Abstract
Objective
To determine whether standard evaluations of pain distinguish subjects with no pain from those with myofascial pain syndromes (MPS) and active trigger points (MTrPs); and to assess whether self-reports of mood, function and health-related quality of life differ between these groups.
Design
Prospective, descriptive study.
Setting
University
Patients
Adults with and without neck pain
Methods
We evaluated adults with MPS and active (painful) MTrPs and those without pain. Subjects in the “Active” (‘A’) group had at least one active MTrP with spontaneous pain which was persistent, lasted more than 3 months and had characteristic pain on palpation. Subjects in the “No pain” (‘Np’) group had no spontaneous pain. However, some had discomfort on MTrP palpation (latent MTrP) while others in the Np group had no discomfort on palpation of nodules or had no nodules.
Outcome Measures
Each participant underwent range of motion (ROM) measurement, 10-point manual muscle test, and manual and algometric palpation. The latter determined the pain/pressure threshold using an algometer of 4 pre-determined anatomical sites along the upper trapezius. Participants rated pain using a verbal analogue scale (0–10); completed the Brief Pain Inventory and Oswestry Disability Scale (ODS), which included a sleep sub-scale; Short Form 36(SF36) and the Profile of Mood States (POMS).
Results
here were 24 in the ‘A’ group (mean 36 yrs, 16 women) and 26 in the ‘Np’ group (mean 26 yrs, 12 women). Subjects in group ‘A’ differed from ‘Np’ in number of latent MTrPs (p=.0062); asymmetrical cervical ROM (p=.01 side bending and p=.002 rotation); in all pain reports (p<.0001); algometry (p<.03); POMS (p<.038); SF36 (p<.01) and ODS (p<.0001).
Conclusion
A systematic musculoskeletal evaluation of people with MPS reliably distinguishes them from subjects with no pain. The two groups are significantly different in their physical findings and self-reports of pain, sleep disturbance, disability, health status and mood. These findings support the view that a “local” pain syndrome has significant associations with mood, health-related quality of life and function..
INTRODUCTION
Soft tissue pain syndromes are prevalent in our population. It is reported that 15% of routine medical clinic visits are the result of soft tissue pain [22]. The prevalence is considerably higher in pain clinics [7], estimated to account for 85% of these visits. Direct medical costs for non-cancer back pain in the United States were estimated at $90.7 billion in 1998. Lost productivity is also high. Neck pain accounts for less than 33% of all back pain [8,10,25]. What emerges from these studies is that axial pain is expensive and has a great impact on function and disability [13].
The impact of myofascial pain on an individual’s life activity is poorly understood in part because there is no agreed upon definition of myofascial pain syndromes (MPS) or definitive diagnostic criteria. Additionally, treatment response in pain syndromes relies upon the use of patient reported outcomes (PROs) that use descriptors of the pain, its frequency and intensity. These are valid measures, but their sensitivity to change and the variation of interpretation by individual patients makes quantitation difficult.
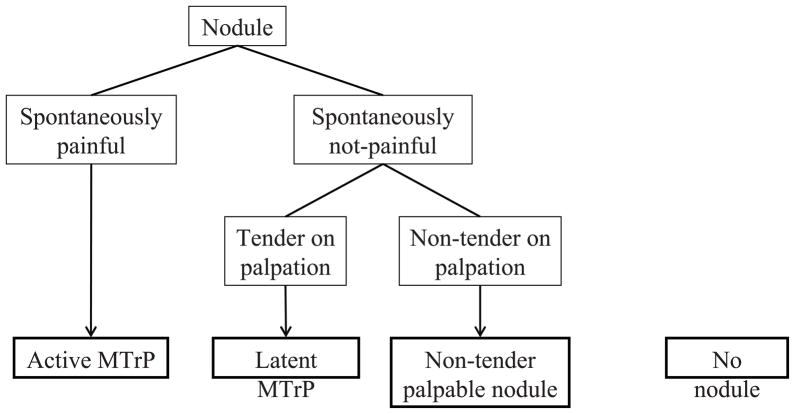
Much has been written about MPS and the myofascial trigger point (MTrP) [2,5,7], but mechanisms generating and perpetuating pain have not yet been fully understood [9,14,23]. Our group has identified a biochemical profile associated with MPS, and in particular with active as compared with latent MTrPs [21]. These findings have been correlated with the classification of MTrPs (active, latent or none), but not with the clinical picture of the MPS, which includes physical findings of cervical and upper extremity range of motion, strength and overall soft tissue palpation.
Our clinical research team is engaged in a controlled clinical trial, whose aim is to assess the pathophysiology of the MTrP. This report presents the systematic approach we use to evaluate people with active MTrPs. For purposes of this study, we recruited subjects who have had persistent cervical pain for more than 3 months and other subjects who have had no spontaneous pain. Subjects in the group with pain were determined (based on history and physical examination) to have at least one active MTrP in the upper trapezius muscle as a contributor to their spontaneous pain complaint. We adopted the classic definition of active and latent MTrPs [24]. The former is a palpable, discrete nodule within a taut band of skeletal muscle that is spontaneously painful and its palpation reproduces the typical pain. The pain may, but need not radiate. A latent MTrP is a nodule with the same physical characteristics as an active one but requires palpation to elicit pain. Some nodules are not tender to palpation 1).
