Abstract
Objective
To investigate the nature of cognitive impairments and underlying brain mechanisms in older female fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome (FXTAS).
Methods
Extensive neuropsychological testing and cognitive event-related brain potentials (ERPs, particularly, the auditory P300) were examined in 84 female participants: 33 fragile X premutation carriers with FXTAS (mean age = 62.8), 25 premutation carriers without FXTAS (mean age = 55.4) and 26 normal healthy controls (mean age = 59.3).
Results
Both premutation groups exhibited executive dysfunction on the Behavioral Dyscontrol Scale (BDS), with subtle impairments in inhibition and performance monitoring in female carriers without FXTAS, and more substantial deficits in FXTAS women. However, the female carrier group without FXTAS showed more pronounced deficiencies in working memory.
Abnormal ERPs were recorded over the frontal lobes, where FXTAS patients showed both P300 amplitude reduction and latency prolongation, while only decreased frontal P300 amplitudes were found in carriers without FXTAS. These frontal P3 measures correlated with executive function and information processing speed.
Interpretation
The neuropsychological testing and ERP results of the present study provide support for the hypothesis that executive dysfunction is the primary cognitive impairment among older female premutation carriers both with and without FXTAS, although these deficits are relatively mild compared to those in FXTAS males. These findings are consistent with a synergistic effect of the premutation and aging on cognitive impairment among older female fragile X premutation carriers, even in those without FXTAS symptoms.
Introduction
Studies on premutation carriers of the fragile X mental retardation 1 (FMR1) gene (with 55–200 CGG trinucleotide repeats) have identified the fragile X-associated tremor/ataxia syndrome (FXTAS), characterized by cerebellar ataxia, intention tremor, neuropathy and cognitive deficits progressing to dementia in up to 50% of older males1, 2. Neuropathologic abnormalities in FXTAS include eosinophilic intranuclear inclusions in neurons and astrocytes, gray matter loss in cerebellum and frontal cortex, and hyperintensities and connectivity alterations of white matter3–7. Research in males has indicated that executive dysfunction appears to be the primary cognitive impairment of FXTAS8, 9, with deficits in general intelligence (IQ), attention, memory, and visual spatial processing also common9–12.
Female premutation carriers over age 50 generally show a lower penetrance (8–16%) of FXTAS with milder symptoms, whereas ~20% of them present with primary ovarian insufficiency (FX-POI)13. A recent study comparing a brother-sister pair with similar premutation CGG length showed more significant psychiatric and cognitive deficiencies in the male sibling even though his sister was a homozygous premutation carrier14, suggesting somewhat distinct and female-favorable mechanisms mediating behavioral phenotypes in female carriers. While male carriers without FXTAS exhibit deficits in executive functioning (e.g., inhibition, attentional control and performance monitoring), visual spatial processing and working memory, only mildly impaired arithmetic, perceptual organization and memory retrieval have been found in female carriers without FXTAS15–18. One study even showed faster reaction time (RT) in young female premutation carriers than normal controls on a simple RT task19. It has been proposed that female carriers could benefit from the normal FMR1 allele in the additional X-chromosome and/or from putative protective effects of estrogen, thus manifest milder symptoms20.
Nonetheless, evidence is emerging for more variable phenotypes among female carriers. Greater prevalence of co-morbidities in female than male carriers, particularly psychiatric21, 22 (e.g., anxiety, depression) and autoimmune disorders23 (e.g., hypothyroidism), has been documented. As for cognitive function, female carriers without FXTAS have demonstrated lower verbal IQ4 and more frequently reported attention problems24, 25 than males. Strikingly, a neuropathological case series reported dementia in 4 of the 8 autopsied female carriers, and found intranuclear inclusions in all of them, even in the two without FXTAS26. This suggests a higher prevalence of neural anomalies in female carriers than originally thought. Moreover, since most studies of female carriers without FXTAS have been conducted in those under age 45, little is known about the interacting effects of aging and the fragile X premutation on cognitive functions in older females.
In the present study, extensive neuropsychological testing and cognitive event-related potentials (ERP) were integrated to characterize cognitive deficits and underlying brain mechanisms in older female premutation carriers with and without FXTAS. The oddball P300 (P3) ERP paradigm employed has been shown sensitive to the core cognitive deficit, namely executive dysfunction, in predominantly-male FXTAS patients27. Prior studies have also demonstrated relationships between P3 and frontal executive function in normal subjects28, 29. We hypothesized that both female premutation carrier groups would have mild but noticeable executive dysfunction as primary cognitive deficits, and that P3 measures over the frontal lobes would track their executive dysfunction.
