Abstract
Background and Objective
Evidence has shown that matrix metalloproteinases-3 (MMP3) is important for cancer progression. Recent studies about the association between the -1171(5A>6A) polymorphism in MMP3 promoter region and cancer risk have yielded conflicting results.
Methodology/Principal Findings
We performed a meta-analysis of 41 studies including 11112 cases and 11091 controls to determine whether the -1171(5A>6A) polymorphism of MMP3 was associated with cancer risk. We assessed the strength of association and performed sub-group analyses by cancer types, ethnicity, smoking status, genotyping method, source of controls and sample size. The pooled results revealed that no significant association of the -1171(5A>6A) polymorphism with overall cancer risk in any of four models. Further sub-group analysis revealed that individuals with the 6A allele had lower risk of gastrointestinal cancer in two models: heterozygote comparison (6A/5A vs. 5A/5A: OR = 0.74, 95%CI: 0.60—0.91; I2 = 1.9%), and dominant model (6A/6A+6A/5A vs. 5A/5A: OR = 0.77, 95%CI: 0.64—0.94; I2 = 29.0%). Additionally, the associations were significant in Asian populations for three models: homozygote comparison (6A/6A vs. 5A/5A, OR = 0.68, 95%CI: 0.52—0.90; I2 = 26.7%), heterozygote comparison (6A/5A vs. 5A/5A: OR = 0.75, 95%CI: 0.58—0.98; I2 = 0.0%), and dominant model (6A/6A+6A/5A vs. 5A/5A: OR = 0.69, 95%CI: 0.54—0.88; I2 = 0.5%). It was noteworthy that we had a contrary finding in non-smokers: the variant 6A/6A homozygote might statistically increase cancer risk compared with 6A/5A+5A/5A genotype (OR = 1.92, 95%CI: 1.25—2.96; I2 = 72.7%).
Conclusion
This meta-analysis suggests that the -1171(5A>6A) polymorphism in MMP3 promoter region is not associated with overall cancer risk, but it may contribute to decreased cancer risk in Asian population when compared with Caucasian population and significantly reduce the risk of gastrointestinal cancer.
Introduction
The matrix metalloproteinases (MMPs), a family of highly conserved zinc-dependent proteolytic enzymes that degrade many different components of the extracellular matrix (ECM) and basement membrane, have been involved in the regulation of various cell behaviors with relevance to tumor development and metastasis [1]–[3]. MMPs are divided into five subgroups according to their structure and substrate specificity: collagenases, stromelysins, gelatinases, membrane-type MMPs, and other MMPs [4]. MMPs are classified into 24 enzymes according to substrate specificity and structural similarities [5]. Expression of most MMPs in tumors is regulated primarily at the transcriptional level, but there is also evidence of modulation of mRNA stability in response to growth factors and cytokines secreted by tumor-infiltrating inflammatory cells as well as by tumor and stromal cells [6].
MMP3 (stromelysin-1) is known to lyse basal membrane collagen and induce the synthesis of other MMPs such as MMP1 and MMP9 [7], [8]. The MMP3 gene is localized on 11q22 adjacent to the MMP1 gene, produced by stromal fibroblasts, macrophages and synovial cells [9]. A single adenine insertion/deletion polymorphism (5A>6A) at the 1171 position of the MMP3 promoter region could modulate its transcription [10]. In vitro assays of promoter activity showed that the 5A allele had a two-fold higher promoter activity than the 6A allele [10]. A large number of studies have demonstrated the association between MMP3 -1171(5A>6A) polymorphism and cancer risk, including colorectal, lung, head and neck, esophagus, breast, ovarian cancers and so on [6], [11]–[15]. However, these studies yielded different or even controversial results. For example, Ghilardi et al. [11] found a significant association between MMP3 -1171 5A allele and increased cancer risk, but Su et al. [15] reported no significant correlation.
Meta-analysis is a means of increasing the effective sample size through pooling of data from individual studies, thus enhancing the statistical power of the analysis for the estimation of genetic effects [16]. To clarify the association between MMP3 -1171(5A>6A) polymorphism and cancer risk, we performed this meta-analysis by pooling eligible studies to calculate the estimate of overall cancer risk and evaluated influence of cancer types, ethnicity, smoking status, genotyping method, source of controls and sample size.
Methods
Literature Search Strategy and Selection Criteria
This meta-analysis was designed, conducted, and reported according to the PRISMA guideline [17]. We carried out literature search in the PubMed, EMBASE and CNKI (Chinese National Knowledge Infrastructure) without language, time period and sample size limitations, covering all papers published up to August 21, 2013, with a combination of the following keywords: MMP3 gene (e.g.: ‘‘MMP3’’, or ‘‘matrix metalloproteinase-3’’); cancer (e.g.: ‘‘cancer’’, ‘‘carcinoma’’, ‘‘tumor’’ or ‘‘neoplasms’’) and polymorphism or variation. Before searching Pubmed database, we searched MeSH database to find the most matched searching items. And for the descriptor “polymorphism”, we used MeSH word “Polymorphism, Single Nucleotide” in the searching strategy. In addition, we performed manual search of references of relative articles and reviews. The following criteria was used for the literature selection: (a) case–control studies or cohort studies; (b) investigating the association between the -1171(5A>6A) polymorphism in MMP3 promoter region and cancer risk; (c) sufficient genotype distribution information in cases and controls. The major reasons for exclusion of studies were (a) reviews and duplicated reports from the same study; (b) study design other than case-control method; (c) studies without detailed genotype frequencies.
Data Extraction
Data were extracted from all eligible publications independently by two of the authors (Yang and Hu) according to the selection criteria from each of the eligible papers: name of first author, publication year, country where the study was conducted, ethnicity, source of controls, cancer types and genotyping methods, total number of cases and controls, genotype frequency in cases and controls. Different ethnicities were categorized as Asian and Caucasian. Cancer types were classified as Gynecological cancer (GC), including ovarian, cervical and endometrial cancer; Gastrointestinal cancer (GIC), including gastric and colorectal cancer; Breast cancer (BC); Head and neck cancer (HNC); Hepatocellular carcinoma(HC); Lung cancer(LC); Oral cancer(OC); Others (renal cell carcinoma, esophageal cancer, bladder cancer, brain astrocytoma and nasopharyngeal carcinoma). All eligible studies were defined as hospital-based(HB), population-based(PB), friends and spouse-based(FASB) according to the source of controls. The Hardy–Weinberg equilibrium (HWE) was calculated by Chi-square test (p<0.05 was considered as significant disequilibrium) based on -1171 5A>6A polymorphism genotyping distribution in controls [18].