We devised a systematic approach to describe the physical findings in subjects identifying spontaneous pain in the upper trapezius or neck region and those with no pain, permitting us to compare individuals with and without MPS and active MTrPs. The aim is to develop a standardized approach to assess MPS, using objective and self-report data. This paper presents results of the application of the systematic approach.
METHODS
The study was approved by the Chesapeake Institutional Review Board. Subjects were recruited by posting flyers on the campus of George Mason University, Fairfax, VA. Participants were almost exclusively faculty, students and staff of the University. The study subjects were classified as either “Active” or “No pain”. Those in the “Active” group (‘A’ group) received the standard physical examination and ultrasound imaging evaluation plus the prescribed 3 consecutive weekly treatments of dry needling into the most active MTrP (i.e., the MTrP that upon palpation reproduced and/or exacerbated the subjects’ spontaneous pain complaint; and if there were more than one active MTrP, the most symptomatic was selected). Only one was treated. Those in the “No pain” group (‘Np’ group) were control research subjects and received a physical examination and ultrasound imaging evaluation only. Subjects were entered into the ‘A’ group if they have had neck pain (upper trapezius) for more than 3 months’ duration; and their pain was present without provocation. Additionally, on physical examination there had to be a palpable nodule in the upper trapezius whose palpation reproduced or exacerbated their spontaneous pain symptoms. Radicular pain on MTrP palpation to other regions of the head and neck was acceptable, but not required for acceptance into the “Active” group. Those in the ‘Np’ group were subjects who did not have neck or low back pain. However, they could have non-tender palpable nodules or nodules that were tender to palpation. The latter are classified as latent MTrPs. The definition of a latent MTrP is a tender palpable nodule dependent upon palpation to produce local and/or referred pain. Our MTrP classification scheme is presented in Figure 1.
FIGURE 1.
Classification of Nodules Assessed by Palpation
Exclusions for study entry included: presence of chronic fatigue syndrome, Lyme disease, other chronic pain conditions, recent medication change and non-pharmacological interventions such as the use of acupuncture and chiropractic, among others. These criteria are presented in Table 1.
TABLE 1.
Inclusion, Exclusion Criteria and Standard Assessments
1. Inclusion Criteria
|
2. Exclusion Criteria:
|
3. Assessments:
|
Evaluations for all subjects included a thorough musculoskeletal history and physical examination of the neck and shoulder girdle as well as any treatment history. Medical history also included questions about medication, supplements, nature of work, leisure activity and whether the subjects participated in routine physical therapies and regular exercise. An assessment of pain was determined by asking the subject to rate the current level of pain in the neck/trapezius region of both sides, as well as a recalled average level of pain over the past week. Pain was verbally rated from 0 (none)-10 (worst possible) using an analogue scale (VAS).
The physical examination included manual palpation of the cervical spine, neck extensor muscles and trapezius. An effort was made to assess whether the tissue was homogeneous or not based on surface palpation along the upper trapezius (Figure 1). Measures of active range of motion (AROM) and manual muscle testing, using the Kendall 10 point scale, of the cervical spine and shoulders were performed with subjects in the seated position [12]. (Figure 2) Cervical range of motion was measured using a CROM™ device [28]. The subjects were told to sit erect in a straight back chair to prevent substitution movements from the thoracic and lumbar spine. They were instructed to keep their arms by their sides and to position their feet flat on the floor. Cervical range of motion was then measured in the sagittal, frontal and rotational planes. Normal cervical range of motion was determined using the standards provided by the 6th American Medical Association Guide to Permanent Impairment [19]. Assessment of the symmetry of soft tissue by visual inspection was performed. Subjects were identified as having symmetrical or asymmetrical cervical range of motion in the rotational and frontal planes. This was defined as a 10% or greater difference between the 3 planar movements to the left when compared with the right.
Figure 2.