Methods
Participants
Study participants were 84 females, including 33 FXTAS patients (FXTAS+, mean FXTAS stage =3.0, range: 2–5), 25 premutation carriers without FXTAS (FXTAS−, mean FXTAS stage =0.4, range: 0–1) and 26 normal controls (NC) (FXTAS stages range from 0 (normal function) through 6 (bedridden)30). FXTAS− carriers were younger than the FXTAS+ group (Table 1), but neither premutation group differed significantly from NC subjects in age or education levels. All participants provided informed consent for a protocol approved by local Institutional Review Board. Exclusion criteria include history of substance abuse/dependence, traumatic brain injury, or any other central nervous system (CNS) disorder. Participants with CNS-active medications for anxiety/depression and neuropathic pain were not excluded because these problems are part of the premutation phenotype, and these medications thus have a high prevalence of use in representative samples of premutation carriers. Procedures described elsewhere were performed to measure the CGG repeat length31 and activation ratio32 (AR) indicating the percentage of cells with the normal X-chromosome active.
Table 1.
Demographic and genetic profiles.
|
p values(ANCOVAs) |
|||||||
|---|---|---|---|---|---|---|---|
| NC (N=26) |
FXTAS− (N=25) |
FXTAS+ (N=33) |
Overall | NC vs FXTAS− |
NC vs FXTAS+ |
FXTAS− vs FXTAS+ |
|
| Age | 59.3 (7.3) | 55.4 (6) | 62.8(9.8) | .004** | .27 | .31 | .003** |
| Education | 16.9 (2.5) | 15.9 (3) | 15 (3.1) | .07 | .72 | .07 | .83 |
| Left Handedness | 3 | 2 | 2 | .75 | -- | -- | -- |
| CGG repeats | 32.1 (7) | 85.8 (20) | 82 (20) | -- | -- | -- | 1.0 |
| CGG range | 20–50 | 52–134 | 55–150 | -- | -- | -- | -- |
| Activation Ratio | -- | 0.49 (0.24) | 0.49 (0.15) | -- | -- | -- | .98 |
Abbreviations: NC = Normal Controls, FXTAS− = female premutation carriers without FXTAS, FXTAS+ = female premutation carriers with FXTAS.
p≤ 0.01.
Neuropsychological testing
Each participant was administrated an extensive test battery to assess global cognitive abilities [Mini-Mental State Examination (MMSE), Wechsler Adult Intelligence Scale (WAIS-III)], executive function [Behavioral Dyscontrol Scale (BDS, a well validated 9-item instrument measuring the intentional control of simple voluntary motor behavior)33, Stroop test, and Controlled Oral Word Association Test (COWAT)], information processing speed [Symbol Digit Modalities Test (SDMT)] and verbal memory [California Verbal Learning Test (CVLT)].
EEG/ERP data collection
In an auditory oddball paradigm requiring dual-response, lower (113 Hz) and higher (200 Hz) frequency pure tone stimuli were presented at 40dB above individual hearing level. In response to infrequent target tones (25% high or low tones, counterbalanced across blocks), participants were instructed to press a button and also mentally count the number of targets. At the end of each block, the subjects’ mental count of target tones was reported. 32-channel Electroencephalogram (EEG) was recorded with a Nicolet-SM-2000 amplifier (bandpass =0.016–100 Hz, sampling rate =250 Hz). Artifact-rejected trials with a 100 ms pre-stimulus baseline were averaged by experimental condition to obtain the ERPs (for more details, see Yang et al.27).
Data analyses
Four ERP components were measured: N100 (70–150 ms), P200 (160–260 ms), N200 (170–290 ms), and P3 (300–650 ms). Waveforms to both target and standard tones were used to measure N100. The P200 and N200 components were measured from standard tones and difference waves (targets minus standards), respectively. P3 measures were obtained from target tones with a correct response. ERP data were low-pass filtered offline at 30 Hz, and components were quantified by local peak amplitudes and local peak latencies.
Three-group ANCOVAs were performed on the neuropsychological data (see covariates below). Repeated-measures ANCOVAs (SPSS 20, IBM) were performed on ERP data. Analyses of N100 and P200 included 4 fronto-central electrodes (Fz, Cz, FC1/2). Five channels (Cz, FC1/2, CP1/2) were used for N200 comparisons. P3 analyses were first performed on 26 scalp electrodes (all except FP1/2), then on 8 parasagittal electrodes (F3/4, FC1/2, CP1/2, P3/4) with additional within-subjects factors of hemisphere and anterior/posterior. Anterior/posterior effects were further tested on 3 midline electrodes (Fz, Cz, Pz). The Greenhouse-Geiser and the Bonferroni corrections were used to adjust for violations of sphericity and multiple-comparison in ANCOVAs, respectively. Partial correlations were calculated to test the relationship between midline P3 measures and neuropsychological test scores. The NC/carrier discriminability of P3 measures was examined with logistic regression models.
Results
Molecular and genetic measures
There were no significant differences in CGG repeat length or AR between the two premutation groups (Table 1).
