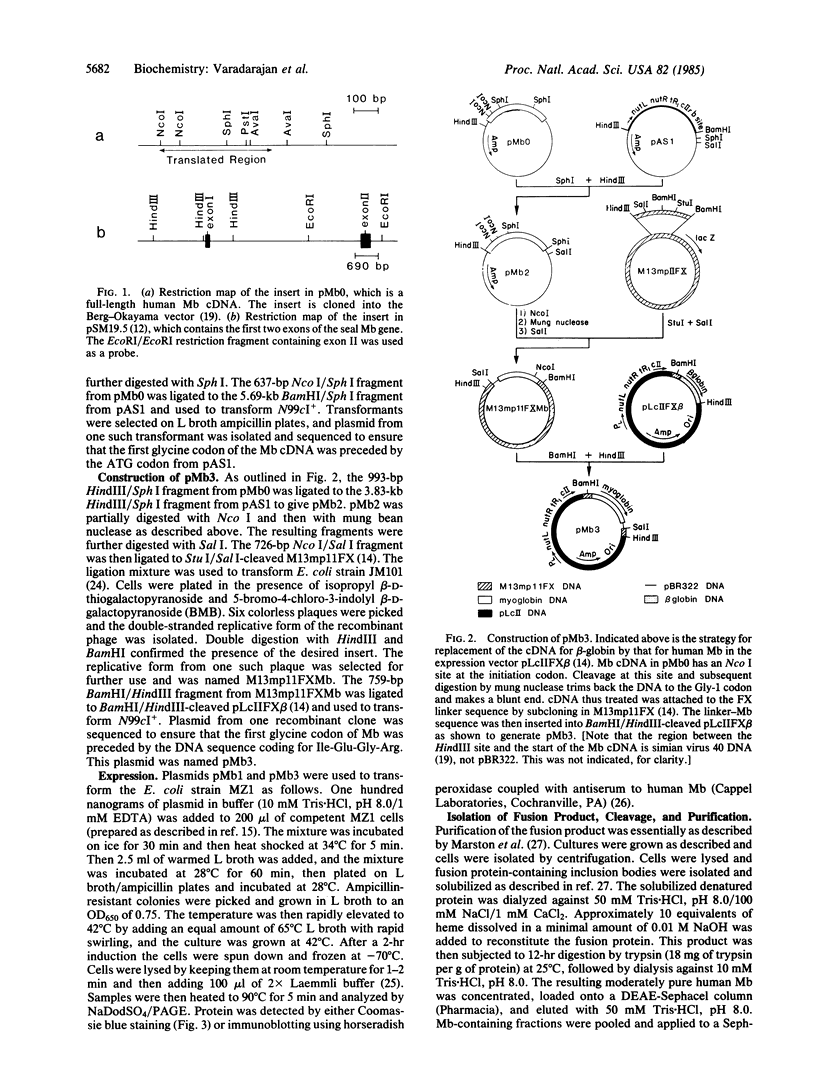

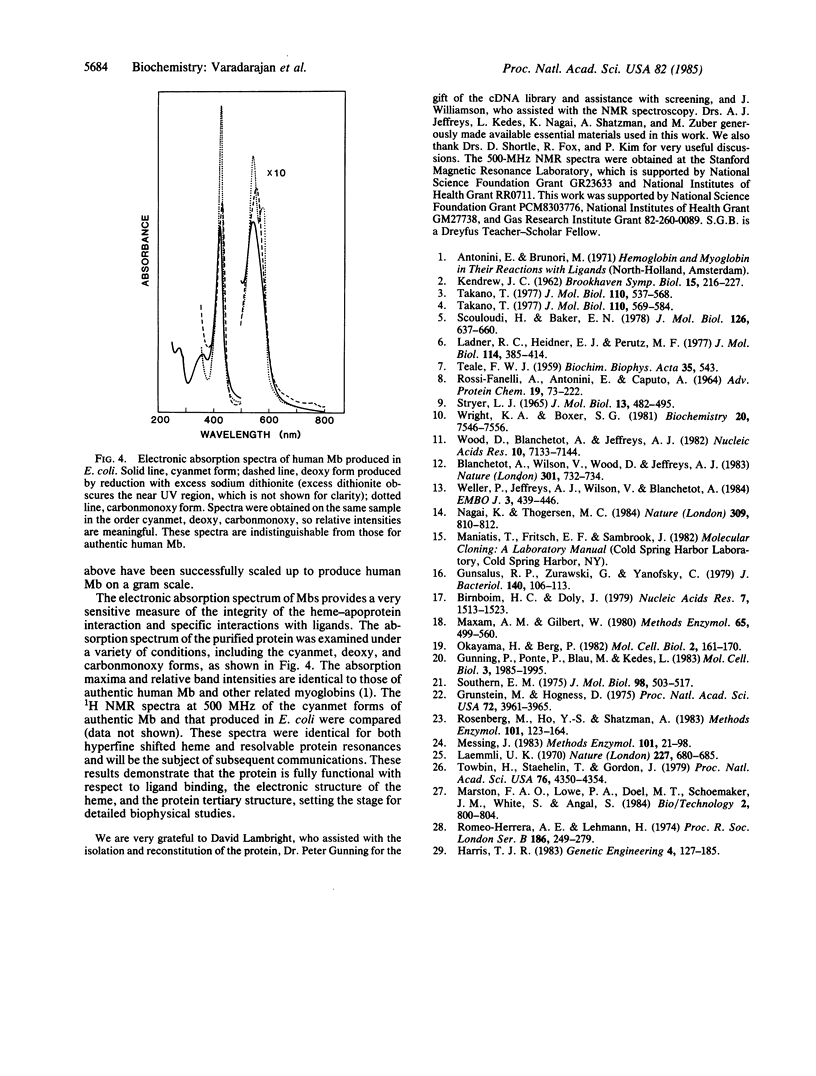
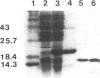
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchetot A., Wilson V., Wood D., Jeffreys A. J. The seal myoglobin gene: an unusually long globin gene. Nature. 1983 Feb 24;301(5902):732–734. doi: 10.1038/301732a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Blau H., Kedes L. alpha-skeletal and alpha-cardiac actin genes are coexpressed in adult human skeletal muscle and heart. Mol Cell Biol. 1983 Nov;3(11):1985–1995. doi: 10.1128/mcb.3.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Zurawski G., Yanofsky C. Structural and functional analysis of cloned deoxyribonucleic acid containing the trpR-thr region of the Escherichia coli chromosome. J Bacteriol. 1979 Oct;140(1):106–113. doi: 10.1128/jb.140.1.106-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENDREW J. C. Side-chain interactions in myoglobin. Brookhaven Symp Biol. 1962 Dec;15:216–228. [PubMed] [Google Scholar]
- Ladner R. C., Heidner E. J., Perutz M. F. The structure of horse methaemoglobin at 2-0 A resolution. J Mol Biol. 1977 Aug 15;114(3):385–414. doi: 10.1016/0022-2836(77)90256-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Generation of beta-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. 1984 Jun 28-Jul 4Nature. 309(5971):810–812. doi: 10.1038/309810a0. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSIFANELLI A., ANTONINI E., CAPUTO A. HEMOGLOBIN AND MYOGLOBIN. Adv Protein Chem. 1964;19:73–222. doi: 10.1016/s0065-3233(08)60189-8. [DOI] [PubMed] [Google Scholar]