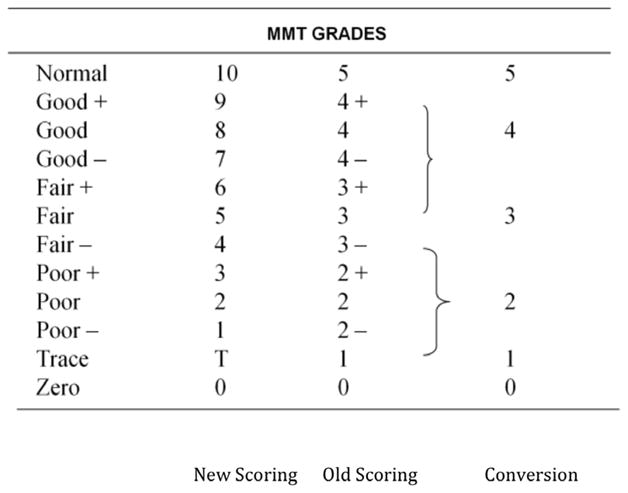
Kendall 10 Point Manual Muscle Test (MMT)
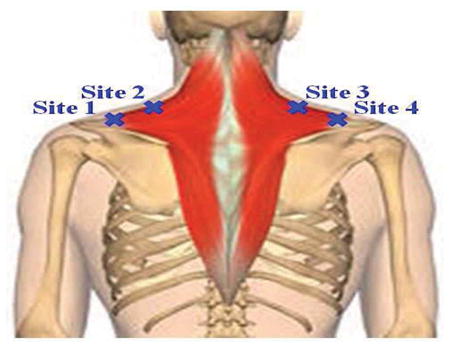
Two sites for evaluation are identified on the left and right sides of the upper trapezius (total of 4 sites). They are: 2cm medial to the acromioclavicular joint and along the medial border of the upper trapezius as it moves cephalad from the shoulder girdle (Table 2). A measure of pain pressure threshold was obtained at the 4 sites using a pressure algometer (Commander Algometer, JTech Medical, Salt Lake City, Utah) (Table 2). Subjects were instructed to identify when the algometer was inducing pain rather than pressure. The pressure in pounds was recorded for each site.
Table 2.
Physical Exam Data Form
|
Each participant completed 4 questionnaires. These included the Brief Pain Inventory (BPI) [3]; Oswestry Disability Scale (ODS), a measure of disability related to the spine and adjacent musculoskeletal system which includes sub-scales for musculoskeletal pain, sleep disturbance and functional activities related to the musculoskeletal system [4]; MOS Short Form 36 (SF36), a health status questionnaire [26]; and short version of the Profile of Mood States, a symptom check list of moods [20].
We devised a series of questions, administered to all subjects, to help standardize the evaluation and characterize the nature of the pain in the neck/upper trapezius. These consisted of descriptive terms and were aimed at trying to characterize the pain the individual was experiencing. This assessment is presented in Table 2.
DATA ANALYSIS
We conducted two-sample comparisons of variables of interest between the “Active” group and the “No pain” group. For all continuous variables, we first evaluated the normality assumption by using the Shapiro-Wilk test. If we fail to reject the null hypothesis of normality in both groups for a variable, a two-sample t-test will be used to compare the mean value of the variable; otherwise, a Wilcoxon rank sum test will be carried out. For binary variables, we used the Fisher’s Exact test to assess whether there is significant difference between group proportions. The Pearson’s chi-square test for homogeneity and the Mantel-Haenszel chi-square test for trend were used for nominal and ordinal categorical variables, respectively. For each variable of interest, subjects with missing data were excluded from the data analysis. All tests are two-sided and a p-value of < .05 was considered statistically significant. All data analyses were conducted using the SAS software, version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
In this analysis, we report the results of 24 subjects who meet criteria for active MTrPs(‘A’ group) and 26 who were placed into the control group, having no spontaneous pain in the upper trapezius, even in the presence of a palpable nodule (‘Np’ group). Members of the ‘Np’ group had no active MTrPs in the upper trapezius. However, there were various physical findings within the group including latent MTrPs, non-tender nodules and no palpable nodules. Seventeen out of the 24 subjects in the ‘A’ group describe their pain as aching/dull. Ten out of the 24 ‘A’ group subjects describe their pain as nagging. Nine out of the 24 subjects in the ‘A’ group describe their pain as tender. Most of the ‘A’ group subjects indicated that the pain increased in intensity as the day wore on. This was partially influenced by the kind of activity in which they were engaged. Eighteen of the 24 ‘A’ group subjects reported that computer work aggravated their neck pain and 14 reported it was aggravated by prolonged sitting. Fifteen of the 24 subjects in the ‘A’ group reported that lifting weights worsened their neck pain. Twenty of the 26 subjects in the ‘Np’ group and 18 of 24 in the ‘A’ group reported having a sedentary job. Additionally, 19 out of the 24 among the ‘A’ subjects reported that their sleep is occasionally disturbed by pain. There is concordance between the hand of dominance and the side of neck pain in 7 out of 10 with unilateral neck pain in ‘A’ group subjects. Fourteen of 24 ‘A’ group subjects presented with bilateral neck pain at the initial evaluation.
Members of the ‘A’ Group used analgesic medication more frequently than the ‘Np’ group (63% vs 7%, respectively). They also used mood regulators more frequently (25% vs 0%). Members of both groups used dietary supplements frequently (45% vs 34%).
A distribution of descriptive (categorical) variables of interest in the ‘A’ group is presented in Table 3.
Table 3.