Neuropsychological testing
Table 2 summarizes group performance on neuropsychological tests. Both carrier groups had WAIS-III Full-Scale IQ scores within normal range, while NC subjects had a higher than average IQ. Therefore, age and IQ were used as covariates in group comparisons on all neuropsychological tests except the MMSE, WAIS-III subscales, and the categorical BDS item scores. Relative to normal controls, both female carrier groups demonstrated impaired executive functioning as measured by BDS total scores33, with more pronounced deficits found in the FXTAS+ group. Nonparametric tests on BDS items revealed significantly decreased scores on motor control, motor inhibition, motor learning and performance monitoring in both premutation carrier groups (Supplementary Table 1. To adjust for multiple comparisons, only p values ≤ 0.01 were considered significant.) Furthermore, the FXTAS+ group had substantially impaired performance on 8 of 9 BDS-items (except attention). However, no significant impairments were observed on the other two executive function tests (i.e., the Stroop and COWAT) in either premutation group, indicating milder executive function deficits in females carriers compared to FXTAS males8, 9, 27.
Table 2.
Neuropsychological testing scores.
|
p values(ANCOVAs) |
|||||||
|---|---|---|---|---|---|---|---|
| NC (N=26) |
FXTAS− (N=25) |
FXTAS+ (N=33) |
Overall | NC vs FXTAS− |
NC vs FXTAS+ |
FXTAS− vs FXTAS+ |
|
| Global Cognitive Abilities | |||||||
| WAIS-III Full-Scale IQ | 120.4(12) | 104.2(12) | 107.3(14) | .001*** | .001*** | .005** | 1.0 |
| WAIS-III Performance IQ | 120.4(14) | 103.1(12) | 104.7(16) | .001*** | .001*** | .002** | 1.0 |
| WAIS-III Verbal IQ | 117.1(11) | 104.6(13) | 108(13) | .009** | .008** | .063 | 1.0 |
| MMSE | 28.8 (1) | 28.1(1.3) | 28.3 (1.2) | .09 | -- | -- | -- |
| Executive Functioning | |||||||
| BDS Total | 23.3(2.1) | 19.3(3) | 16.3(4.1) | <.0001*** | .017* | <.0001*** | .06 |
| Stroop | 51.6(9.2) | 48.5(6.9) | 44.3(10) | .21 | -- | -- | -- |
| COWAT | 49.6 (15) | 44.1(11) | 39.1(13) | .77 | -- | -- | -- |
| SDMT | 57.1(9.1) | 56.1(8) | 47.3(10) | .02* | .58 | .52 | .02* |
| WAIS-III Subscales | |||||||
| Information Processing Speed | 117.9(13) | 103.6(12) | 102.9(18) | .04* | .06 | .08 | 1.0 |
| Working Memory | 110.9(13) | 98.4(12) | 104.7(13) | .035* | .06 | 1.0 | .11 |
| Perceptual Organization | 118.7(16) | 103.8(13) | 105.8(16) | .03* | .03* | .13 | 1.0 |
| Verbal Comprehension | 118.2(9) | 107.8(15) | 108(13) | .10 | -- | -- | -- |
| CVLT LD Free Recall | 11.4(2.7) | 10.6(3.5) | 9.6(3.3) | .92 | -- | -- | -- |
| CVLT LD Cued Recall | 11.7(2.3) | 11.7(3.2) | 10.8(2.5) | .75 | -- | -- | -- |
| CVLT SD Free Recall | 11.3 (2.7) | 10.3(3) | 9.3(3) | .96 | -- | -- | -- |
| CVLT SD Cued Recall | 12(2.4) | 11.4(2.6) | 10.9(2.7) | .99 | -- | -- | -- |
Means (SDs) reported above are raw scores, except for the WAIS-III scores which are age-adjusted. ANCOVA Covariates: none for IQ and MMSE, education for WAIS-III subscales, age and IQ for other tests.
Abbreviations: NC = Normal Controls, FXTAS− = female premutation carriers without FXTAS, FXTAS+ = female premutation carriers with FXTAS, WAIS-III = Wechsler Adult Intelligence Scale, MMSE = Mini-Mental State Examination, BDS = Behavioral Dyscontrol Scale, COWAT = Controlled Oral Word Association Test, SDMT = Symbol Digit Modalities Test, CVLT = California Verbal Learning Test, LD = long-delay, SD = short-delay.
p≤ 0.05,
p≤ 0.01,
p≤ 0.001.
Italic p values: borderline significance.
Since WAIS-III subscale index scores have been adjusted based on age-specific norms, and the FSIQ is derived from those scores, ANCOVAs with education (but not age and IQ) as covariate were performed on WAIS-III subscales. Both carrier groups showed a trend of slower information processing speed on WAIS-III. Nonetheless, neither premutation group differed from controls on SDMT (adjusted means: NC = 52.5, FXTAS− = 56.2, FXTAS+ = 48.8), another test for processing speed. FXTAS− women appeared to have the poorest working memory performance on WAIS-III. This trend was further supported by findings on the |count-hit| (discrepancy between the number of correct button-presses and mental counting to target tones), an inverse measure of working memory during our oddball task.
No significant group differences were found for the CVLT, indicating intact verbal learning/memory in female premutation carriers.
