Abstract
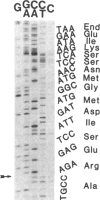
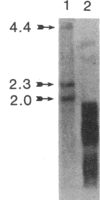
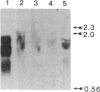
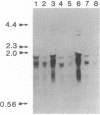
Several recombinant cDNA clones specific for the mitochondrial iron-sulfur protein adrenodoxin have been identified in a bovine adrenocortical cDNA library. One clone (pBAdx4) contains a 900-base-pair insert that includes the entire amino acid coding region of the adrenodoxin precursor protein. The amino acid sequence of mature adrenodoxin deduced from the nucleotide sequence of pBAdx4 is identical with that determined by protein sequencing except for three amide changes. The previously undetermined sequence of the adrenodoxin NH2-terminal precursor segment (58 amino acids) contains several basic residues, a characteristic feature of the precursor segment of proteins destined for mitochondria. In addition, a 14 amino acid extension is present at the COOH terminus of the mature adrenodoxin sequence. Whether this represents a COOH-terminal precursor segment is not clear. Three different adrenodoxin mRNAs are present [1.75, 1.4, and 0.95 kilobase(s) long] in bovine adrenocortical RNA. RNA from bovine corpus luteum, liver, and kidney contains transcripts that hybridize to adrenodoxin cDNA. Accumulation of adrenodoxin mRNA occurs in cultured bovine adrenocortical cells after treatment with ACTH or dibutyryl-cAMP, similar to that observed for the mitochondrial steroid hydroxylases that it services--namely, the cholesterol side-chain-cleavage cytochrome P-450 and the steroid 11 beta-hydroxylase cytochrome P-450.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns D. M., Beacham I. R. A method for the ligation of DNA following isolation from low melting temperature agarose. Anal Biochem. 1983 Nov;135(1):48–51. doi: 10.1016/0003-2697(83)90728-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fessler L. I., Morris N. P., Fessler J. H. Procollagen: biological scission of amino and carboxyl extension peptides. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4905–4909. doi: 10.1073/pnas.72.12.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geren L. M., O'Brien P., Stonehuerner J., Millett F. Identification of specific carboxylate groups on adrenodoxin that are involved in the interaction with adrenodoxin reductase. J Biol Chem. 1984 Feb 25;259(4):2155–2160. [PubMed] [Google Scholar]
- Hiwatashi A., Nishii Y., Ichikawa Y. Purification of cytochrome P-450D1 alpha (25-hydroxyvitamin D3-1 alpha-hydroxylase) of bovine kidney mitochondria. Biochem Biophys Res Commun. 1982 Mar 15;105(1):320–327. doi: 10.1016/s0006-291x(82)80047-8. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Williams K. R., Kalousek F., Kraus J. P., Doolittle R. F., Konigsberg W., Rosenberg L. E. Structure and expression of a complementary DNA for the nuclear coded precursor of human mitochondrial ornithine transcarbamylase. Science. 1984 Jun 8;224(4653):1068–1074. doi: 10.1126/science.6372096. [DOI] [PubMed] [Google Scholar]
- John M. E., John M. C., Ashley P., MacDonald R. J., Simpson E. R., Waterman M. R. Identification and characterization of cDNA clones specific for cholesterol side-chain cleavage cytochrome P-450. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5628–5632. doi: 10.1073/pnas.81.18.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M. E., John M. C., Simpson E. R., Waterman M. R. Regulation of cytochrome P-45011 beta gene expression by adrenocorticotropin. J Biol Chem. 1985 May 10;260(9):5760–5767. [PubMed] [Google Scholar]
- Kimura T., Suzuki K., Padmanabhan R., Samejima T., Tarutani O., Ui N. Studies on steroid hydroxylase. Molecular properties of adrenal iron-sulfur protein. Biochemistry. 1969 Oct;8(10):4027–4031. doi: 10.1021/bi00838a020. [DOI] [PubMed] [Google Scholar]
- Kramer R. E., Anderson C. M., Peterson J. A., Simpson E. R., Waterman M. R. Adrenodoxin biosynthesis by bovine adrenal cells in monolayer culture. Induction by adrenocorticotropin. J Biol Chem. 1982 Dec 25;257(24):14921–14925. [PubMed] [Google Scholar]
- Kramer R. E., Du Bois R. N., Simpson E. R., Anderson C. M., Kashiwagi K., Lambeth J. D., Jefcoate C. R., Waterman M. R. Cell-free synthesis of precursor forms of mitochondrial steroid hydroxylase enzymes of the bovine adrenal cortex. Arch Biochem Biophys. 1982 May;215(2):478–485. doi: 10.1016/0003-9861(82)90106-0. [DOI] [PubMed] [Google Scholar]
