Abstract
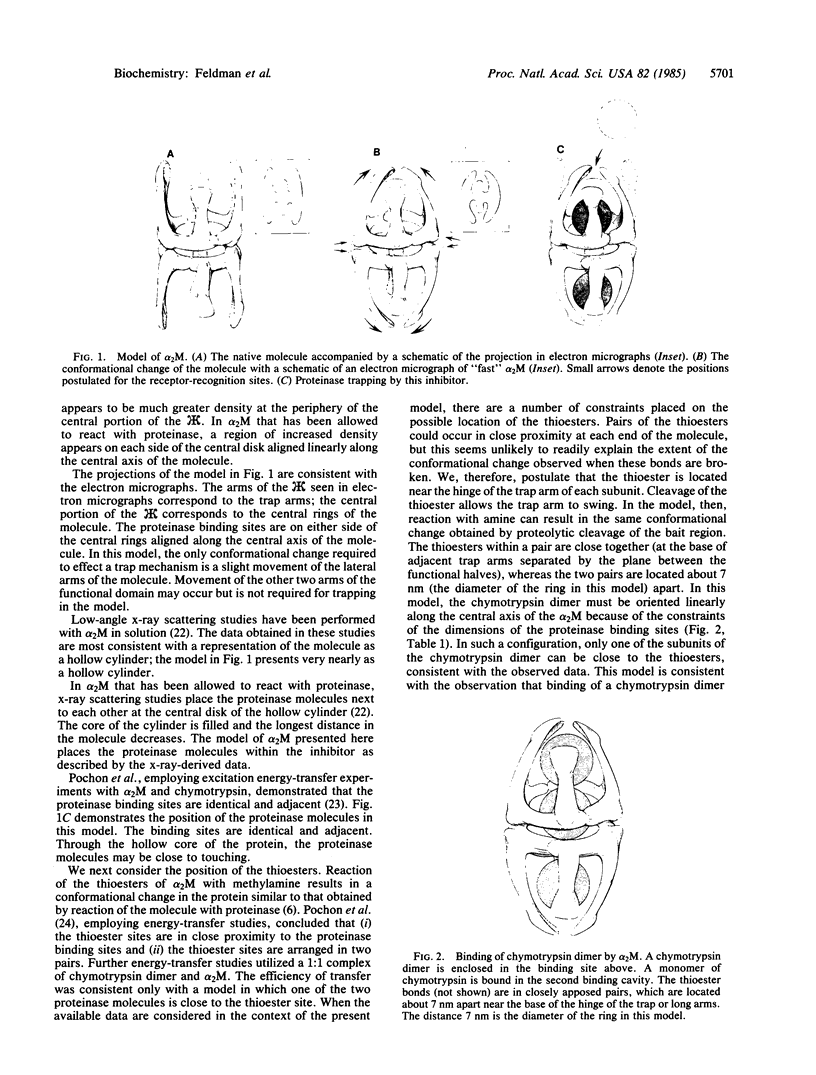
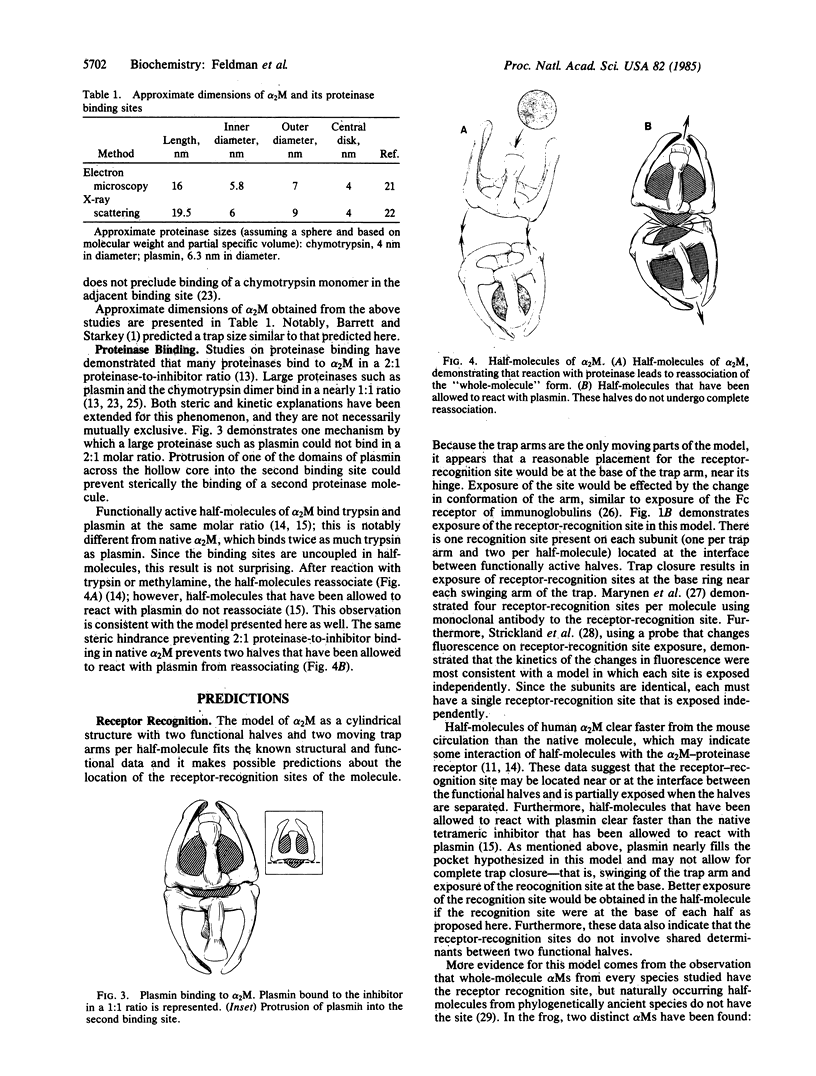
A model of alpha 2-macroglobulin is presented that is compatible with previous structural, functional, and phylogenetic studies of the protein. The model of the molecule resembles a hollow cylinder and is comprised of two identical functional halves with three C2 axes of symmetry and no mirror planes. The "trap mechanism" of this proteinase inhibitor is effected by slight movement of two trap arms per "half-molecule." Evidence for this model is obtained from the study of the structure and proteinase binding of the molecule. By using this model, predictions are made concerning proteinase binding ratios, receptor recognition, and "slow-to-fast" conformational change of the molecule.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings H. S., Castellino F. J. Interaction of human plasmin with human alpha 2-macroglobulin. Biochemistry. 1984 Jan 3;23(1):105–111. doi: 10.1021/bi00296a017. [DOI] [PubMed] [Google Scholar]
- Dangott L. J., Cunningham L. W. Residual alpha 2-macroglobulin in fetal calf serum and properties of its complex with thrombin. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1243–1251. doi: 10.1016/s0006-291x(82)80131-9. [DOI] [PubMed] [Google Scholar]
- Debanne M. T., Bell R., Dolovich J. Uptake of proteinase-alpha-macroglobulin complexes by macrophages. Biochim Biophys Acta. 1975 Dec 5;411(2):295–304. doi: 10.1016/0304-4165(75)90309-8. [DOI] [PubMed] [Google Scholar]
- Feldman S. R., Gonias S. L., Ney K. A., Pratt C. W., Pizzo S. V. Identification of "embryonin" as bovine alpha 2-macroglobulin. J Biol Chem. 1984 Apr 10;259(7):4458–4462. [PubMed] [Google Scholar]
- Feldman S. R., Ney K. A., Gonias S. L., Pizzo S. V. In vitro binding and in vivo clearance of human alpha 2-macroglobulin after reaction with endoproteases from four different classes. Biochem Biophys Res Commun. 1983 Jul 29;114(2):757–762. doi: 10.1016/0006-291x(83)90845-8. [DOI] [PubMed] [Google Scholar]
- Feldman S. R., Pizzo S. V. Circular dichroic spectroscopy of non-human alpha-macroglobulins. Biochem Biophys Res Commun. 1984 Sep 17;123(2):771–777. doi: 10.1016/0006-291x(84)90296-1. [DOI] [PubMed] [Google Scholar]