ERP results
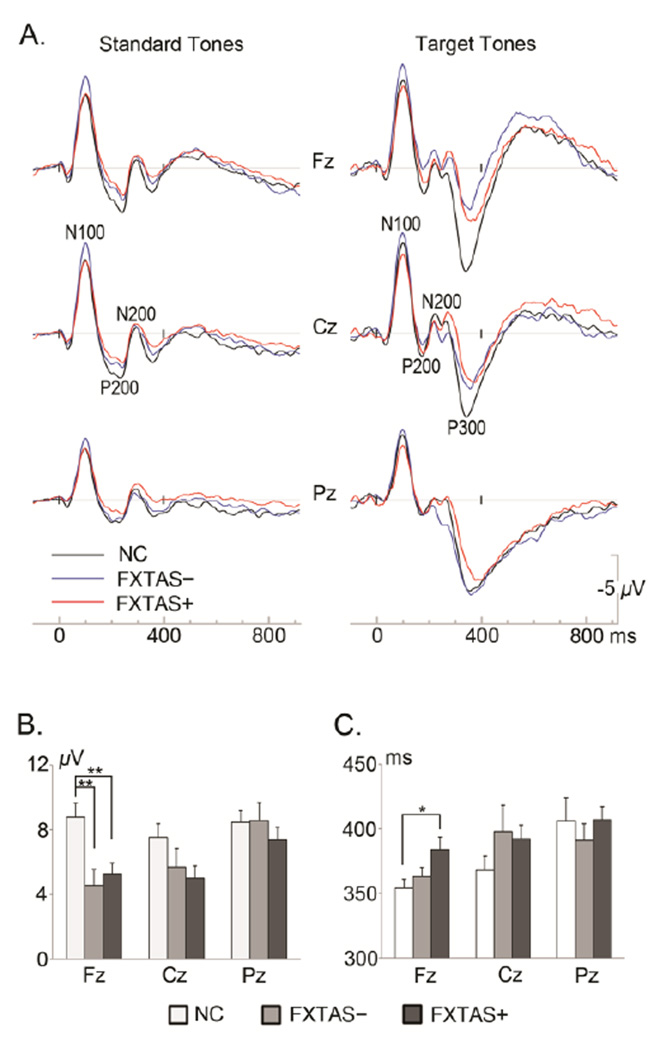
EEG/ERP data from 7 participants (8.3%: 1 NC, 2 FXTAS− and 4 FXTAS+) were excluded from analyses due to excessive eye-movement artifacts. Table 3 presents behavioral performance in the oddball task. Figure 1A depicts the grand-average ERP waveforms.
Table 3.
Behavioral performance in the oddball task.
|
p values(ANCOVAs) |
|||||||
|---|---|---|---|---|---|---|---|
| NC (N=25) |
FXTAS− (N=23) |
FXTAS+ (N=29) |
Overall | NC vs FXTAS− |
NC vs FXTAS+ |
FXTAS− vs FXTAS+ |
|
| Accuracy | 98.4(4.9) | 96.9(4.8) | 96.9(4.1) | .40 | -- | -- | -- |
| Reaction Time | 487(79) | 471(91) | 521(101) | .03* | .95 | .25 | .03* |
| |Count-hit| | 1.1(1.6) | 2.2(2.4) | 1.3(1.3) | .019* | .035* | 1.0 | .041* |
| Artifact-free Target Trials | 58.2(21.5) | 53.3(20.4) | 56.7(18.3) | .68 | -- | -- | -- |
Abbreviations: NC = Normal Controls, FXTAS− = female premutation carriers without FXTAS, FXTAS+ = female premutation carriers with FXTAS.
p≤ 0.05.
Figure 1.
Grand-average ERP waveforms elicited by standard tones and correct target tones (A). Bar graphs show P3 peak amplitude (B) and peak latency (C) at 3 midline electrodes with significant group differences at the frontal site indicated (*p = 0.027, ** p < 0.01). Error bars = standard errors, NC = Normal Controls, FXTAS− = female premutation carriers without FXTAS, FXTAS+ = female premutation carriers with FXTAS.