- Kramer R. E., Rainey W. E., Funkenstein B., Dee A., Simpson E. R., Waterman M. R. Induction of synthesis of mitochondrial steroidogenic enzymes of bovine adrenocortical cells by analogs of cyclic AMP. J Biol Chem. 1984 Jan 25;259(2):707–713. [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth J. D., Geren L. M., Millett F. Adrenodoxin interaction with adrenodoxin reductase and cytochrome P-450scc. Cross-linking of protein complexes and effects of adrenodoxin modification by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide. J Biol Chem. 1984 Aug 25;259(16):10025–10029. [PubMed] [Google Scholar]
- Marder J. B., Goloubinoff P., Edelman M. Molecular architecture of the rapidly metabolized 32-kilodalton protein of photosystem II. Indications for COOH-terminal processing of a chloroplast membrane polypeptide. J Biol Chem. 1984 Mar 25;259(6):3900–3908. [PubMed] [Google Scholar]
- Maruya N., Hiwatashi A., Ichikawa Y., Yamano T. Purification and characterization of renal ferredoxin from bovine renal mitochondria. J Biochem. 1983 May;93(5):1239–1247. doi: 10.1093/oxfordjournals.jbchem.a134258. [DOI] [PubMed] [Google Scholar]
- Matocha M. F., Waterman M. R. Discriminatory processing of the precursor forms of cytochrome P-450scc and adrenodoxin by adrenocortical and heart mitochondria. J Biol Chem. 1984 Jul 10;259(13):8672–8678. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morohashi K., Fujii-Kuriyama Y., Okada Y., Sogawa K., Hirose T., Inayama S., Omura T. Molecular cloning and nucleotide sequence of cDNA for mRNA of mitochondrial cytochrome P-450(SCC) of bovine adrenal cortex. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4647–4651. doi: 10.1073/pnas.81.15.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi N., Ishikawa T., Ohashi M., Omura T. Contributions of cytoplasmic free and membrane-bound ribosomes to the synthesis of NADPH-adrenodoxin reductase and adrenodoxin of bovine adrenal cortex mitochondria. J Biochem. 1983 Nov;94(5):1505–1515. [PubMed] [Google Scholar]
- Nabi N., Omura T. In vitro synthesis of adrenodoxin and adrenodoxin reductase: existence of a putative large precursor form of adrenodoxin. Biochem Biophys Res Commun. 1980 Nov 28;97(2):680–686. doi: 10.1016/0006-291x(80)90318-6. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan R., Kimura T. Studies on adrenal steroid hydroxylases. Extrinsic properties of the optical activity in adrenal iron-sulfur protein (adrenodoxin). J Biol Chem. 1970 May 25;245(10):2469–2475. [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Patzelt C., Tager H. S., Carroll R. J., Steiner D. F. Identification and processing of proglucagon in pancreatic islets. Nature. 1979 Nov 15;282(5736):260–266. doi: 10.1038/282260a0. [DOI] [PubMed] [Google Scholar]
- Sagara Y., Ito A., Omura T. Partial purification of a metalloprotease catalyzing the processing of adrenodoxin precursor in bovine adrenal cortex mitochondria. J Biochem. 1984 Dec;96(6):1743–1752. doi: 10.1093/oxfordjournals.jbchem.a135007. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Waterman M. R. Regulation by ACTH of steroid hormone biosynthesis in the adrenal cortex. Can J Biochem Cell Biol. 1983 Jul;61(7):692–707. doi: 10.1139/o83-088. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T., Kimura T. The amino acid sequence of bovine adrenodoxin. J Biol Chem. 1973 Feb 25;248(4):1141–1157. [PubMed] [Google Scholar]
- Tosi M., Young R. A., Hagenbüchle O., Schibler U. Multiple polyadenylation sites in a mouse alpha-amylase gene. Nucleic Acids Res. 1981 May 25;9(10):2313–2323. doi: 10.1093/nar/9.10.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey R. C., Stevenson P. M. Properties of ferredoxin reductase and ferredoxin from the bovine corpus luteum. Int J Biochem. 1984;16(5):489–495. doi: 10.1016/0020-711x(84)90165-4. [DOI] [PubMed] [Google Scholar]
- Waterman M. R. ACTH-mediated induction of synthesis and activity of cytochrome P-450s and related enzymes in cultured bovine adrenocortical cells. Xenobiotica. 1982 Nov;12(11):773–786. doi: 10.3109/00498258209038949. [DOI] [PubMed] [Google Scholar]
- Wikvall K. Hydroxylations in biosynthesis of bile acids. Isolation of a cytochrome P-450 from rabbit liver mitochondria catalyzing 26-hydroxylation of C27-steroids. J Biol Chem. 1984 Mar 25;259(6):3800–3804. [PubMed] [Google Scholar]