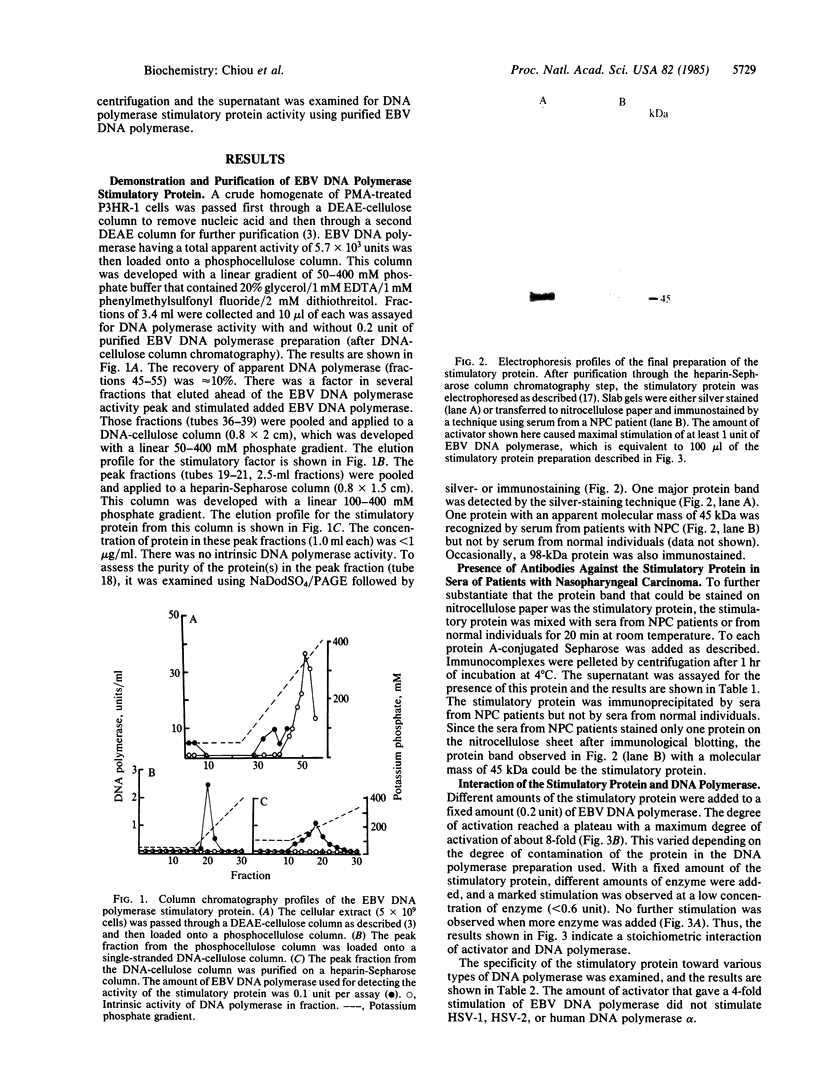
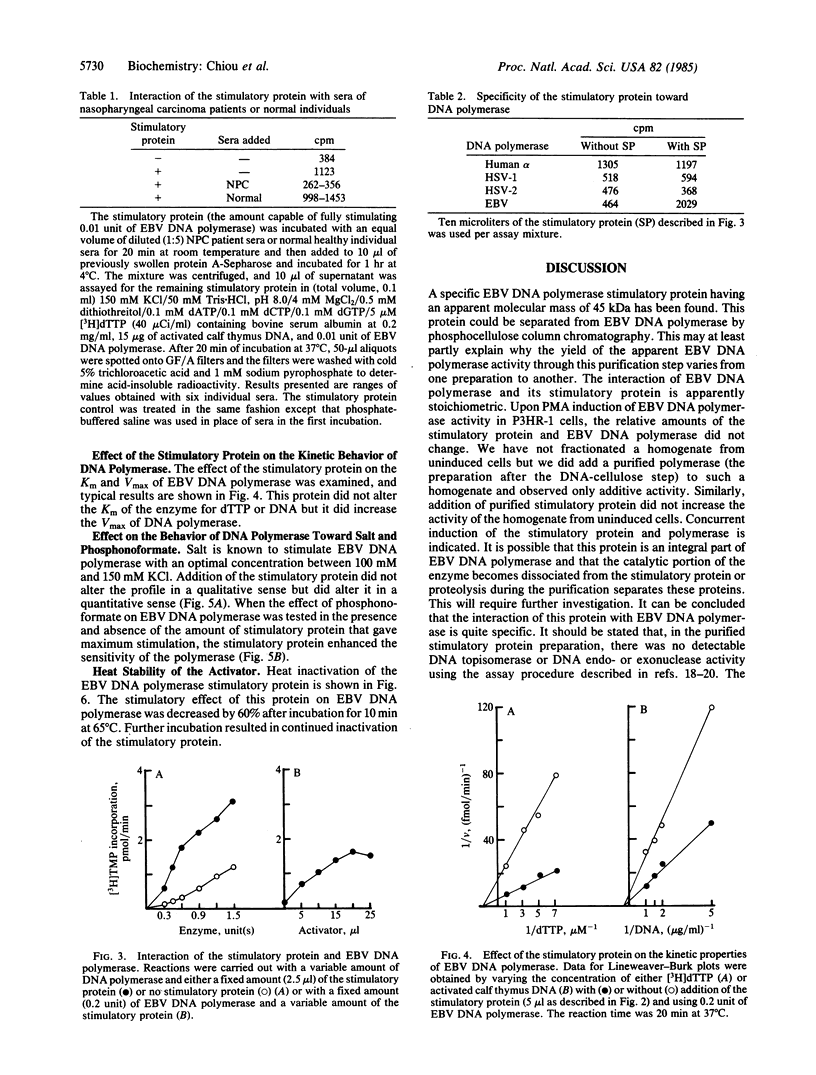
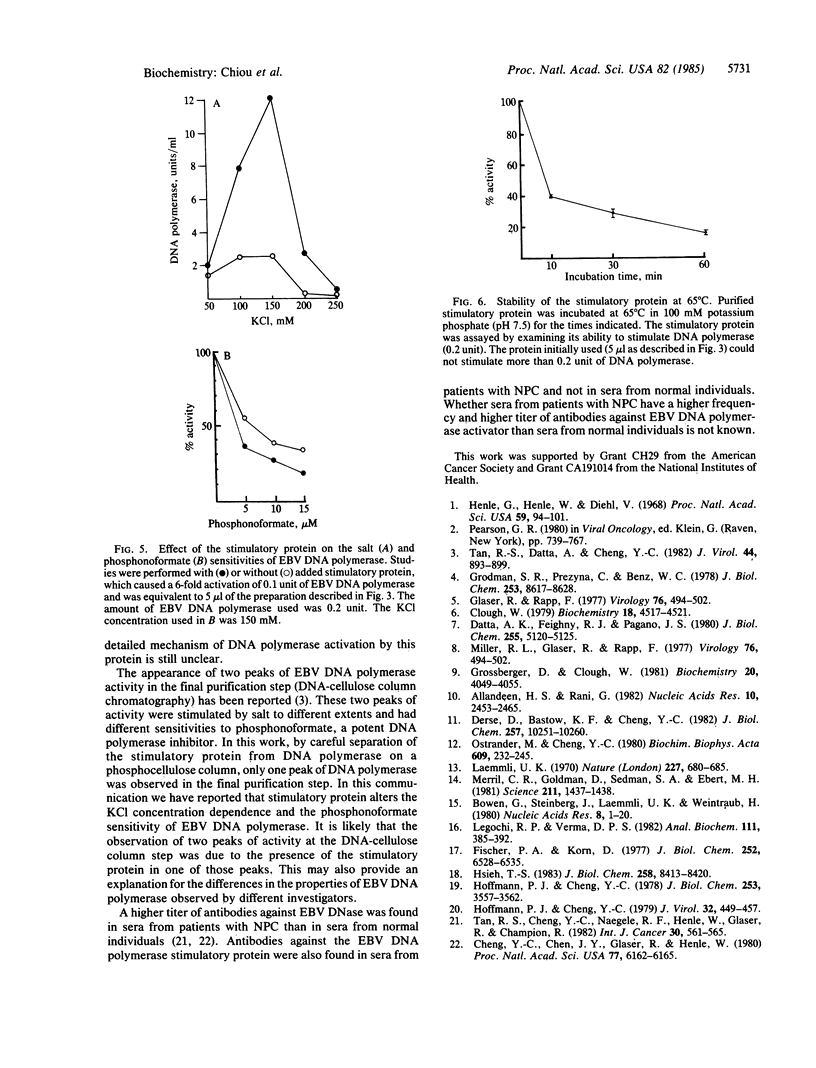
Abstract
A heat-labile Epstein-Barr virus-specific DNA polymerase stimulatory protein having a molecular mass of 45 kDa was purified from phorbol 12-myristate 13-acetate-treated P3HR-1 cells by column chromatography. The virus DNA polymerase stimulatory protein was precipitated by sera from patients with nasopharyngeal carcinoma but not by sera from healthy donors. The interaction of the stimulatory protein with DNA polymerase was stoichiometric. Furthermore, this protein stimulated Epstein-Barr virus DNA polymerase but not herpes simplex virus type 1 or type 2 or human DNA polymerase alpha. The stimulatory protein did not alter the Km value of dTTP or DNA but did increase the Vmax of DNA polymerase. Salt concentrations between 100 mM and 150 mM KCl were optimal for this protein-induced stimulation of Epstein-Barr virus DNA polymerase activity. The presence of the stimulatory protein in the reaction mixture enhanced the sensitivity of virus DNA polymerase to phosphonoformate.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaudeen H. S., Rani G. Cellular and Epstein-Barr virus specific DNA polymerases in virus-producing Burkitt's lymphoma cell lines. Nucleic Acids Res. 1982 Apr 10;10(7):2453–2465. doi: 10.1093/nar/10.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Chen J. Y., Glaser R., Henle W. Frequency and levels of antibodies to Epstein-Barr virus-specific DNase are elevated in patients with nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6162–6165. doi: 10.1073/pnas.77.10.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough W. Deoxyribonuclease activity found in Epstein--Barr virus producing lymphoblastoid cells. Biochemistry. 1979 Oct 16;18(21):4517–4521. doi: 10.1021/bi00588a009. [DOI] [PubMed] [Google Scholar]