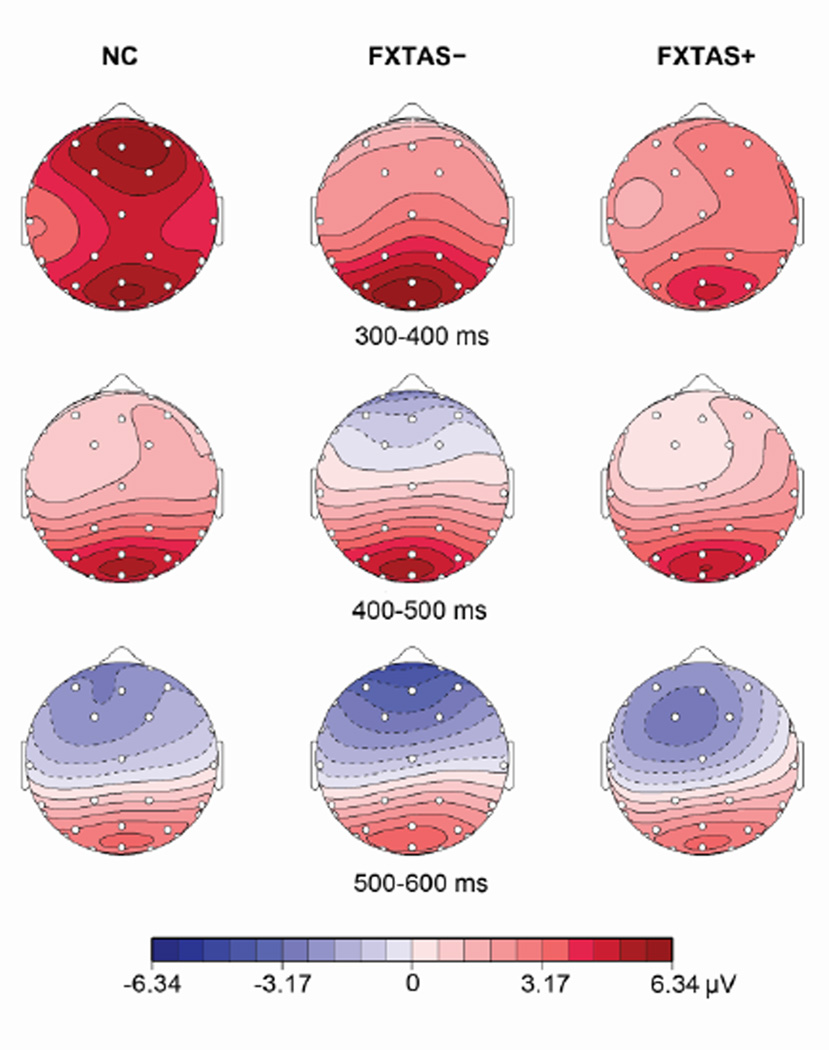
ANCOVAs controlling for age were performed on the 26 channel ERP data. This revealed a significant group×electrode interaction on P3 amplitude (F50,1825 =3.4, p =.001). Further ANCOVAs on the 8 parasagittal electrodes showed a significant anterior/posterior×group interaction on P3 amplitude (F =9.48, p =.0002), with smaller P3 amplitudes across anterior electrodes in both premutation carrier groups (NC =8.2 µV, FXTAS− =4.58 µV, FXTAS+ =5.17 µV) (Fig. 2), as well as a marginally significant anterior/posterior×group interaction on P3 latency (F =3.01, p =.056) with a trend of longer latency in FXTAS+ females (NC =355 ms, FXTAS− =365 ms, FXTAS+ =384 ms). Since no hemisphere effects were obtained in the parasagittal electrode analyses (p ≥ .18), follow-up group comparisons focused on the 3 midline electrodes (Fz, Cz and Pz). Both FXTAS− and FXTAS+ carriers demonstrated significantly decreased P3 amplitudes at the frontal electrode Fz (Fig. 1B), whereas P3 latency at Fz was only delayed in FXTAS+ females (Fig. 1C). There were no significant group differences in P3 amplitudes or latencies at the central (Cz) or parietal (Pz) sites.
Figure 2.
Scalp distribution of ERPs elicited by correct target tones (anterior = up, left = left). Color scale indicates mean voltage across 100 ms period. NC = Normal Controls, FXTAS− = female premutation carriers without FXTAS, FXTAS+ = female premutation carriers with FXTAS.
No significant group differences were obtained in analyses of N100 (F2,73 ≤ 1.78, p ≥ .18), P200 (F2,73 ≤ 2.52, p ≥ .09), or N200(F2,73 ≤ 1.36, p ≥ .26). These normal early ERP components may indicate preserved processing of auditory sensory information, and may also suggest sparing of early stage auditory attention in both premutation groups.
To exclude possible confounding of CNS-active medications on the frontal P3 abnormalities obtained above, ANOVAs were performed on P3 data from Fz, FC1/2 and F3/4. Comparisons between NC (n =20) and a combined group of all carriers (n =22) free of CNS-medications (amplitude: F1,36 =4.19, p =.048; latency: F1,36 =4.53, p =.040) suggest that the general pattern of smaller P3 amplitude and longer latency in frontal channels is not likely due to the CNS-active medications taken by this subset of participants. In addition, no differences were found between female carriers with and without (n =30) CNS-medications (amplitude: F1,46 =1.71, p =.20; latency: F1,46 =1.03, p =.32).
Correlational results
In partial correlation analyses (Table 4) performed across all subjects (covariates: age and IQ), P3 latency at Fz correlated with BDS, Stroop and WAIS-III information processing speed scores, and P3 amplitude at Fz correlated with processing speed. P3 latency at Cz was associated with both working memory measures (i.e., the WAIS-III working memory subscale and the |count-hit|). P3 amplitude at Pz correlated with BDS and SDMT performance.
Table 4.