Statistical Analysis
The strength of the association between MMP3 -1171(5A>6A) polymorphism and cancer risk was estimated by calculating odds ratio (OR) with 95% confidence intervals (95% CI), based on the genotype frequencies in cases and controls. The pooled ORs were calculated for four models: homozygote comparison (6A/6A vs. 5A/5A), heterozygote comparison (6A/5A vs. 5A/5A), dominant model (6A/6A+6A/5A vs. 5A/5A) and recessive model (6A/6A vs. 6A/5A+5A/5A). The fixed effects model (Mantel-Haenszel method) was used when there was no significant heterogeneity [19]; otherwise, the random effects model (the Der Simonian and Laird method) was utilized [20]. According to the Cochrane Handbook for Systematic Reviews of Interventions, a useful statistic for quantifying inconsistency is I = [(Q –df)/Q]×100%, where Q is the chi-squared statistic and df is its degrees of freedom. This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error. The value of I2>50% indicates substantial heterogeneity. Sensitivity analysis was conducted by deleting each individual study in turn from the total and reanalyzing the remainder [21]. Sub-group analyses and logistic meta-regression analyses were conducted to explore the source of heterogeneity among variables, such as cancer types, ethnicity, genotyping method, source of controls and sample size (studies with more than 1000 participants were defined as ‘‘large’’, and studies with less 1000 participants were defined as ‘‘small’’). Publication bias was both examined with Begg’s funnel plot [22] and Egger’s regression method [23] (p<0.05 was considered representative of statistically significant publication bias). All p values are two-sided. Data were analyzed using STATA software (version 12.1; Stata Corp, College Station, Texas USA).
Results
Characteristics of Studies
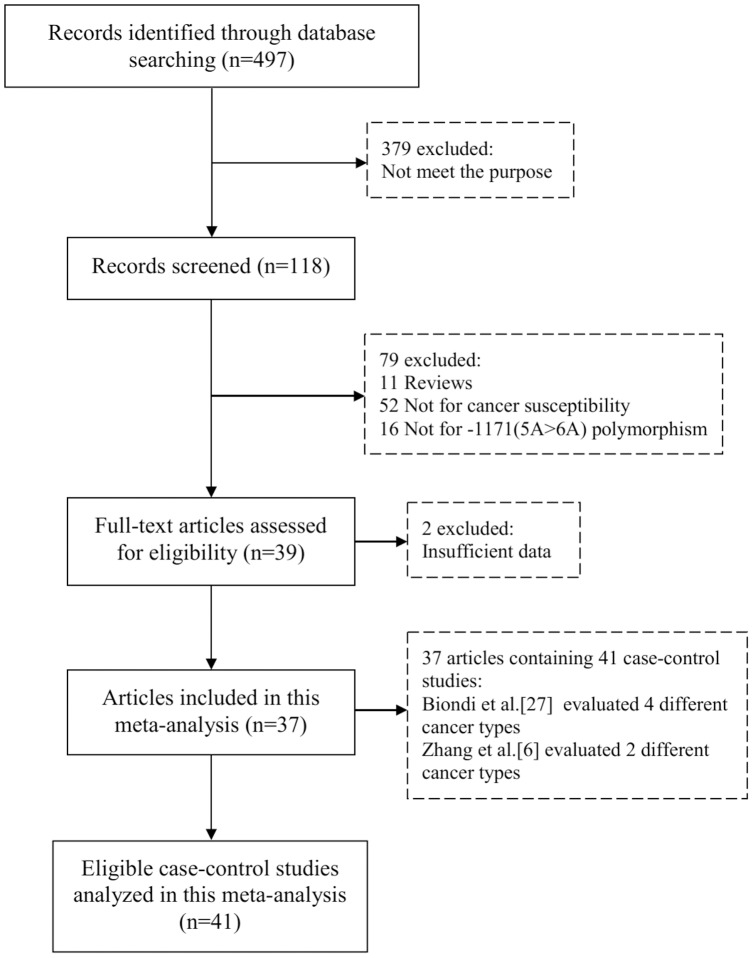
The detailed screening process was shown in Figure 1. Finally, there are a total of 41 eligible case-control studies included in this meta-analysis, containing 11112 cases and 11091 controls [6], [9], [11]–[15], [24]–[53]. In the study reported by Biondi et al. [27], the cancer types contained breast, colorectal, ovarian and lung cancer; and in another study reported by Zhang et al. [6], the cancer types included esophageal and gastric cancer. And the genotype frequencies were presented separately, thus each of them was considered as a separate study in this meta-analysis. There were 23 studies conducted in Asians, and 18 studies conducted in Caucasians. Population-based controls were used in 15 studies and hospital-based controls were used in 24 studies. There were 7 studies of large sample size and 34 studies of small sample size. The detailed characteristics of the eligible studies included in this meta-analysis are shown in Table 1.
Figure 1. PRISMA Flow Chart.
Table 1. Characteristics of Eligible Studies.
| First author | Year | Ethnicity | Cancer types | Control | Genotyping method | Cases | Controls | HWE | ||||
| 5A/5A | 5A/6A | 6A/6A | 5A/5A | 5A/6A | 6A/6A | |||||||
| Biondi [27] | 2000 | Caucasian | Breast cancer | PB | AS-PCR | 15 | 22 | 6 | 42 | 74 | 48 | 0.22 |
| Biondi [27] | 2000 | Caucasian | Colorectal cancer | PB | AS-PCR | 11 | 35 | 17 | 42 | 74 | 48 | 0.22 |
| Biondi [27] | 2000 | Caucasian | Ovarian cancer | PB | AS-PCR | 3 | 19 | 3 | 42 | 74 | 48 | 0.22 |
| Biondi [27] | 2000 | Caucasian | Lung cancer | PB | AS-PCR | 9 | 14 | 6 | 42 | 74 | 48 | 0.22 |
| Lei [26] | 2002 | Caucasian | Breast cancer | Mixed | AS-PCR | 58 | 127 | 61 | 47 | 92 | 43 | 0.88 |
| Ghilardi [11] | 2002 | Caucasian | Breast cancer | PB | AS-PCR | 24 | 47 | 15 | 22 | 54 | 34 | 0.95 |
| Hinoda [12] | 2002 | Asian | Colorectal cancer | HB | PCR-RFLP | 3 | 19 | 79 | 3 | 44 | 80 | 0.28 |
| Smolarz [13] | 2003 | Caucasian | Ovarian cancer | PB | AS-PCR | 37 | 46 | 35 | 26 | 52 | 32 | 0.59 |