- Romero-Herrera A. E., Lehmann H. The amino acid sequence of human myoglobin and its minor fractions. Proc R Soc Lond B Biol Sci. 1974 Jul 9;186(1084):249–279. doi: 10.1098/rspb.1974.0048. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Ho Y. S., Shatzman A. The use of pKc30 and its derivatives for controlled expression of genes. Methods Enzymol. 1983;101:123–138. doi: 10.1016/0076-6879(83)01009-5. [DOI] [PubMed] [Google Scholar]
- Scouloudi H., Baker E. N. X-ray crystallographic studies of seal myoglobin. The molecule at 2.5 A resolution. J Mol Biol. 1978 Dec 25;126(4):637–660. doi: 10.1016/0022-2836(78)90013-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stryer L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965 Sep;13(2):482–495. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P., Jeffreys A. J., Wilson V., Blanchetot A. Organization of the human myoglobin gene. EMBO J. 1984 Feb;3(2):439–446. doi: 10.1002/j.1460-2075.1984.tb01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. D. Antifibrillatory drugs: the case for lidocaine and procainamide. Ann Emerg Med. 1984 Sep;13(9 Pt 2):802–804. doi: 10.1016/s0196-0644(84)80442-4. [DOI] [PubMed] [Google Scholar]
- Wood D., Blanchetot A., Jeffreys A. J. Molecular cloning of seal myoglobin mRNA. Nucleic Acids Res. 1982 Nov 25;10(22):7133–7144. doi: 10.1093/nar/10.22.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. A., Boxer S. G. Solution properties of synthetic chlorophyllide--and bacteriochlorophyllide--apomyoglobin complexes. Biochemistry. 1981 Dec 22;20(26):7546–7556. doi: 10.1021/bi00529a033. [DOI] [PubMed] [Google Scholar]