Correlation coefficients across all subjects (age and IQ controlled#).
| P3 Amplitude | P3 Latency | |||||
|---|---|---|---|---|---|---|
| Fz | Cz | Pz | Fz | Cz | Pz | |
| BDS | .25 | .26 | .28* | −.40** | −.06 | −.03 |
| Stroop | .05 | .03 | .13 | −.39** | −.17 | −.15 |
| SDMT | .18 | .19 | .27* | −.22 | −.06 | −.10 |
| |Count-hit| | −.23 | −.17 | −.11 | .17 | .32* | .12 |
| WAIS- Working Memory | .15 | .01 | −.11 | −.13 | −.32* | −.20 |
| WAIS- Information Processing Speed | .29* | .25 | .18 | −.32* | −.06 | −.001 |
Except for analyses on two the WAIS-III subscales with age as the only covariate.
Abbreviations: BDS = Behavioral Dyscontrol Scale, SDMT = Symbol Digit Modalities Test, WAIS = Wechsler Adult Intelligence Scale-III.
p≤ 0.05,
p≤ 0.01.
Italic P values: .05 <p< 0.10.
Similar correlations were found in analyses which included all female carriers. P3 latency at Fz was correlated with performance on the BDS (r =−.47, p =.003) and Stroop test (r =−.38, p =.016). P3 latency at Cz was associated with the |count-hit| (r =.37, p =.02). P3 amplitude at Cz was correlated with the WAIS-III information processing speed (r =.34, p =.043). P3 amplitude at Pz correlated with processing speed (r =.39, p =.02), as well as the BDS (r =.33, p =.04). The main P3 measures did not correlate with CGG repeat length or AR (p > .26), whereas CGG repeat length correlated inversely with processing speed (r =−.35, p =.03) and verbal memory recognition/discriminability (r =−.41, p =.01) on the CVLT.
Group discrimination
A logistic regression model with three P3 measures (latency at Fz, amplitudes at Fz and Pz) classified premutation carriers and normal controls with an overall accuracy of 84.4% (χ2 =23.7, df =3, p=.0003; sensitivity =94.2%, 49/52; specificity =64%, 16/25).
Discussion
Recent studies have suggested that dissociable mechanisms may be mediating distinct phenotypes for male and female fragile X premutation carriers 4, 14, 21–24. Using extensive neuropsychological testing and cognitive ERP, the current study is the first that revealed mild, yet statistically robust executive dysfunction and associated frontal electrophysiological abnormalities in older female premutation carriers with and without FXTAS. An unexpected finding was that female carriers without FXTAS demonstrated more pronounced deficits in working memory. A logistic regression model using P3 measures as predictors identified premutation carriers with a high sensitivity of 94%.
The executive dysfunction found in the present study are consistent with prior neuropsychological studies done predominantly on male carriers8, 9, 18, and extend the notion that executive dysfunction is the primary cognitive impairment among fragile X premutation carriers8 regardless of gender and FXTAS diagnosis. Loesch et al.16 observed poorer BDS total score in male carriers, whereas female carriers only showed decrements on two items of the BDS. The milder impairments in their female carriers could be attributed to the younger age of these women (mean=41) relative to our sample. This age effect could also explain the negative findings for executive dysfunction in two studies examining female carriers around age 4015, 34. However, executive functions as evaluated by the Stroop and COWAT both showed decrements in our prior study on older FXTAS patients27 but not in the present study. This inconsistency is likely due to the different percentage of females examined (29% vs 100%) in the two studies, indicating milder executive deficits in female premutation carriers primarily involving motor regulation and performance monitoring, with executive processes such as interference control and rapid information generation relatively preserved. The milder cognitive deficits in older female fragile X premutation carriers compared to male carriers are likely due to neuroprotective effects of the normal FMR1 allele in the additional X chromosome. The FMR1 mRNA toxic gain-of-function is generally believed to be the pathogenic mechanism of FXTAS35, 36, and FMR1 mRNA levels in female carriers are usually lower than those in their male counterparts (e.g., Adams et al.4).
The finding of more noticeable working memory deficits in FXTAS− is novel and needs to be replicated in independent samples. Although working memory deficits are generally associated with male FXTAS8, 9, FXTAS− men have been found to have preserved working memory across studies8–10, 18. In one study in a larger group of younger (< 50 years of age) female and male asymptomatic carriers, Hunter et al.24 reported no evidence of deficiencies in various cognitive domains including working memory. Therefore, the marked working memory deficits found in our older FXTAS− women (mean age = 55, range: 47–70) suggest synergistic effects of gender, aging and the fragile X premutation, which warrant further systematic investigation.