| Hirata [24] | 2004 | Asian | Renal cell carcinoma | HB | PCR-RFLP | 3 | 38 | 115 | 4 | 67 | 159 | 0.31 |
| Hashimoto [25] | 2004 | Asian | Head and neck cancer | HB | PCR-RFLP | 3 | 30 | 107 | 5 | 63 | 155 | 0.63 |
| Zinzindohoue [14] | 2004 | Caucasian | Head and neck cancer | HB | AS-PCR | 36 | 70 | 19 | 60 | 121 | 68 | 0.67 |
| Krippl [28] | 2004 | Caucasian | Breast cancer | PB | Taqman | 103 | 259 | 138 | 115 | 233 | 145 | 0.26 |
| Zhang [6] | 2004 | Asian | Esophageal cancer | HB | PCR-RFLP | 1 | 73 | 160 | 8 | 105 | 237 | 0.36 |
| Zhang [6] | 2004 | Asian | Gastric cancer | HB | PCR-RFLP | 5 | 42 | 136 | 8 | 105 | 237 | 0.36 |
| Okamoto [33] | 2005 | Asian | Hepatocellular carcinoma | HB | PCR-RFLP | NA | NA | 60 | NA | NA | 137 | NA |
| Fang [9] | 2005 | Asian | Lung cancer | HB | PCR-RFLP | 7 | 73 | 163 | 8 | 105 | 237 | 0.36 |
| Li [34] | 2005 | Asian | Ovarian cancer | PB | PCR-RFLP | 4 | 53 | 94 | 4 | 34 | 84 | 0.81 |
| Kader [31] | 2006 | Caucasian | Bladder cancer | HB | Taqman | 134 | 285 | 136 | 136 | 277 | 143 | 0.94 |
| Elander [30] | 2006 | Caucasian | Colorectal cancer | PB | PCR-RFLP | 37 | 52 | 38 | 48 | 115 | 45 | 0.13 |
| Su [15] | 2006 | Caucasian | Lung cancer | FASB | Taqman | 485 | 1012 | 517 | 325 | 648 | 350 | 0.47 |
| Tu [42] | 2006 | Asian | Oral cancer | HB | AS-PCR | 0 | 31 | 119 | 1 | 12 | 85 | 0.45 |
| Lievre [29] | 2006 | Caucasian | Colorectal cancer | HB | AS-PCR | 158 | 271 | 166 | 130 | 291 | 126 | 0.13 |
| Li [39] | 2006 | Asian | Ovarian cancer | HB | PCR-RFLP | 4 | 34 | 84 | 4 | 53 | 94 | 0.28 |
| Xu [32] | 2006 | Asian | Colorectal cancer | HB | PCR-RFLP | 1 | 23 | 102 | 1 | 27 | 98 | 0.56 |
| Lu [43] | 2007 | Asian | Brain astrocytoma | HB | PCR-RFLP | 5 | 71 | 145 | 8 | 109 | 249 | 0.32 |
| Vairaktaris [35] | 2007 | Caucasian | Oral cancer | PB | PCR-RFLP | 36 | 40 | 84 | 30 | 51 | 75 | <0.01 |
| Woo [36] | 2007 | Asian | Colorectal cancer | PB | PCR-RFLP | 5 | 52 | 128 | 4 | 69 | 231 | 0.65 |
| Zhou [37] | 2007 | Asian | Nasopharyngeal carcinoma | PB | AS-PCR | 8 | 149 | 635 | 5 | 154 | 604 | 0.15 |
| Lei [40] | 2007 | Caucasian | Breast cancer | PB | Taqman | 203 | 478 | 273 | 206 | 478 | 262 | 0.66 |
| Zhai [38] | 2007 | Asian | Hepatocellular carcinoma | HB | AS-PCR | 8 | 64 | 360 | 3 | 77 | 399 | 0.73 |
| Nishizawa [41] | 2007 | Asian | Oral cancer | HB | Taqman | 3 | 50 | 117 | 8 | 54 | 102 | 0.81 |
| Han [51] | 2008 | Asian | Cervical cancer | HB | AS-PCR | 1 | 16 | 43 | 3 | 35 | 62 | 0.46 |
| Vairaktaris [45] | 2009 | Caucasian | Oral cancer | PB | PCR-RFLP | 36 | 84 | 40 | 30 | 75 | 51 | 0.80 |
| Okamoto [50] | 2010 | Asian | Hepatocellular carcinoma | HB | PCR-RFLP | 3 | 29 | 60 | 4 | 27 | 55 | 0.77 |
| Yi [47] | 2010 | Asian | Endometrial cancer | HB | PCR-RFLP | 4 | 35 | 79 | 6 | 51 | 172 | 0.35 |
| Chaudhary [49] | 2010 | Asian | Head and neck cancer | HB | PCR-RFLP | 6 | 23 | 106 | 2 | 14 | 110 | 0.07 |
| Fakhoury [44] | 2012 | Asian | Lung cancer | PB | PCR-RFLP | 26 | 15 | 0 | 20 | 24 | 7 | 0.96 |
| Gonzalez-Arriaga [46] | 2012 | Caucasian | Lung cancer | HB | PCR-RFLP | 164 | 367 | 185 | 119 | 276 | 139 | 0.42 |
| Dey [48] | 2012 | Asian | Gastric cancer | HB | PCR-RFLP | 16 | 70 | 132 | 7 | 38 | 130 | 0.06 |
| Motovali-Bashi [53] | 2012 | Asian | Colorectal cancer | HB | PCR-RFLP | 54 | 55 | 11 | 24 | 50 | 26 | 1.00 |
| Grudny [52] | 2013 | Caucasian | Lung cancer | HB | PCR-RFLP | 16 | 19 | 18 | 9 | 36 | 9 | 0.01 |
PB: population-based; HB: hospital-based; FASB: friends and spouse-based; HWE: Hardy–Weinberg equilibrium.
Association between -1171(5A>6A) polymorphism and Overall Cancers Risk
As shown in Table 2, we found no significant association of the -1171(5A>6A) polymorphism in MMP3 promoter region with overall cancer risk in any of four models.
Table 2. Meta-analysis Results.