Descriptive Features of Pain in Subjects with Active Myofascial Trigger Points
| Variable | Frequency |
|---|---|
| Duration of Pain | |
| Intermittent | 7 |
| Extended | 15 |
| Temporal Occurrence of Pain | |
| Morning | 5 |
| Afternoon | 0 |
| Evening | 9 |
| Night | 3 |
| Other | 4 |
| Pain influences activity | |
| Yes or somewhat | 14 |
| No | 6 |
| Nature of Pain | |
| Local | 16 |
| Widespread | 6 |
| Other | 2 |
| Sleep Difficulties(Oswestry Disability Index)) | |
| 0 (none) | 4 |
| 1 (some) | 18 |
| 2 (frequent) | 1 |
| Pain Disrupts Sleep | |
| 1(yes) | 14 |
| 2(no) | 8 |
| Trigger Point Location | |
| Both sides | 14 |
| Left side | 2 |
| Right side | 6 |
| Duration of Pain | |
| 3–11 months | 3 |
| 1–2 years | 4 |
| 3 years or more | 15 |
| Exercise Frequency | |
| 3x/week or more | 15 |
| < 3x/week | 7 |
The distribution of nodules in all subjects was assessed. A greater percentage of subjects have one latent MTrP in the ‘A’ group compared to the ‘Np’ group (p=.0062); and a greater percentage of subjects have 4 normal sites in the ‘Np’ group compared to the ‘A’ group (p<.001). No one in the ‘Np’ group had an active MTrP, by definition. Table 4 presents MTrP types in the ‘A’ group compared with those in the ‘Np’ group. The sites were classified (1. active MTrPs, 2. latent MTrPs, 3. palpable but asymptomatic nodules on compression and 4. normal sites) with their frequencies at 4 possible site distributions.
Table 4.
| Table 4a: Distribution of Number Of Sites With Latent Trigger Points | |||||
|---|---|---|---|---|---|
| Group | No. of Latent Sites | ||||
| Frequency* | 0 | 1 | 2 | 3 | Total |
| Active Group | 10 | 9 | 4 | 1 | 24 |
| No Pain Group | 20 | 1 | 4 | 0 | 25 |
| Total | 30 | 10 | 8 | 1 | 49 |
| Frequency Missing = 1 | |||||
| Table 4 b: Distribution of Number of Normal Sites | ||||||
|---|---|---|---|---|---|---|
| Group | No. of Normal Sites | |||||
| Frequency** | 0 | 1 | 2 | 3 | 4 | Total |
| Active Group | 1 | 7 | 14 | 2 | 0 | 24 |
| No Pain Group | 0 | 0 | 4 | 1 | 20 | 25 |
| Total | 1 | 7 | 18 | 3 | 20 | 49 |
| Frequency Missing = 1 | ||||||
A greater percentage of subjects in the ‘Active’ group have one latent site compared to the ‘Np’ group. (p=0.0062)
A greater percentage of subjects have four normal sites in the ‘Np’ group compared to the ‘A’ group. (p<0.0001).
We have also examined cervical and shoulder range of motion and strength in both groups. The cervical strength of subjects in the ‘A’ and ‘Np’ groups was measured using the 10 point Kendall scale. We tested cervical flexion, extension, side bending and rotation. At the initial evaluation, 10 out of the 24 subjects in ‘A’ group presented with cervical strength limitations, compared to 0 out of the 26 subjects in the ‘Np’ group. Comparison of side bending or rotation of the neck in the ‘A’ group who had one MTrP versus more than one MTrP demonstrated no significant difference using Wilcoxon rank sum. (p=0.6). However, comparing the asymmetry between left and right sides for side-bending and rotation members of the ‘A’ group and ‘Np’ group were significantly different for side bending (p=0.01) and rotation (p=0.002).
Additional comparisons between the 2 study groups include analysis of continuous variables (Table 5).
Table 5a.
Pain Measures
| Variable | Mean (S.D.) Active | Mean (S.D.) No pain | p-value |
|---|---|---|---|
| Age | 35.79 (12.96) | 25.62 (7.75) | 0.0015 ** |
| VAS Current Pain Score, Right | 2.091 (1.900) | 0 (0) | <0.0001 *** |
| VAS Current Pain Score, Left | 1.545 (1.625) | 0 (0) | <0.0001 *** |
| VAS Average Pain Score, Right | 3.363 (1.891) | 0.115 (0.588) | <0.0001 *** |
| VAS Average Pain Score, Left | 2.455 (1.945) | 0.077 (0.392) | <0.0001 *** |
| BPI Score | 3.326 (1.695) | 0.702 (1.134) | <0.0001 *** |
| BPI P! Interference Sleep Score | 2.522 (2.874) | 0.385 (1.235) | <0.0001 *** |
| PPT Score Site 1 | 10.06 (2.79) | 13.52 (3.90) | 0.0013 ** |
| PPT Score Site 2 | 9.09 (3.15) | 11.62 (4.33) | 0.0296 * |
| PPT Score Site 3 | 8.29 (2.77) | 11.76 (4.69) | 0.0038 ** |
| PPT Score Site 4 | 10.38 (3.58) | 13.28 (4.24) | 0.0180 ** |
p-value < 0.05;
p-value <= 0.01;
p-value <= 0.001
VAS=Visual Analogue Scale
BPI=Brief Pain Inventory
PPT=Pain Pressure Threshold (algometry). Sites correspond to anatomical areas across the upper trapezius
DISCUSSION
Significant efforts have been made to better understand MPS and the role of MTrPs in their pathogenesis. Progress has been made in understanding the dynamic roles of peripheral and central sensitization [14,21,23,24] in the unique neurobiology of muscle pain [15]. Such information is essential for identifying the pathogenesis of MPS, its relationship to the MTrP and developing effective treatments for active MTrPs. Research in this area is ongoing.