The brain potential results shed light on the neural mechanisms underlying cognitive impairment in female premutation carriers. The abnormal P3 measured over the frontal lobes in both carrier groups provides electrophysiological evidence for executive dysfunction as the cardinal cognitive deficit in fragile X premutation carriers. Frontal-executive function involves the capacity for autonomous regulation of behavior and attention, and includes processes such as maintaining and updating task-related information in working memory, inhibiting irrelevant information, switching task goals, and performance monitoring37, 38. Based on volumetric MRI findings of significant frontal gray matter loss in FXTAS males12 and the presence of intranuclear inclusions in frontal cortex of female carriers26, the abnormal frontal P3 findings in both female and male carriers27 suggest an association between behavioral and neural signatures of executive dysfunction in fragile X premutation carriers, regardless of gender or FXTAS symptoms.
Unlike the parietal P3 amplitude reduction found in a male-predominant older FXTAS group27, our FXTAS females had decreased and delayed frontal P3 but did not manifest significant abnormalities over posterior brain regions. Following a recent proposed P3 framework39, Yang et al.27 postulated that the frontal P3 (P3a) reflects attentional processing whereas the parietal P3 (P3b) indexes working memory updating. Nonetheless, the current P3 results not only indicate a milder alteration of brain dynamics in female carriers, but also suggest that compromised frontal brain processes may underlie working memory impairments as well (cf. D’Esposito40). It should be noted that only the female FXTAS group had delayed P3 latencies which are associated with slower information processing speed, both of which could originate in the white matter abnormalities highly prevalent in this disorder4. Perhaps, white matter disease or degeneration is critical for the expression of FXTAS, both its motor features and its “fronto-subcortical” type cognitive impairments, but further longitudinal MRI/DTI studies are needed to confirm this hypothesis.
Interestingly, neither group differences in FMR1 activation ratio between the FXTAS+ and FXTAS− subgroups, nor significant correlations between activation ratio and P3 measures were Observed, in agreement with prior reports indicating that FXTAS symptoms in female carriers appear not to be directly modulated by activation ratio4, 21, 23, 26. The mechanisms underlying FMR1 activation ratio and mRNA toxicity are thus likely more complex than originally thought, and additional pathophysiological mechanisms may also be operant (cf. Hagerman41).
The current study provides electrophysiological and neuropsychological evidence of impaired frontal lobe processes among older female fragile X premutation carriers. However, due to the technical limitations of scalp ERP recordings, the present results do not provide direct evidence for impaired frontal lobe substrates. This study limitation also applies to the analyses on earlier auditory components (i.e., N100, P200 and N200). Source localization algorithms such as LORETA (low resolution electromagnetic tomography) might be applied to the current dataset to probe brain structures underlying the observed ERP abnormalities. Further experiments using techniques with higher spatial resolution (e.g., fMRI, MEG) and studies designed to examine individual components of executive function may also advance our understanding of the cognitive and neural phenotypes of FMR1 premutation carriers. Finally, because of the likely synergistic effects of gender, aging, and the premutation on cognition, careful consideration of gender and age effects is highly recommended for future research among the fragile X premutation carriers.
Supplementary Material
Acknowledgements
We would like to thank Yi (Lisa) Mu, Danh Nguyen and Kylee Cook for their great help with data retrieval.
Funding: This work was supported by the National Institutes of Health Roadmap Interdisciplinary Research Consortium Grant, by grant numbers UL1DE019583 (NIDCR), RL1AG032115 (National Institutes on Aging), and RL1AG032119 (National Institutes on Aging), and by National Institutes on Aging grant R01 AG18442.
Footnotes
Disclosures: Dr. Randi J. Hagerman received research support from Seaside therapeutics, Forest and Roche and consultation with Novartis and Roche for fragile X syndrome treatment studies.
References
- 1.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 2.Seritan AL, Nguyen DV, Farias ST, et al. Dementia in fragile X-associated tremor/ataxia syndrome (FXTAS): comparison with Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1138–1144. doi: 10.1002/ajmg.b.30732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 4.Adams JS, Adams PE, Nguyen D, et al. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69:851–859. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- 5.Berry-Kravis E, Abrams L, Coffey SM, et al. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord. 2007;22:2018–2030. doi: 10.1002/mds.21493. quiz 2140. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto R, Javan AK, Tassone F, et al. A voxel-based morphometry study of grey matter loss in fragile X-associated tremor/ataxia syndrome. Brain. 2011;134:863–878. doi: 10.1093/brain/awq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang JY, Hessl DH, Hagerman RJ, et al. Age-dependent structural connectivity effects in fragile x premutation. Arch Neurol. 2012;69:482–489. doi: 10.1001/archneurol.2011.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brega AG, Goodrich G, Bennett RE, et al. The primary cognitive deficit among males with fragile X-associated tremor/ataxia syndrome (FXTAS) is a dysexecutive syndrome. J Clin Exp Neuropsychol. 