| 6A/6A vs. 5A/5A | 6A/5A vs. 5A/5A | 6A/6A+6A/5A vs. 5A/5A | 6A/6A vs. 6A/5A+5A/5A | ||||||||
| N | OR | I2 | OR | I2 | OR | I2 | OR | I2 | |||
| Total | 41 | 0.92(0.84, 1.01) | 23.7% | 0.95(0.87, 1.03) | 14.4% | 0.94(0.87, 1.01) | 14.2% | 0.94(0.85, 1.04) | 56.2% | ||
| Cancer Types | |||||||||||
| GIC | 9 | 0.86(0.68, 1.09) | 57.3% | 0.74(0.60, 0.91) * | 1.9% | 0.77(0.64, 0.94) * | 29.0% | 0.99(0.70, 1.38) | 77.9% | ||
| GC | 6 | 0.86(0.53, 1.39) | 0.0% | 1.00(0.64, 1.55) | 26.2% | 0.93(0.61, 1.42) | 0.0% | 0.92(0.66, 1.29) | 46.4% | ||
| BC | 5 | 0.98(0.81, 1.18) | 55.3% | 1.06(0.90, 1.25) | 0.0% | 1.03(0.88, 1.20) | 9.8% | 0.87(0.66, 1.13) | 54.1% | ||
| HNC | 3 | 0.51(0.29, 0.88) * | 0.0% | 0.91(0.57, 1.44) | 0.0% | 0.75(0.49, 1.16) | 0.0% | 0.73(0.35, 1.52) | 80.3% | ||
| HC | 3 | 0.61(0.24, 1.60) | 49.2% | 0.58(0.22, 1.55) | 51.0% | 0.61(0.24, 1.58) | 50.3% | 0.80(0.48, 1.32) | 66.8% | ||
| LC | 6 | 0.95(0.81, 1.11) | 0.0% | 0.97(0.84, 1.12) | 44.1% | 0.96(0.84, 1.09) | 39.4% | 0.99(0.80, 1.22) | 39.8% | ||
| OC | 4 | 0.94(0.63, 1.40) | 40.1% | 0.93(0.62, 1.38) | 35.8% | 0.94(0.65, 1.35) | 25.9% | 0.94(0.64, 1.38) | 55.1% | ||
| Others | 5 | 0.99(0.74, 1.34) | 0.0% | 1.05(0.81, 1.37) | 0.0% | 1.03(0.80, 1.32) | 0.0% | 1.01(0.88, 1.16) | 0.0% | ||
| Ethnicity | |||||||||||
| Caucasian | 18 | 0.96(0.87, 1.06) | 4.3% | 0.97(0.89, 1.06) | 33.7% | 0.97(0.89, 1.05) | 1.8% | 0.95(0.83,1.09) | 49.3% | ||
| Asian | 23 | 0.68(0.52, 0.90) * | 26.7% | 0.75(0.58, 0.98) * | 0.0% | 0.69(0.54, 0.88) * | 0.5% | 0.93(0.79, 1.09) | 61.8% | ||
| Smoking status | |||||||||||
| Smoker | 7 | 0.95(0.75, 1.19) | 0.0% | 0.93(0.76, 1.15) | 51.1% | 0.94(0.77, 1.14) | 0.0% | 1.51(0.78, 2.92) | 90.7% | ||
| Non-smoker | 6 | 1.01(0.84, 1.21) | 0.0% | 1.01(0.86, 1.19) | 11.4% | 1.01(0.87, 1.18) | 0.0% | 1.92(1.25, 2.96) * | 72.7% | ||
| Genotyping method | |||||||||||
| PCR-RFLP | 23 | 0.81(0.67, 0.97) * | 18.9% | 0.78(0.66, 0.92) * | 0.0% | 0.78(0.66, 0.91) * | 0.0% | 0.94(0.80, 1.12) | 64.4% | ||
| AS-PCR | 13 | 0.83(0.68, 1.02) | 31.6% | 0.89(0.75, 1.06) | 23.5% | 0.88(0.74, 1.03) | 7.4% | 0.86(0.69, 1.07) | 55.0% | ||
| Taqman | 5 | 1.03(0.90, 1.17) | 0.0% | 1.07(0.95, 1.20) | 0.0% | 1.05(0.94, 1.17) | 0.0% | 0.99(0.89, 1.09) | 0.0% | ||
| Source of Controls | |||||||||||
| PB | 15 | 0.90(0.77, 1.05) | 18.8% | 0.96(0.83, 1.10) | 30.4% | 0.93(0.82, 1.07) | 24.5% | 0.88(0.74, 1.04) | 44.1% | ||
| HB | 24 | 0.88(0.76, 1.03) | 32.3% | 0.87(0.76, 1.00) | 0.3% | 0.87(0.76, 0.99) * | 7.2% | 0.97(0.83, 1.13) | 64.8% | ||
| FASB | 1 | 0.99(0.81, 1.20) | NA | 1.05(0.88, 1.24) | NA | 1.03(0.87, 1.21) | NA | 0.96(0.82, 1.12) | NA | ||
| Mixed | 1 | 1.15(0.66, 1.99) | NA | 1.12(0.70, 1.79) | NA | 1.13(0.72, 1.76) | NA | 1.07(0.68, 1.67) | NA | ||
| Sample Size | |||||||||||
| Largea | 7 | 1.01(0.90, 1.13) | 0.0% | 1.00(0.91, 1.11) | 4.5% | 1.10(0.92, 1.11) | 0.0% | 1.01(0.93, 1.10) | 0.0% | ||
| Smallb | 34 | 0.75(0.63, 0.88) * | 24.3% | 0.83(0.72, 0.97) * | 7.8% | 0.79(0.69, 0.91) * | 6.9% | 0.90(0.77, 1.04) | 61.0% | ||
GIC: Gastrointestinal cancer; GC: Gynecological cancer; BC: Breast cancer; HNC: Head and neck cancer; HC: Hepatocellular carcinoma; LC: Lung cancer; OC: Oral cancer; PB: population-based; HB: hospital-based; FASB: friends and spouse-based ; N: number of studies included; OR: odds ratio;
OR with statistical significance; a: studies with more than 1000 participants; b: studies with less than 1000 participants.
Stratified Analyses
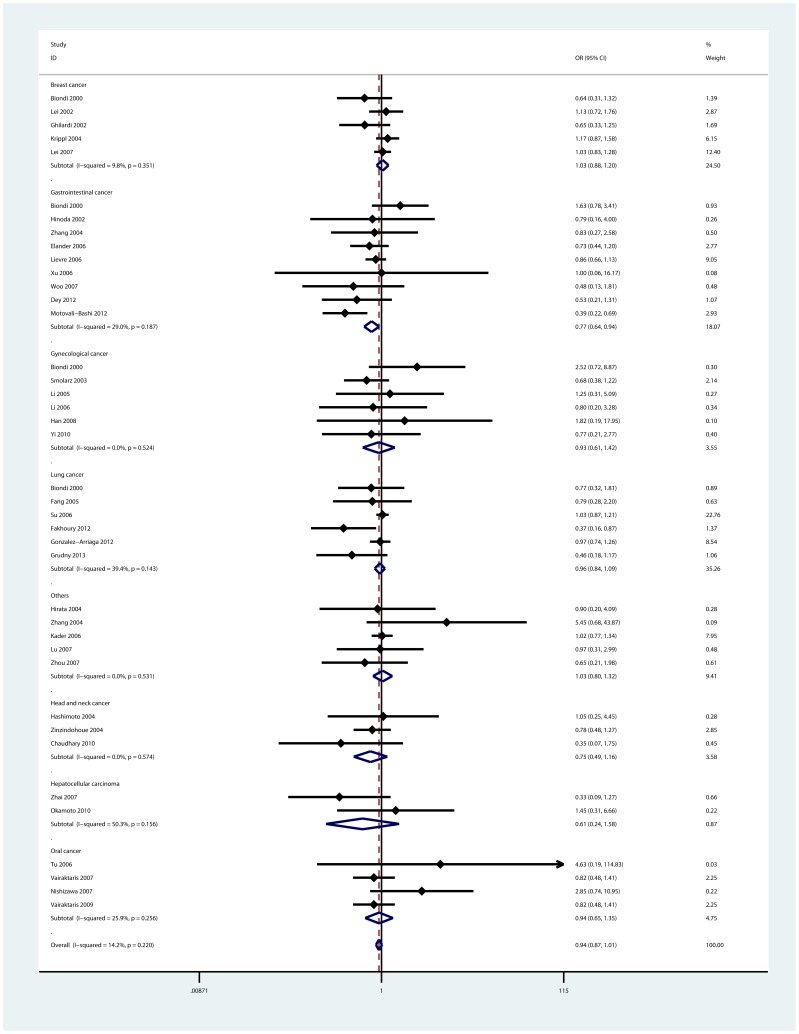
When stratified by cancer types, it was found that individuals with the 6A allele had lower risk of gastrointestinal cancer in two models: heterozygote comparison (6A/5A vs. 5A/5A: OR = 0.74, 95%CI: 0.60—0.91; I2 = 1.9%), and dominant model (6A/6A+6A/5A vs. 5A/5A: OR = 0.77, 95%CI: 0.64—0.94; I2 = 29.0%, Figure 2). In addition, we also found the -1171(5A>6A) polymorphism was associated with decreased risk of head and neck cancer in homozygote comparison (6A/6A vs. 5A/5A, OR = 0.51, 95%CI: 0.29—0.88; I2 = 0.0%). However, no significant association was observed for other cancer types.