- Datta A. K., Feighny R. J., Pagano J. S. Induction of Epstein-Barr virus-associated DNA polymerase by 12-O-tetradecanoylphorbol-13-acetate. Purification and characterization. J Biol Chem. 1980 Jun 10;255(11):5120–5125. [PubMed] [Google Scholar]
- Derse D., Bastow K. F., Cheng Y. Characterization of the DNA polymerases induced by a group of herpes simplex virus type I variants selected for growth in the presence of phosphonoformic acid. J Biol Chem. 1982 Sep 10;257(17):10251–10260. [PubMed] [Google Scholar]
- Fisher P. A., Korn D. DNA polymerase-alpha. Purification and structural characterization of the near homogeneous enzyme from human KB cells. J Biol Chem. 1977 Sep 25;252(18):6528–6535. [PubMed] [Google Scholar]
- Goodman S. R., Prezyna C., Benz W. C. Two Epstein-Barr virus-associated DNA polymerase activities. J Biol Chem. 1978 Dec 10;253(23):8617–8628. [PubMed] [Google Scholar]
- Grossberger D., Clough W. Characterization of purified Epstein--Barr virus induced deoxyribonucleic acid polymerase: nucleotide turnover, processiveness, and phosphonoacetic acid sensitivity. Biochemistry. 1981 Jul 7;20(14):4049–4055. doi: 10.1021/bi00517a016. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. DNase induced after infection of KB cells by herpes simplex virus type 1 or type 2. II. Characterization of an associated endonuclease activity. J Virol. 1979 Nov;32(2):449–457. doi: 10.1128/jvi.32.2.449-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. The deoxyribonuclease induced after infection of KB cells by herpes simplex virus type 1 or type 2. I. Purification and characterization of the enzyme. J Biol Chem. 1978 May 25;253(10):3557–3562. [PubMed] [Google Scholar]