2008;30:853–869. doi: 10.1080/13803390701819044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigsby J, Brega AG, Engle K, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- 10.Cornish KM, Kogan CS, Li L, et al. Lifespan changes in working memory in fragile X premutation males. Brain Cogn. 2009;69:551–558. doi: 10.1016/j.bandc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olichney JM, Chan S, Wong LM, et al. Abnormal N400 word repetition effects in fragile X-associated tremor/ataxia syndrome. Brain. 2010;133:1438–1450. doi: 10.1093/brain/awq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto R, Backer KC, Tassone F, et al. An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the fragile X mental retardation 1 gene with and without fragile X-associated tremor/ataxia syndrome (FXTAS) J Psychiatr Res. 2011;45:36–43. doi: 10.1016/j.jpsychires.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacquemont S, Hagerman RJ, Leehey MA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 14.Basuta K, Narcisa V, Chavez A, et al. Clinical phenotypes of a juvenile sibling pair carrying the fragile X premutation. Am J Med Genet A. 2011;155A:519–525. doi: 10.1002/ajmg.a.33446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke P, Leboyer M, Hardt J, et al. Neuropsychological profiles of FMR-1 premutation and full-mutation carrier females. Psychiatry Res. 1999;87:223–231. doi: 10.1016/s0165-1781(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 16.Loesch DZ, Bui QM, Grigsby J, et al. Effect of the fragile X status categories and the fragile X mental retardation protein levels on executive functioning in males and females with fragile X. Neuropsychology. 2003;17:646–657. doi: 10.1037/0894-4105.17.4.646. [DOI] [PubMed] [Google Scholar]
- 17.Hocking DR, Kogan CS, Cornish KM. Selective spatial processing deficits in an at-risk subgroup of the fragile X premutation. Brain Cogn. 2012;79:39–44. doi: 10.1016/j.bandc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Hunter JE, Sherman S, Grigsby J, et al. Capturing the fragile X premutation phenotypes: a collaborative effort across multiple cohorts. Neuropsychology. 2012;26:156–164. doi: 10.1037/a0026799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodrich-Hunsaker NJ, Wong LM, McLennan Y, et al. Enhanced Manual and Oral Motor Reaction Time in Young Adult Female Fragile X Premutation Carriers. J Int Neuropsychol Soc. 2011:1–5. doi: 10.1017/S1355617711000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagerman RJ, Leavitt BR, Farzin F, et al. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum Genet. 2004;74:1051–1056. doi: 10.1086/420700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams PE, Adams JS, Nguyen DV, et al. Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:775–785. doi: 10.1002/ajmg.b.31046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter JE, Rohr JK, Sherman SL. Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet. 2010;77:374–381. doi: 10.1111/j.1399-0004.2009.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coffey SM, Cook K, Tartaglia N, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter JE, Allen EG, Abramowitz A, et al. No evidence for a difference in neuropsychological profile among carriers and noncarriers of the FMR1 premutation in adults under the age of 50. Am J Hum Genet. 2008;83:692–702. doi: 10.1016/j.ajhg.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter JE, Epstein MP, Tinker SW, et al. The FMR1 premutation and attention-deficit hyperactivity disorder (ADHD): evidence for a complex inheritance. Behav Genet. 2012;42:415–422. doi: 10.1007/s10519-011-9520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tassone F, Greco CM, Hunsaker MR, et al. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav. 2012;11:577–585. doi: 10.1111/j.1601-183X.2012.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang JC, Chan S, Khan S, et al. Neural Substrates of Executive Dysfunction in Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): a Brain Potential Study. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs251. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J Cogn Neurosci. 2006;18:766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- 29.Fjell AM, Walhovd KB, Fischl B, et al. Cognitive function, P3a/P3b brain potentials, and cortical thickness in aging. Hum Brain Mapp. 2007;28:1098–1116. doi: 10.1002/hbm.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tassone F, Pan R, Amiri K, et al. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10:43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tassone F, Hagerman RJ, Ikle DN, et al. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999;84:250–261. [PubMed] [Google Scholar]
- 33.Grigsby J, Kaye K. The Behavioral Dyscontrol Scale: Manual. 2nd ed. 1996. [Google Scholar]
- 34.Bennetto L, Pennington BF, Porter D, et al. Profile of cognitive functioning in women with the fragile X mutation. Neuropsychology. 2001;15:290–299. [PubMed] [Google Scholar]
- 35.Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Human Molecular Genetics. 2010;19:R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willemsen R, Levenga J, Oostra BA. CGG repeat in the FMR1 gene: size matters. Clinical Genetics. 2011;80:214–225. doi: 10.1111/j.1399-0004.2011.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilbert SJ, Burgess PW. Executive function. Current Biology. 2008;18:R110–R114. doi: 10.1016/j.cub.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Miyake A, Friedman NP, Emerson MJ, et al. The unity and diversity of executive functions and their contributions to complex"frontal lobe" tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 39.Polich J. In: Neuropsychology of P300. Luck SJ, Kappenman ES, editors. New York (NY): Oxford University Press; 2012. [Google Scholar]
- 40.D'Esposito M. From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society B-Biological Sciences. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagerman PJ. Current Gaps in Understanding the Molecular Basis of FXTAS. Tremor Other Hyperkinet Mov (N Y) 2012;2 doi: 10.7916/D80C4TH0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.