Figure 2. Forest plot of dominant model for overall comparison by cancer types (6A/6A+6A/5A vs. 5A/5A).
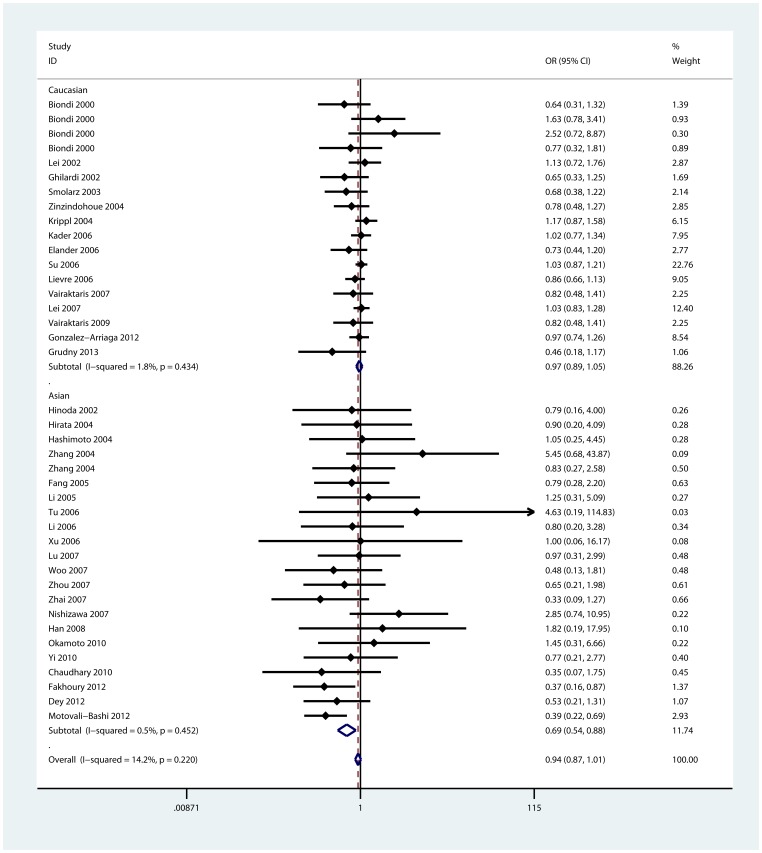
In the stratified analyses by ethnicities, the associations were significant in Asian population for three models: homozygote comparison (6A/6A vs. 5A/5A, OR = 0.68, 95%CI: 0.52—0.90; I2 = 26.7%), heterozygote comparison (6A/5A vs. 5A/5A: OR = 0.75, 95%CI: 0.58—0.98; I2 = 0.0%), and dominant model (6A/6A+6A/5A vs. 5A/5A: OR = 0.69, 95%CI: 0.54—0.88; I2 = 0.5%, Figure 3). But in Caucasian population, there were no significant associations found by this sub-group analysis. In terms of sub-group analyses by genotyping method and sample size, we found significant decreased risk of cancer in the studies using PCR-RFLP method and the studies of small sample size for three models(Table 2).
Figure 3. Forest plot of dominant model for overall comparison by ethnicities (6A/6A+6A/5A vs. 5A/5A).
Sensitivity Analyses and Publication Bias
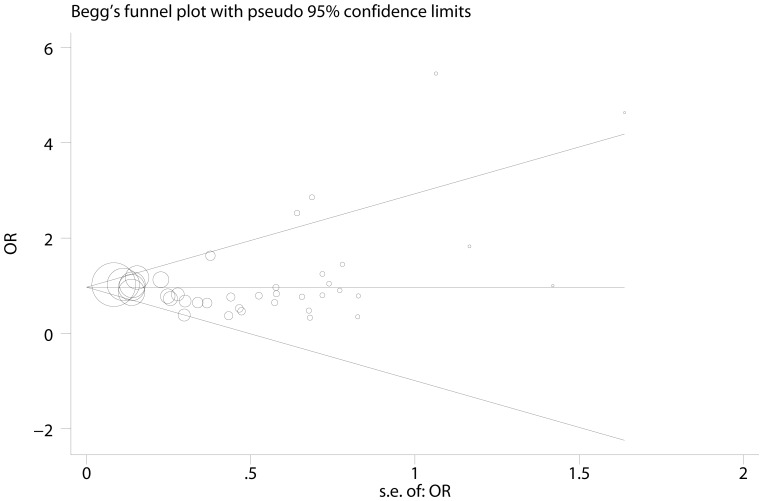
One single study involved in this meta-analysis was deleted each time to reflect the influence of the individual dataset to the pooled ORs [54], and the corresponding pooled ORs were not altered (Figure S1), suggesting stability of the meta-analyses. Begg’s funnel plot and Egger’s test were performed to assess the publication bias of studies. The shape of Begg’s funnel plot was roughly symmetrical (Figure 4). The statistical results still did not show publication bias by Egger’s test (p = 0.682).
Figure 4. Funnel plot analysis to detect publication bias in 41 eligible studies.
Discussion
To our knowledge, the first meta-analysis which provided comprehensive assessment of the -1171(5A>6A) polymorphism in MMP3 promoter region and cancer risk was performed in 2010 [55]. Compared with previous meta-analysis, we updated 15 new studies(41 vs. 26). In this meta-analysis, 41 eligible studies, including 11112 cases and 11091 controls, were included and analyzed. Although numerous studies supported -1171(5A>6A) polymorphism could decrease different cancer risk, while the pooled ORs of this meta-analysis failed to confirm this association. It is worth noting that the association was significant in Asian population when compared with Caucasian population, especially in gastrointestinal cancer.