However, research on pathogenesis does not address important components of how to classify clinical syndromes and findings, evaluate patients and assess outcomes following treatment. One obstacle to developing a comprehensive approach to MPS, and the subject of this paper, is the need for a standard approach to the clinical examination. Most clinicians and investigators have accepted the definition of active and latent MTrPs [24]. Agreement about what constitutes this pain syndrome, and whether it is attributable to or the result of the MTrP is not resolved.
This manuscript presents data gathered from two groups of research subjects, one with and the other without active MTrPs. The outcome sought was to identify pain measures both sensitive and specific to distinguish the two study groups with respect to pain, and identify clinical change over time. Additionally, a variety of measures were selected to further characterize symptoms, physical findings and function in subjects with active MTrPs (i.e., spontaneous pain), as well as to determine whether they reliably distinguish the two groups. To the best of our knowledge, this has not yet been shown, although significant effort and some progress have been made [8,17].
We standardized the physical examination by identifying 4 sites along the upper trapezius: at its origin in the region of the acromioclavicular joint; and the other at the base of the neck. Years of clinical experience have shown that the former infrequently has active MTrPs and the latter frequently does. These observations enable us to compare to structurally different parts of the muscle. We excluded including the vertical portion of the muscle because the splenii and cervical structures would make it difficult to assess their contribution.
What should be included in a standard evaluation of people with MPS? Clinical practice necessitates judicious and efficient use of patient and health professional time in the evaluation process. Since most patients seek pain relief and return to or maintenance of their usual function, both concerns should be assessed. Measurements should be brief, specific and sensitive. This paper presents the results of a systematic prospective assessment employed as part of a research project, hence lengthy and addressing impairments, performance and mood/perception of MPS. This assessment reliably distinguishes subjects with active MTrPs from those without pain. Data gathered on the 50 subjects reported here show that the 2 groups are different in physical findings, pain measures, sleep disturbance, disability, health status and mood. What is typically thought of and reported as a “local” pain problem primarily involving the shoulder girdle is associated with more symptoms and disability. It is always possible that there are other physical/physiological contributors to this. Nonetheless, these observations suggest that evaluations of people with MPS should include measures of pain, function, health status and mood because they distinguish people with MPS from those with no pain, and provide measures that could demonstrate improvement in symptoms and functional status likely to be associated with this condition. The latter findings are important to identify and treat.
Self-reports of pain are essential for identifying the origin and type of pain an individual experiences. Many questionnaires exist and specific ones seem to be chosen for individual diseases and syndromes. Consensus has not yet been achieved for a universal pain assessment. However, substantial progress has been made in identifying common elements through the use of item reduction and other computer assisted technologies, and many believe that a single dimensional instrument is inadequate to assess pain (www.nihpromis.org). In this study, we measured pain using the VAS and BPI, and tenderness using algometry. We used standard questions to describe the nature of pain. All 3 measures were able to distinguish the ‘A’ group from ‘Np’ group. The language the ‘A’ group used to describe their pain is different from how many describe neuropathic pain.
The descriptors we selected may help differentiate MPS/MTrP pain from other types of pain. This has yet to be proven. Nonetheless, the quality of the pain, its location and its temporal pattern (Table 3), in addition to the physical findings, may help differentiate it from neuropathic pain. Careful assessment of the upper trapezius using palpation for MTrPs and neck range of motion, especially side-bending, provide specific outcome measures to target, since they are significantly different when comparing subjects with and without pain. We selected a 10% difference in ROM laterality as a way of identifying asymmetry, which was an arbitrary decision and based on convenience. However, the data were also analyzed using a Spearman rank sum order of degrees of movement.
Describing pain quality, location and temporal features (qualitative and quantitative characteristics) are also valuable for informing clinicians of patients’ pain statuses. It is the hope that this combination of findings will have a high degree of sensitivity and specificity for this pain syndrome.
Published work suggests that women sewing machine operators are more likely to have shoulder/neck symptoms if they have little social support. Length of employment and ergonomics are risk factors [11]. These observations support the view that shoulder and neck problems are not likely to be confined to a single, localized symptom; and have associations with global activity and participation in a variety of life activities. This study supports these findings because the ODS data suggest that there is significant disability associated with active MTrPs. Our findings similarly suggest that the active MTrP, while a specific physical finding, is associated with overall health status. Evaluations should assess the associations among an individual’s daily routines and their mood and health status. Treatments should target the findings that are abnormal, including those obtained from self-reports and physical findings.
