Abstract
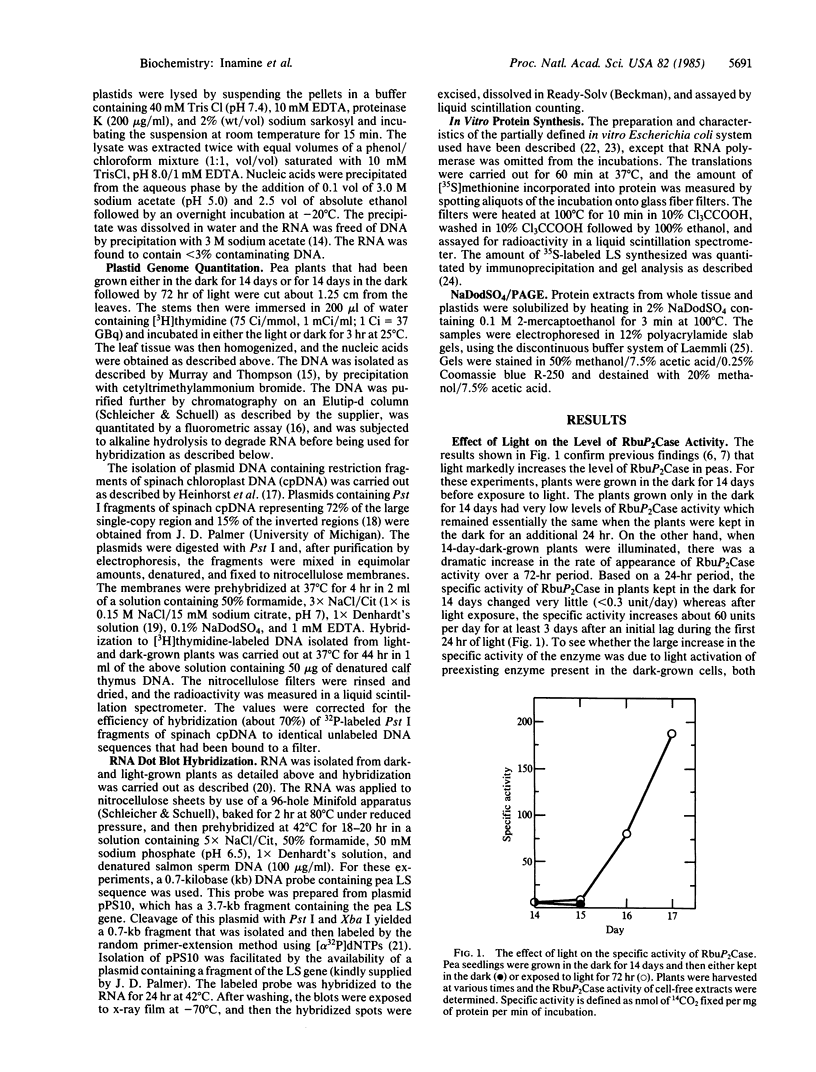
The specific activity of ribulose-1,5-bisphosphate carboxylase (EC 4.1.1.39) increases 30- to 50-fold when dark-grown pea seedlings are shifted into the light. The large subunit (LS) of this multimeric protein is known to be synthesized in the chloroplast, but plastids from dark-grown cells contain relatively low levels of LS. However, despite the low level of LS synthesis in the plastids of dark-grown plants, these organelles contain significant levels of LS mRNA. Hybridization studies showed that the amount of LS mRNA increased about 3-fold, relative to total plant RNA, when dark-grown plants were illuminated. This increase in LS mRNA can be accounted for by a similar increase in chloroplast genome copy number. It was found that the amount of translatable LS mRNA per μg of plastid RNA is similar when isolated from either dark-grown plants or dark-grown plants subjected to light. These results suggest that although light can increase the level of LS mRNA by increasing the copy number of this gene, the primary regulation of LS synthesis by light in pea chloroplasts is at the level of translation.