MMP3 is known to play a key role in both local invasiveness and metastasis, the latter of which involves the ability of neoplastic cells to cross the basal membrane of both the epithelium and the vascular endothelium. This is due to MMP3 can also activate gelatinase B and the collagenases and release several cell surface molecules, including E-cadherin, a known contributor to cancer development [56]. MMP3 overexpression by some cancer types is consistent with this hypothesis [57]. Apoptosis is suppressed in the presence of intact ECM basement membrane [58]. MMPs may therefore be involved in apoptosis by their ability to degrade the ECM. The insertion of an adenosine in the MMP3 gene promoter sequence halves its transcriptional activity [10]. It is conceivable that the higher transcriptional activity associated with the 5A allele may enhance tumor invasiveness. It was confirmed in this meta-analysis.
Among 41 eligible studies, carriers of the variant 6A allele were only reported with a significantly decreased cancer risk compared with those of 5A allele in gastrointestinal cancer [6], [12], [15], [27], [29], [30], [36], [48], [53]. In dominant model, there was only one study suggested the 5A allele significantly contributed to the susceptibility of lung cancer [44], but the pooled ORs failed to confirm the association in each corresponding group classified by cancer types. Furthermore, we found a significant association in head and neck cancer for homozygote comparison.
When stratified by ethnicities, we found the association between the -1171(5A>6A) polymorphism in MMP3 promoter region and cancer risk was only significant in Asians for three genetic models. The differences may be explained by genetic diversities, such as different risk factors in life styles, and various of environmental exposure [59]–[61]. Additionally, in the sub-group analysis of genotyping method, the positive result was only observed in studies using PCR-RFLP method, but not in studies using AS-PCR or Taqman method. Thus, the differences in methodology might contribute to the results in this meta-analysis.
Further analyses showed few significant results in studies of different smoking status. However, we had a contrary finding in non-smokers: the variant 6A/6A homozygote might statistically increase cancer risk compared with 6A/5A+5A/5A genotype(OR = 1.92, 95%CI: 1.25—2.96; I2 = 72.7%), which seemed to be in confliction with the previous single studies [9], [35]. The conventional view was that the genotypes containing the wild 5A allele might remarkably increase the risk of oral and lung cancer development in smokers. One possible explanation is that the effect of MMPs polymorphisms on cancer risk may be overwhelmed by the effect of cigarette smoking among smokers. Alternatively, cigarettes smoking is a major source of extracellular matrix and may induce mRNA levels of MMPs and tissue inhibitors of metalloproteases [62]. Therefore, the effect of polymorphisms affecting expression of MMP genes in smokers may depend upon the balance between MMPs and tissue inhibitors of metalloproteases [15].
Heterogeneity between studies in each model is shown in Table 2. The source of heterogeneity across studies was explored among covariables, such as cancer types, ethnicities, source of controls, sample size and genotyping method. Meta-regression results revealed that no covariables contributed to the heterogeneity across studies in the overall result. However, sub-group analyses suggested the cancer types and sample size might be the main source of heterogeneity in this meta-analysis. The studies of small sample size may contribute to a small-study effect, in which effects reported are larger, and lead to between studies variance. Publication biases were assessed by Begg’s funnel plots and their symmetries were further evaluated by Egger’s linear regression tests. The data suggested that no evident biases were observed, indicating the credibility and stability of the results.
Several limitations of this meta-analysis should be addressed. First, individual data was not available and a more precise analysis should be conducted on other covariates such as age, sex, and environmental factors. Secondly, the sample size was still relatively small for some stratified analyses. In spite of these limitations, we included 11112 cases and 11091 controls in this meta-analysis, which can increase the statistical power and strengthen the reliability of results.
In conclusion, we demonstrate that the -1171(5A>6A) polymorphism in MMP3 promoter region is not associated with overall cancer risk, but it may contribute to decreased cancer risk in Asian population when compared with Caucasian population and significantly reduce the risk of gastrointestinal cancer. To confirm these results, large scale case-control studies are required.
Supporting Information
Sensitivity Analyses. The pooled odds ratios were calculated by omitting each data set at a time.
(TIF)
PRISMA checklist.
(DOC)
Funding Statement
This work was supported by the National Natural Science Foundation of China (81201830, 81372321), and the special fund of Jiangsu Key laboratory of Cancer Molecular Biology and Translational Medicine(BM2013007). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Basset P, Okada A, Chenard MP, Kannan R, Stoll I, et al. (1997) Matrix metalloproteinases as stromal effectors of human carcinoma progression: therapeutic implications. Matrix Biol 15: 535–541. [DOI] [PubMed] [Google Scholar]
- 2. Johnsen M, Lund LR, Romer J, Almholt K, Dano K (1998) Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr Opin Cell Biol 10: 667–671. [DOI] [PubMed] [Google Scholar]
- 3. Curran S, Murray GI (2000) Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. European journal of cancer (Oxford, England : 1990) 36: 1621–1630. [DOI] [PubMed] [Google Scholar]
- 4. Massova I, Kotra LP, Fridman R, Mobashery S (1998) Matrix metalloproteinases: structures, evolution, and diversification. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 12: 1075–1095. [PubMed] [Google Scholar]