There are several weaknesses in this study. The investigators were not naïve to who had pain. However, we were able to mitigate this using two independent examiners who are experienced clinicians and have good inter-rater reliability. Additionally, the study used three pain measures, VAS, BPI and algometry. Each showed significant discrimination between the two groups.
We purposely accepted subjects into the “Active” group who had chronic MPS to provide us with a steady baseline. However, the natural history of this syndrome is poorly understood, and we therefore have little data about how universal the signs, symptoms and self-reports would be in people with acute MPS (<3 months’ duration of symptoms). The importance of measures of disability, mood and abnormalities of ROM may be present only in people who have had protracted symptoms.
Another weakness in this study is the fact that we recruited subjects using advertising on a college campus. Almost all of our subjects are students and staff at the University. Therefore, the population may be atypical. The mean age of the ‘Np’ group and ‘A’ group are 10 years apart and both groups are well under 40 years of age. This is quite a young population. Age may be an important, confounding variable. Some of the data collected show that the groups are significantly different with respect to scores on several subscales of the POMS (depression, confusion, fatigue and tension), health status measures and some physical findings (PPT, cervical side-bending). There are intergroup differences on the health status measure (SF36), but both groups’ scores are above the mean of the US population, suggesting their health status was normal or better than the general population. Nonetheless, the scores on the questionnaires from the ‘Np’ group were significantly different from the group with active MTrPs.
Clinical research is likely to inform practice and improve desired treatment outcomes when we are able to classify correctly different clinical conditions. In this study, there is some heterogeneity in the physical findings among members of the ‘Np’ group. They qualify if they have no spontaneous pain and any of the following on palpation: no nodule, a non-tender nodule or a latent MTrP. Analysis of the subjects in the ‘Np’ group was not performed with these subsets in mind. Hence, there may be some imprecision introduced by combining subjects on the basis of no spontaneous pain rather than on MTrP presence or absence. This would separate a group with active or latent MTrPs from another with no nodules or non-tender nodules.
Chronic pain syndromes such as MPS exhibit profound neuroplastic changes, altering neuronal excitability and architecture in structures of the pain matrix (e.g., the spinal cord, thalamic nuclei, cortical areas, limbic system and periaqueductal gray area). This process can fundamentally alter pain threshold, pain intensity and affect [29]. A dynamic balance exists between supraspinal descending facilitation and inhibition, as the rostral ventral medulla (RVM) is a pivotal relay area between the periaqueductal gray and the spinal cord. The RVM contains a population of “on cells” and “off cells” which can either increase or decrease the level of pain and sensitization, respectively. It does so through projections that modulate activity in the dorsal horn. In chronic musculoskeletal pain conditions, there appears to be an overall shift to a decrease in inhibition, presumably due to an imbalance of “on cell” and “off cell” activity [27].
Muscle pain also impairs diffuse noxious inhibitory control (DNIC) [1]. Disrupted descending inhibition in chronic musculoskeletal pain may lead to an increased pain sensitivity of muscle tissue [6]. Accordingly, clinical manifestations such as diffuse muscle tenderness and the findings of active and latent MTrPs on palpation could occur irrespective of events in the periphery. Our clinical findings of latent MTrPs in both the ‘A’ group and ‘Np’ group suggest that palpable nodules may exist along a spectrum involving varying degrees of sensitization. Presumably, even subjects who do not have spontaneous pain may also exhibit varying degrees of sensitization, manifesting as latent MTrPs and non-tender nodules, as we found in the ‘Np’ group. Current data suggest that MTrPs are not merely a peripheral phenomenon but rather, they activate and sensitize wide dynamic range neurons in the dorsal horn and higher brain centers and may, in turn, be dynamically modulated by these structures, leading to a spectrum of clinical findings [16,18].
Despite the shortcomings of this study, we present a careful, systematic and comprehensive approach to the evaluation of research subjects with MTrPs. The pain measures selected are sensitive. Measures that assess physical findings (strength, range of motion, palpation), self-reports of pain, fatigue, mood and health status clearly distinguish the 2 study groups. The findings suggest that people with chronic, active MTrPs have local findings that impact many aspects of life activity, mood and health status.
CONCLUSION
People with active MTrPs have pain that is evaluable using standardized tests. They also have more functional and health status abnormalities. When compared with controls without active MTrPs. Patients with MPS should receive a multi-dimensional systematic evaluation. This evaluation should use standardized, reliable measures designed to assess physical findings, including range of motion and strength of the neck and shoulders; and self-reports of mood and function and health status.
Table 5b.