- Feldman S. R., Pizzo S. V. Comparison of the binding of chicken alpha-macroglobulin and ovomacroglobulin to the mammalian alpha 2-macroglobulin receptor. Arch Biochem Biophys. 1984 Nov 15;235(1):267–275. doi: 10.1016/0003-9861(84)90275-3. [DOI] [PubMed] [Google Scholar]
- Feldman S. R., Pizzo S. V. Purification and characterization of frog alpha-macroglobulin: receptor recognition of an amphibian glycoprotein. Biochemistry. 1985 May 7;24(10):2569–2575. doi: 10.1021/bi00331a026. [DOI] [PubMed] [Google Scholar]
- Feldman S. R., Rosenberg M. R., Ney K. A., Michalopoulos G., Pizzo S. V. Binding of alpha 2-macroglobulin to hepatocytes: mechanism of in vivo clearance. Biochem Biophys Res Commun. 1985 Apr 30;128(2):795–802. doi: 10.1016/0006-291x(85)90117-2. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Balber A. E., Hubbard W. J., Pizzo S. V. Ligand binding, conformational change and plasma elimination of human, mouse and rat alpha-macroglobulin proteinase inhibitors. Biochem J. 1983 Jan 1;209(1):99–105. doi: 10.1042/bj2090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonias S. L., Einarsson M., Pizzo S. V. Catabolic pathways for streptokinase, plasmin, and streptokinase activator complex in mice. In vivo reaction of plasminogen activator with alpha 2-macroglobulin. J Clin Invest. 1982 Aug;70(2):412–423. doi: 10.1172/JCI110631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonias S. L., Pizzo S. V. Characterization of functional human alpha 2-macroglobulin half-molecules isolated by limited reduction with dithiothreitol. Biochemistry. 1983 Feb 1;22(3):536–546. doi: 10.1021/bi00272a003. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Pizzo S. V. Chemical and structural modifications of alpha 2-macroglobulin: effects on receptor binding and endocytosis studied in an in vivo model. Ann N Y Acad Sci. 1983;421:457–471. doi: 10.1111/j.1749-6632.1983.tb18139.x. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Pizzo S. V. Reaction of human alpha 2-macroglobulin half-molecules with plasmin as a probe of protease binding site structure. Biochemistry. 1983 Oct 11;22(21):4933–4940. doi: 10.1021/bi00290a009. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Reynolds J. A., Pizzo S. V. Physical properties of human alpha 2-macroglobulin following reaction with methylamine and trypsin. Biochim Biophys Acta. 1982 Aug 10;705(3):306–314. doi: 10.1016/0167-4838(82)90252-7. [DOI] [PubMed] [Google Scholar]