- 5. Somerville RP, Oblander SA, Apte SS (2003) Matrix metalloproteinases: old dogs with new tricks. Genome Biol 4: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang J, Jin X, Fang S, Li Y, Wang R, et al. (2004) The functional SNP in the matrix metalloproteinase-3 promoter modifies susceptibility and lymphatic metastasis in esophageal squamous cell carcinoma but not in gastric cardiac adenocarcinoma. Carcinogenesis 25: 2519–2524. [DOI] [PubMed] [Google Scholar]
- 7. Brinckerhoff CE, Rutter JL, Benbow U (2000) Interstitial collagenases as markers of tumor progression. Clinical cancer research : an official journal of the American Association for Cancer Research 6: 4823–4830. [PubMed] [Google Scholar]
- 8. Van Themsche C, Potworowski EF, St-Pierre Y (2004) Stromelysin-1 (MMP-3) is inducible in T lymphoma cells and accelerates the growth of lymphoid tumors in vivo. Biochem Biophys Res Commun 315: 884–891. [DOI] [PubMed] [Google Scholar]
- 9. Fang S, Jin X, Wang R, Li Y, Guo W, et al. (2005) Polymorphisms in the MMP1 and MMP3 promoter and non-small cell lung carcinoma in North China. Carcinogenesis 26: 481–486. [DOI] [PubMed] [Google Scholar]
- 10. Ye S, Watts GF, Mandalia S, Humphries SE, Henney AM (1995) Preliminary report: genetic variation in the human stromelysin promoter is associated with progression of coronary atherosclerosis. British heart journal 73: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghilardi G, Biondi ML, Caputo M, Leviti S, DeMonti M, et al. (2002) A single nucleotide polymorphism in the matrix metalloproteinase-3 promoter enhances breast cancer susceptibility. Clin Cancer Res 8: 3820–3823. [PubMed] [Google Scholar]
- 12. Hinoda Y, Okayama N, Takano N, Fujimura K, Suehiro Y, et al. (2002) Association of functional polymorphisms of matrix metalloproteinase (MMP)-1 and MMP-3 genes with colorectal cancer. Int J Cancer 102: 526–529. [DOI] [PubMed] [Google Scholar]
- 13. Smolarz B, Szyllo K, Romanowicz-Makowska H, Niewiadomski M, Kozlowska E, et al. (2003) PCR analysis of matrix metalloproteinase 3 (MMP-3) gene promoter polymorphism in ovarian cancer. Pol J Pathol 54: 233–238. [PubMed] [Google Scholar]
- 14. Zinzindohoue F, Blons H, Hans S, Loriot MA, Houllier AM, et al. (2004) Single nucleotide polymorphisms in MMP1 and MMP3 gene promoters as risk factor in head and neck squamous cell carcinoma. Anticancer Res 24: 2021–2026. [PubMed] [Google Scholar]
- 15. Su L, Zhou W, Asomaning K, Lin X, Wain JC, et al. (2006) Genotypes and haplotypes of matrix metalloproteinase 1, 3 and 12 genes and the risk of lung cancer. Carcinogenesis 27: 1024–1029. [DOI] [PubMed] [Google Scholar]
- 16. Pan HF, Leng RX, Ye DQ (2011) Lack of association of interleukin-18 gene promoter -607 A/C polymorphism with susceptibility to autoimmune diseases: a meta-analysis. Lupus 20: 945–951. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Altman DG, Liberati A, Tetzlaff J (2011) PRISMA statement. Epidemiology (Cambridge, Mass.) 22: 128; author reply 128. [DOI] [PubMed]
- 18. Qiu MT, Hu JW, Ding XX, Yang X, Zhang Z, et al. (2012) Hsa-miR-499 rs3746444 Polymorphism Contributes to Cancer Risk: A Meta-Analysis of 12 Studies. PLoS One 7: e50887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boissel JP, Sacks HS, Leizorovicz A, Blanchard J, Panak E, et al. (1988) Meta-analysis of clinical trials: summary of an international conference. Eur J Clin Pharmacol 34: 535–538. [DOI] [PubMed] [Google Scholar]
- 20. Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127: 820–826. [DOI] [PubMed] [Google Scholar]
- 21. Yang X, Qiu MT, Hu JW, Wang XX, Jiang F, et al. (2013) GSTT1 null genotype contributes to lung cancer risk in asian populations: a meta-analysis of 23 studies. PLoS One 8: e62181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 23. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirata H, Okayama N, Naito K, Inoue R, Yoshihiro S, et al. (2004) Association of a haplotype of matrix metalloproteinase (MMP)-1 and MMP-3 polymorphisms with renal cell carcinoma. Carcinogenesis 25: 2379–2384. [DOI] [PubMed] [Google Scholar]
- 25. Hashimoto T, Uchida K, Okayama N, Imate Y, Suehiro Y, et al. (2004) Association of matrix metalloproteinase (MMP)-1 promoter polymorphism with head and neck squamous cell carcinoma. Cancer Lett 211: 19–24. [DOI] [PubMed] [Google Scholar]
- 26. Lei H, Zaloudik J, Vorechovsky I (2002) Lack of association of the -1171 (5A) allele of the MMP3 promoter with breast cancer. Clin Chem 48: 798–799. [PubMed] [Google Scholar]
- 27. Biondi ML, Turri O, Leviti S, Seminati R, Cecchini F, et al. (2000) MMP1 and MMP3 polymorphisms in promoter regions and cancer. Clin Chem 46: 2023–2024. [PubMed] [Google Scholar]
- 28. Krippl P, Langsenlehner U, Renner W, Yazdani-Biuki B, Koppel H, et al. (2004) The 5A/6A polymorphism of the matrix metalloproteinase 3 gene promoter and breast cancer. Clin Cancer Res 10: 3518–3520. [DOI] [PubMed] [Google Scholar]
- 29. Lievre A, Milet J, Carayol J, Le CD, Milan C, et al. (2006) Genetic polymorphisms of MMP1, MMP3 and MMP7 gene promoter and risk of colorectal adenoma. BMC Cancer 6: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elander N, Soderkvist P, Fransen K (2006) Matrix metalloproteinase (MMP) -1, -2, -3 and -9 promoter polymorphisms in colorectal cancer. Anticancer Res 26: 791–795. [PubMed] [Google Scholar]
- 31. Kader AK, Shao L, Dinney CP, Schabath MB, Wang Y, et al. (2006) Matrix metalloproteinase polymorphisms and bladder cancer risk. Cancer Res 66: 11644–11648. [DOI] [PubMed] [Google Scholar]
- 32. Xu E, Lai M, Lu B, Xing X, Huang Q (2006) No association between the polymorphisms in matrix metalloproteinase-1 and matrix metalloproteinase-3 promoter regions and colorectal cancer in Chinese. Dis Colon Rectum 49: 1439–1444. [DOI] [PubMed] [Google Scholar]
- 33. Okamoto K, Mandai M, Mimura K, Murawaki Y, Yuasa I (2005) The association of MMP-1, -3 and -9 genotypes with the prognosis of HCV-related hepatocellular carcinoma patients. Res Commun Mol Pathol Pharmacol 117–118: 77–89. [PubMed] [Google Scholar]
- 34.Li Y, Kang S, Guo W, Jin X (2005) Association of single nucleotide polymorphism in matrix metalloproteinases promoter with susceptibility to ovarian cancer. Chin J Obstet Gyneco : 472–475. [PubMed]
- 35. Vairaktaris E, Yapijakis C, Vasiliou S, Derka S, Nkenke E, et al. (2007) Association of -1171 promoter polymorphism of matrix metalloproteinase-3 with increased risk for oral cancer. Anticancer Res 27: 4095–4100. [PubMed] [Google Scholar]