Self-reports, Health Status, Function and Mood
| Variable | Mean (S.D.) Active | Mean (S.D.) No pain | p-value |
|---|---|---|---|
| SF-36, Bodily Pain Score | 58.76 (20.20) | 96.10 (10.40) | <0.0001 *** |
| SF-36, General Health Score | 67.24 (19.14) | 86.38 (11.82) | 0.0016 ** |
| SF-36, Mental Health Score | 71.47 (17.75) | 85.00 (7.42) | 0.0044 ** |
| SF-36, Physical Function. Score | 85.00 (17.59) | 99.05 (3.01) | 0.0001 *** |
| SF-36, Emotional Score | 77.94 (27.47) | 96.43 (7.71) | 0.0108 * |
| SF-36, Physical Score | 77.20 (19.64) | 99.70 (1.36) | <0.0001 *** |
| SF-36, Social Functioning Score | 79. 41 (25.36) | 97.02 (7.81) | 0.0064 ** |
| SF-36, Vitality Score | 47.43 (16.40) | 71.73 (13.92) | <0.0001 *** |
| POMS Anger Score | 0.087 (0.191) | 0.131 (0.450) | 0.2006 |
| POMS Confusion Score | 0.452 (0.483) | 0.176 (0.323) | 0.0167 * |
| POMS Depression Score | 0.185 (0.388) | 0.065 (0.229) | 0.0382 * |
| POMS Fatigue Score | 0.922 (0.962) | 0.272 (0.404) | 0.0075 ** |
| POMS Tension Score | 0.457 (0.498) | 0.227 (0.438) | 0.0104 * |
| POMS Total Mood Disturbance Score | 0.660 (2.257) | −1.082 (1.787) | 0.0024 * |
| Oswestry Score | 12.22 (6.54) | 1.667 (4.072) | <0.0001 *** |
p-value < 0.05;
p-value <= 0.01;
p-value <= 0.001
SF36=Short Form 36, a health related quality of life measure
POMS= Profile of Mood States
Oswestry=the Oswestry Disability Scale
Acknowledgments
This research is supported by a grant from the National Institutes of Health> 1RO1-AR057348-01A1
Footnotes
This research was presented, in part, at the Annual Assembly of the American Academy of Physical Medicine and Rehabilitation, November, 2012
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lynn H. Gerber, Center for the Study of Chronic Illness and Disability, College of Health and Human Services, George Mason University.
Siddhartha Sikdar, Departments of Electrical and Computer Engineering and Bioengineering, Volgenau School of Engineering, George Mason University.
Katee Armstrong, Center for the Study of Chronic Illness and Disability, College of Health and Human Services, George Mason University.
Guoqing Diao, Department of Statistics, Volgenau School of Engineering, George Mason University.
Juliana Heimur, Department of Rehabilitation Medicine, Clinical Center, National Institutes of Health.
John Kopecky, College of Science, George Mason University.
Diego Turo, Department of Bioengineering, Volgenau School of Engineering, George Mason University.
Paul Otto, Departments of Electrical and Computer Engineering and Bioengineering, Volgenau School of Engineering, George Mason University.
Tadesse Gebreab, Department of Rehabilitation Medicine, Clinical Center, National Institutes of Health.
Jay Shah, Department of Rehabilitation Medicine, Clinical Center, National Institutes of Health.
References
- 1.Arendt-Nielsen L, Sluka KA, Nie HL. Experimental muscle pain impairs descending inhibition. Pain. 2008;140:465–471. doi: 10.1016/j.pain.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett R. Myofascial Pain Syndromes and Their Evaluation. Baillière’s best practice & research. Clinical rheumatology. 2007;21(3):427–45. doi: 10.1016/j.berh.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Borg-Stein J, Simons DG. Myofascial Pain: Focused Review. Arch Phys Med Rehabil. 2002;83(Suppl 1):S40–S49. doi: 10.1053/apmr.2002.32155. [DOI] [PubMed] [Google Scholar]
- 4.Bron C, Dommerholt JD. Etiology of Myofascial Trigger Points. Curr Pain Headache Rep. 2012;16(5):439–44. doi: 10.1007/s11916-012-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to Assess Pain in Cancer and Other Diseases. Pain. 1983;17(2):197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA. Advances in Neuropathic Pain: Diagnosis, Mechanisms, and Treatment Recommendations. Archives of neurology. 2003;60(11):1524. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 7.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry Low Back Pain Disability Questionnaire. Physiotherapy. 1980;66(8):271–73. [PubMed] [Google Scholar]
- 8.Fricton JR, Kroening R, Haley D, Siegert R. Myofascial Pain Syndrome of the Head and Neck: A Review of Clinical Characteristics of 164 Patients. Oral surgery, oral medicine, oral pathology. 1985;60(6):615–23. doi: 10.1016/0030-4220(85)90364-0. [DOI] [PubMed] [Google Scholar]
- 9.Ge HY, Fernández-de-las-Peñas C, Yue SW. Myofascial trigger points: spontaneous electrical activity and its consequences for pain induction and propagation. Chinese Medicine. 2011;6(13) doi: 10.1186/1749-8546-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerwin RD, Dommerholt J, Shah JP. An Expansion of Simons’ Integrated Hypothesis of Trigger Point Formation. Current pain and headache reports. 2004;8(6):468–75. doi: 10.1007/s11916-004-0069-x. [DOI] [PubMed] [Google Scholar]