- Howell J. B., Beck T., Bates B., Hunter M. J. Interaction of alpha 2-macroglobulin with trypsin, chymotrypsin, plasmin, and papain. Arch Biochem Biophys. 1983 Feb 15;221(1):261–270. doi: 10.1016/0003-9861(83)90143-1. [DOI] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981 Aug 10;256(15):8134–8139. [PubMed] [Google Scholar]
- Marynen P., Van Leuven F., Cassiman J. J., Van den Berghe H. A monoclonal antibody to a neo-antigen on alpha 2-macroglobulin complexes inhibits receptor-mediated endocytosis. J Immunol. 1981 Nov;127(5):1782–1786. [PubMed] [Google Scholar]
- Muirhead H., Cox J. M., Mazzarella L., Perutz M. F. Structure and function of haemoglobin. 3. A three-dimensional fourier synthesis of human deoxyhaemoglobin at 5.5 Angstrom resolution. J Mol Biol. 1967 Aug 28;28(1):117–156. doi: 10.1016/s0022-2836(67)80082-2. [DOI] [PubMed] [Google Scholar]
- Ney K. A., Gidwitz S., Pizzo S. V. Changes in the binding of "fast"-form alpha 2-macroglobulin to 3T3-L1 cells after differentiation to adipocytes. Biochemistry. 1984 Jul 17;23(15):3395–3403. doi: 10.1021/bi00310a003. [DOI] [PubMed] [Google Scholar]
- Osterberg R., Pap S. Structure of alpha 2-macroglobulin in solution and its interaction with proteases: an X-ray scattering study using the contrast variation method. Ann N Y Acad Sci. 1983;421:98–111. doi: 10.1111/j.1749-6632.1983.tb18096.x. [DOI] [PubMed] [Google Scholar]
- Pochon F., Favaudon V., Bieth J. Localization of the proteinase-induced thiol groups in alpha 2-macroglobulin. Biochem Biophys Res Commun. 1983 Mar 29;111(3):964–969. doi: 10.1016/0006-291x(83)91394-3. [DOI] [PubMed] [Google Scholar]
- Pochon F., Favaudon V., Tourbez-Perrin M., Bieth J. Localization of the two protease binding sites in human alpha 2-macroglobulin. J Biol Chem. 1981 Jan 25;256(2):547–550. [PubMed] [Google Scholar]