- 36. Woo M, Park K, Nam J, Kim JC (2007) Clinical implications of matrix metalloproteinase-1, -3, -7, -9, -12, and plasminogen activator inhibitor-1 gene polymorphisms in colorectal cancer. J Gastroenterol Hepatol 22: 1064–1070. [DOI] [PubMed] [Google Scholar]
- 37. Zhou G, Zhai Y, Cui Y, Qiu W, Yang H, et al. (2007) Functional polymorphisms and haplotypes in the promoter of the MMP2 gene are associated with risk of nasopharyngeal carcinoma. Hum Mutat 28: 1091–1097. [DOI] [PubMed] [Google Scholar]
- 38. Zhai Y, Qiu W, Dong XJ, Zhang XM, Xie WM, et al. (2007) Functional polymorphisms in the promoters of MMP-1, MMP-2, MMP-3, MMP-9, MMP-12 and MMP-13 are not associated with hepatocellular carcinoma risk. Gut 56: 445–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Y, Jin X, Kang S, Wang Y, Du H, et al. (2006) Polymorphisms in the promoter regions of the matrix metalloproteinases-1, -3, -7, and -9 and the risk of epithelial ovarian cancer in China. Gynecol Oncol 101: 92–96. [DOI] [PubMed] [Google Scholar]
- 40. Lei H, Hemminki K, Altieri A, Johansson R, Enquist K, et al. (2007) Promoter polymorphisms in matrix metalloproteinases and their inhibitors: few associations with breast cancer susceptibility and progression. Breast Cancer Res Treat 103: 61–69. [DOI] [PubMed] [Google Scholar]
- 41. Nishizawa R, Nagata M, Noman AA, Kitamura N, Fujita H, et al. (2007) The 2G allele of promoter region of matrix metalloproteinase-1 as an essential pre-condition for the early onset of oral squamous cell carcinoma. BMC Cancer 7: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tu HF, Liu CJ, Chang CS, Lui MT, Kao SY, et al. (2006) The functional (-1171 5A-->6A) polymorphisms of matrix metalloproteinase 3 gene as a risk factor for oral submucous fibrosis among male areca users. J Oral Pathol Med 35: 99–103. [DOI] [PubMed] [Google Scholar]
- 43. Lu ZQ, Wang YM, Cao YY, Zhang QJ, Zhang XH, et al. (2007) [Correlations of polymorphisms in matrix metalloproteinase-3 and -7 promoters to susceptibility to brain astrocytoma]. Ai Zheng 26: 463–468. [PubMed] [Google Scholar]
- 44. Fakhoury HM, Noureddine S, Tamim H, Chmaisse H, Makki R (2012) Association of MMP3-1171(5A>6A) polymorphism with lung cancer in Lebanon. Genet Test Mol Biomarkers 16: 988–990. [DOI] [PubMed] [Google Scholar]
- 45. Vairaktaris E, Serefoglou Z, Avgoustidis D, Yapijakis C, Critselis E, et al. (2009) Gene polymorphisms related to angiogenesis, inflammation and thrombosis that influence risk for oral cancer. Oral Oncol 45: 247–253. [DOI] [PubMed] [Google Scholar]
- 46. Gonzalez-Arriaga P, Pascual T, Garcia-Alvarez A, Fernandez-Somoano A, Lopez-Cima MF, et al. (2012) Genetic polymorphisms in MMP 2, 9 and 3 genes modify lung cancer risk and survival. BMC Cancer 12: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yi YC, Chou PT, Chen LY, Kuo WH, Ho ES, et al. (2010) Matrix metalloproteinase-7 (MMP-7) polymorphism is a risk factor for endometrial cancer susceptibility. Clin Chem Lab Med 48: 337–344. [DOI] [PubMed] [Google Scholar]
- 48. Dey S, Stalin S, Gupta A, Saha D, Kesh K, et al. (2012) Matrix metalloproteinase3 gene promoter polymorphisms and their haplotypes are associated with gastric cancer risk in eastern Indian population. Mol Carcinog 51 Suppl 1E42–53. [DOI] [PubMed] [Google Scholar]
- 49. Chaudhary AK, Singh M, Bharti AC, Singh M, Shukla S, et al. (2010) Synergistic effect of stromelysin-1 (matrix metalloproteinase-3) promoter (-1171 5A->6A) polymorphism in oral submucous fibrosis and head and neck lesions. BMC Cancer 10: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Okamoto K, Ishida C, Ikebuchi Y, Mandai M, Mimura K, et al. (2010) The genotypes of IL-1 beta and MMP-3 are associated with the prognosis of HCV-related hepatocellular carcinoma. Intern Med 49: 887–895. [DOI] [PubMed] [Google Scholar]
- 51. Han H, Shao S (2008) Matrix metalloprotease 3 single nucleotide polymorphisms in cervical carcinoma and its clinical significance. Journal of Shanxi Medicine 37: 1088–1090. [Google Scholar]
- 52. Grudny J, Kolakowski J, Kruszewski M, Szopinski J, Sliwinski P, et al. (2013) Association of genetic dependences between lung cancer and chronic obstructive pulmonary disease. Pneumonol Alergol Pol 81: 308–318. [PubMed] [Google Scholar]
- 53. Motovali-Bashi M, Hojati Z, Hajihoseiny S, Hemmati S (2012) The stromelysin-1 5A/5A genotype enhances colorectal cancer cell invasion in Iranian population. J Res Med Sci 17: 962–966. [PMC free article] [PubMed] [Google Scholar]
- 54. Wang J, Zou L, Song Z, Lang X, Huang S, et al. (2012) Meta-analysis of RAGE gene polymorphism and coronary heart disease risk. PLoS One 7: e50790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peng B, Cao L, Wang W, Xian L, Jiang D, et al. (2010) Polymorphisms in the promoter regions of matrix metalloproteinases 1 and 3 and cancer risk: a meta-analysis of 50 case-control studies. Mutagenesis 25: 41–48. [DOI] [PubMed] [Google Scholar]
- 56. Sternlicht MD, Bissell MJ, Werb Z (2000) The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene 19: 1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chambers AF, Matrisian LM (1997) Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 89: 1260–1270. [DOI] [PubMed] [Google Scholar]
- 58. Boudreau N, Sympson CJ, Werb Z, Bissell MJ (1995) Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science (New York, N.Y.) 267: 891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wynder EL (1992) Cancer prevention: optimizing life-styles with special reference to nutritional carcinogenesis. J Natl Cancer Inst Monogr : 87–91. [PubMed]
- 60.Fathy M, Hamed M, Youssif O, Fawzy N, Ashour W (2013) Association Between Environmental Tobacco Smoke Exposure and Lung Cancer Susceptibility: Modification by Antioxidant Enzyme Genetic Polymorphisms. Mol Diagn Ther. [DOI] [PubMed]
- 61. Li D, Walcott FL, Chang P, Zhang W, Zhu J, et al. (2002) Genetic and environmental determinants on tissue response to in vitro carcinogen exposure and risk of breast cancer. Cancer Res 62: 4566–4570. [PubMed] [Google Scholar]
- 62. Yin L, Morita A, Tsuji T (2000) Alterations of extracellular matrix induced by tobacco smoke extract. Arch Dermatol Res 292: 188–194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity Analyses. The pooled odds ratios were calculated by omitting each data set at a time.
(TIF)
PRISMA checklist.
(DOC)