- Hsieh T. Knotting of the circular duplex DNA by type II DNA topoisomerase from Drosophila melanogaster. J Biol Chem. 1983 Jul 10;258(13):8413–8420. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Multiple immunoreplica Technique: screening for specific proteins with a series of different antibodies using one polyacrylamide gel. Anal Biochem. 1981 Mar 1;111(2):385–392. doi: 10.1016/0003-2697(81)90577-7. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Glaser R., Rapp F. Studies of an Epstein-Barr virus-induced DNA polymerase. Virology. 1977 Feb;76(2):494–502. doi: 10.1016/0042-6822(77)90232-x. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Glaser R., Rapp F. Studies of an Epstein-Barr virus-induced DNA polymerase. Virology. 1977 Feb;76(2):494–502. doi: 10.1016/0042-6822(77)90232-x. [DOI] [PubMed] [Google Scholar]
- Ostrander M., Cheng Y. C. Properties of herpes simplex virus type 1 and type 2 DNA polymerase. Biochim Biophys Acta. 1980 Sep 19;609(2):232–245. doi: 10.1016/0005-2787(80)90234-8. [DOI] [PubMed] [Google Scholar]
- Tan R. S., Cheng Y. C., Naegele R. F., Henle W., Glaser R., Champion J. Antibody responses to Epstein-Barr virus-specific DNase in relation to the prognosis of juvenile patients with nasopharyngeal carcinoma. Int J Cancer. 1982 Nov 15;30(5):561–565. doi: 10.1002/ijc.2910300505. [DOI] [PubMed] [Google Scholar]
- Tan R. S., Datta A. K., Cheng Y. C. Identification and characterization of a DNase induced by Epstein-Barr virus. J Virol. 1982 Dec;44(3):893–899. doi: 10.1128/jvi.44.3.893-899.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]