- Salvesen G. S., Sayers C. A., Barrett A. J. Further characterization of the covalent linking reaction of alpha 2-macroglobulin. Biochem J. 1981 May 1;195(2):453–461. doi: 10.1042/bj1950453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm H. J., Schramm W. Computer averaging of single molecules of alpha 2-macroglobulin and the alpha 2-macroglobulin/trypsin complex. Hoppe Seylers Z Physiol Chem. 1982 Aug;363(8):803–812. doi: 10.1515/bchm2.1982.363.2.803. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Petersen T. E., Magnusson S. A thiol-ester in alpha 2-macroglobulin cleaved during proteinase complex formation. FEBS Lett. 1980 Dec 1;121(2):275–279. doi: 10.1016/0014-5793(80)80361-9. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Stepanik T. M., Wierzbicki D. M., Jones C. M., Lønblad P. B., Kristensen T., Mortensen S. B., Petersen T. E., Magnusson S. The primary structure of alpha 2-macroglobulin and localization of a Factor XIIIa cross-linking site. Ann N Y Acad Sci. 1983;421:41–60. doi: 10.1111/j.1749-6632.1983.tb18091.x. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Evolution of alpha 2-macroglobulin. The demonstration in a variety of vertebrate species of a protein resembling human alpha 2-macroglobulin. Biochem J. 1982 Jul 1;205(1):91–95. doi: 10.1042/bj2050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Evolution of alpha 2-macroglobulin. The structure of a protein homologous with human alpha 2-macroglobulin from plaice (Pleuronectes platessa L.) plasma. Biochem J. 1982 Jul 1;205(1):105–115. doi: 10.1042/bj2050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland D. K., Steiner J. P., Feldman S. R., Pizzo S. V. Fluorescent probes as a measure of conformational alterations induced by nucleophilic modification and proteolysis of bovine alpha 2-macroglobulin. Biochemistry. 1984 Dec 18;23(26):6679–6685. doi: 10.1021/bi00321a061. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Characterization of alkylamine-sensitive site in alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4313–4316. doi: 10.1073/pnas.76.9.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelhake J. L. Immunoglobulin structure and effector functions. Immunochemistry. 1978 Sep;15(9):695–714. doi: 10.1016/0161-5890(78)90044-5. [DOI] [PubMed] [Google Scholar]