Keywords: chloroplast gene expression
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. S., Eisenberg D., Eiserling F. A., Weissman L. The structure of form I crystals of D-ribulose-1,5-diphosphate carboxylase. J Mol Biol. 1975 Feb 5;91(4):391–399. doi: 10.1016/0022-2836(75)90267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Edwards C., Chua N. H. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984 Aug;3(8):1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm H. E., Margulies M. M. In vitro protein synthesis by plastids of Phaseolus vulgaris. IV. Amino acid incorporation by etioplasts and effect of illumination of leaves on incorporation by plastids. Plant Physiol. 1970 Apr;45(4):435–442. doi: 10.1104/pp.45.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish L. E., Jagendorf A. T. High rates of protein synthesis by isolated chloroplasts. Plant Physiol. 1982 Oct;70(4):1107–1114. doi: 10.1104/pp.70.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. C., Kekwick R. G. The synthesis of the small subunit of ribulose 1,5-bisphosphate carboxylase in the french bean Phaseolus vulgaris. Eur J Biochem. 1974 May 15;44(2):491–500. doi: 10.1111/j.1432-1033.1974.tb03507.x. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Redfield B., Treadwell B. V., Eskin B., Spears C., Weissbach H. DNA-directed in vitro synthesis of beta-galactosidase. Studies with purified factors. J Biol Chem. 1977 Oct 10;252(19):6889–6894. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamppa G. K., Bendich A. J. Changes in Chloroplast DNA Levels during Development of Pea (Pisum sativum). Plant Physiol. 1979 Jul;64(1):126–130. doi: 10.1104/pp.64.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. E., Jurgenson J. E., Reardon E. M., Price C. A. Plastid translation in organello and in vitro during light-induced development in Euglena. J Biol Chem. 1983 Dec 10;258(23):14478–14484. [PubMed] [Google Scholar]
- Minami E., Watanabe A. Thylakoid membranes: the translational site of chloroplast DNA-regulated thylakoid polypeptides. Arch Biochem Biophys. 1984 Dec;235(2):562–570. doi: 10.1016/0003-9861(84)90230-3. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Price C. A. Photosynthetic activity of spinach chloroplasts after isopycnic centrifugation in gradients of silica. Plant Physiol. 1974 Oct;54(4):532–534. doi: 10.1104/pp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
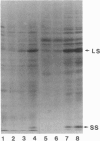
- Palmer J. D., Thompson W. F. Clone banks of the mung bean, pea and spinach chloroplast genomes. Gene. 1981 Oct;15(1):21–26. doi: 10.1016/0378-1119(81)90100-1. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Ovalbumin messenger ribonucleic acid translation. Comparable rates of polypeptide initiation and elongation on ovalbumin and globin messenger ribonucleic acid in a rabbit reticulocyte lysate. J Biol Chem. 1973 Mar 25;248(6):2095–2106. [PubMed] [Google Scholar]
- Sasaki Y., Ishiye M., Sakihama T., Kamikubo T. Light-induced increase of mRNA activity coding for the small subunit of ribulose-1,5-bisphosphate carboxylase. J Biol Chem. 1981 Mar 10;256(5):2315–2320. [PubMed] [Google Scholar]
- Shinozaki K., Sasaki Y., Sakihama T., Kamikubo T. Coordinate light-induction of two mRNAs, encoded in nuclei and chloroplasts, of ribulose 1,5-bisphosphate carboxylase/oxygenase. FEBS Lett. 1982 Jul 19;144(1):73–76. doi: 10.1016/0014-5793(82)80571-1. [DOI] [PubMed] [Google Scholar]
- Siddell S. G., Ellis R. J. Protein synthesis in chloroplasts. Characteristics and products of protein synthesis in vitro in etioplasts and developing chloroplasts from pea leaves. Biochem J. 1975 Mar;146(3):675–685. doi: 10.1042/bj1460675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J. Physical map of polyoma viral DNA fragments produced by cleavage with a restriction enzyme from Haemophilus aegyptius, endonuclease R-HaeIII. J Virol. 1975 Apr;15(4):946–953. doi: 10.1128/jvi.15.4.946-953.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytásek R. A sensitive fluorometric assay for the determination of DNA. Anal Biochem. 1982 Mar 1;120(2):243–248. doi: 10.1016/0003-2697(82)90342-6. [DOI] [PubMed] [Google Scholar]
- Zarucki-Schulz T., Jerez C., Goldberg G., Kung H. F., Huang K. H., Brot N., Weissbach H. DNA-directed in vitro synthesis of proteins involved in bacterial transcription and translation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6115–6119. doi: 10.1073/pnas.76.12.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]