- 11.Hogg-Johnson S, van der Velde G, Carroll LJ, Holm LW, Cassidy JD, Guzman J, Côté P, Haldeman S, Ammendolia C, Carragee E. Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. The Burden and Determinants of Neck Pain in the General Population: Results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(4 Suppl):S39–51. doi: 10.1097/BRS.0b013e31816454c8. [DOI] [PubMed] [Google Scholar]
- 12.Hoheisel U, Unger T, Mense S. Excitatory and Modulatory Effects of Inflammatory Cytokines and Neurotrophins on Mechanosensitive Group Iv Muscle Afferents in the Rat. Pain. 2005;114(1–2):168–76. doi: 10.1016/j.pain.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Hough J. Estimating the Health Care Utilization Costs Associated With People With Disabilities: Data From the 1996 Medical Expenditure Panel Survey (Meps) 2000. [Google Scholar]
- 14.Kaergaard A, Andersen JH. Musculoskeletal Disorders of the Neck and Shoulders in Female Sewing Machine Operators: Prevalence, Incidence, and Prognosis. Occupational and environmental medicine. 2000;57(8):528–34. doi: 10.1136/oem.57.8.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendall Florence Peterson, McCreary Elizabeth Kendall, Provance Patricia Geise, Rodgers Mary McIntyre, Romani William Anthony. North American Edition, editor. Muscles: Testing and Function, With Posture and Pain: Includes a Bonus Primal Anatomy Cd-Rom (Kendall, Muscles) 5. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 16.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD. Expenditures and Health Status Among Adults With Back and Neck Problems. JAMA: the journal of the American Medical Association. 2008;299(6):656–64. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 17.Mense S. The Pathogenesis of Muscle Pain. Curr Pain Headache Rep. 2003;7(6):419–25. doi: 10.1007/s11916-003-0057-6. [DOI] [PubMed] [Google Scholar]
- 18.Mense S, Simons DG, Russell IJ. Muscle Pain: Understanding Its Nature, Diagnosis and Treatment. Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 19.Mense S. How Do Muscle Lesions such as Latent and Active Trigger Points Influence Central Nociceptive Neurons? Journal of Musculoskeletal Pain. 2010;18(4):348–353. [Google Scholar]
- 20.Murphy DR, Hurwitz EL. Application of a Diagnosis-Based Clinical Decision Guide in Patients With Neck Pain. Chiropr Man Therap. 2011;19(1):19. doi: 10.1186/2045-709X-19-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niddam DM, Chan RC, Lee SH, Yeh TC, Hsieh JC. Central modulation of pain evoked from myofascial trigger point. Clinical Journal of Pain. 2007;23:440–448. doi: 10.1097/AJP.0b013e318058accb. [DOI] [PubMed] [Google Scholar]
- 22.Rondinelli Robert. Guides to the Evaluation of Permanent Impairment. 6. Chicago: American Medical Association; 2008. [Google Scholar]
- 23.Shacham S. A Shortened Version of the Profile of Mood States. J Pers Assess. 1983;47(3):305–06. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 24.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in Vivo Microanalytical Technique for Measuring the Local Biochemical Milieu of Human Skeletal Muscle. J Appl Physiol. 2005;99(5):1977–84. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 25.Skootsky SA, Jaeger B, Oye RK. Prevalence of Myofascial Pain in General Internal Medicine Practice. West J Med. 1989;151(2):157–60. [PMC free article] [PubMed] [Google Scholar]
- 26.Sluka KA, Kalra A, Moore SA. Unilateral Intramuscular Injections of Acidic Saline Produce a Bilateral, Long-Lasting Hyperalgesia. Muscle Nerve. 2001;24(1):37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Travell Janet, Simons David. Myofascial Pain and Dysfunction, Vol. 1: The Trigger Point Manual, the Upper Extremities. Williams & Wilkins; 1983. [Google Scholar]
- 28.Trescot AM, Helm S, Hansen H, Benyamin R, Glaser SE, Adlaka R, Patel S, Manchikanti L. Opioids in the Management of Chronic Non- Cancer Pain: An Update of American Society of the Interventional Pain Physicians’(Asipp) Guidelines. Pain physician. 2008;11(2 Suppl):S5–S62. [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Sherbourne CD. The Mos 36-Item Short-Form Health Survey (Sf-36). I. Conceptual Framework and Item Selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 30.Willard F. Basic Mechanisms of Pain.” Future Trends in CAM Research. In: Audette JF, Bailey A, editors. Integrative Pain Medicine: The Science and Practice of Complementary and Alternative Medicine in Pain Management. Humana Press Inc; Totowa: 2008. [Google Scholar]
- 31.Youdas JW, Carey JR, Garrett TR. Reliability of Measurements of Cervical Spine Range of Motion—Comparison of Three Methods. Physical therapy. 1991;71(2):98–104. doi: 10.1093/ptj/71.2.98. [DOI] [PubMed] [Google Scholar]
- 32.Zieglgänsberger W, Berthele A, Tölle TR. Understanding neuropathic pain. CNS Spectrums. 2005;10:298–308. doi: 10.1017/s1092852900022628. [DOI] [PubMed] [Google Scholar]