Abstract
There is substantial evidence for broad cross-reactive immunity and heterotypic protection among human rotavirus strains in children with natural infection or with monovalent Rotarix vaccination. In this commentary, we addressed this same topic by testing sera of guinea pigs and gnotobiotic piglets that were intramuscularly immunized with an inactivated human rotavirus vaccine and also demonstrated a broad cross-protective immunity among human rotavirus strains. Our findings from a single human strain in animal studies bode well for a low cost and efficacious inactivated vaccine to protect children against rotavirus disease throughout the world.
Keywords: rotavirus, IRV, CDC-9, heat inactivation, heterotypic protection
The two currently licensed oral rotavirus vaccines, RotaTeq and Rotarix, which are very effective in reducing cases of severe diarrhea among children in developed and middle income countries,1,2 are much less efficacious (~50%) in low income countries of Africa and Asia.3-5 This same gradient of lower immune responses and protection linked to low socio-economic status of the population is similar to published data from other live oral vaccines tested previously, including early rotavirus vaccines, polio vaccine (OPV) and a cholera vaccine.6 Consequently, the impact of vaccination on rotavirus disease and death in low-income countries of Africa and Asia has not been established. In addition, the two current rotavirus vaccines have been associated with a low risk (1:~50,000) of intussusception among vaccinated infants7,8 and diarrhea in vaccinated and unvaccinated children although the incidence and significance of this vaccine-acquired diarrhea remain to be determined.9,10
To improve the safety and efficacy of oral rotavirus vaccines, we have pursued development of an inactivated rotavirus vaccine (IRV).11 An IRV administered parenterally could avoid some of the problems potentially inherent with live oral vaccines —neutralization from antibodies and other antiviral substances in breast milk, transplacental antibody secreted onto the small intestine or interference from other microorganisms that diminish the efficacy of live oral vaccines. Consequently, a parenteral rotavirus vaccine could be more efficacious for all children, rich and poor. As a parenteral vaccine, an IRV might not be expected a priori to cause intussusception or gastroenteritis or to harbor porcine circoviruses, both issues identified in past licensure studies with oral rotavirus vaccines. If an IRV were able to be combined with other pediatric vaccines (e.g., DTaP, IPV), the incremental cost of vaccine administration would be nil and there would be no need for a separate supply or cold chain, both advantages given the current volume of oral vaccines in the present cold chain.
We have developed a candidate human strain CDC-9 that was isolated from fecal specimen of a child in the United States. The strain is a single gene reassortant with the VP3 gene derived naturally from a G2P4 virus and the other 10 genes from a G1P8 virus, the most common genotype throughout the world.12 The strain was selected by serial passages and plaque purification, grows to high titer (up to 108 ffu/ml) in Vero cells and produces predominantly (>90%) triple-layered virus particles which demonstrate robust stability during upstream production and downstream purification processes. These unique characteristics have not been found with other rotavirus strains and could have added value in producing a low cost and efficacious IRV.
We then developed a novel, thermal method for inactivation. We used this approach because inactivation with β-propiolactone (BPL), an agent commonly used for the inactivation of many viruses, has been shown to cause severe damage to the integrity and biochemical composition of rotavirus particles (Fig. 1). In addition, BPL-treated rotavirus showed reduced viral hemagglutinating activity and intramuscular injection with this material in mice evoked less neutralizing antibody than immunization with live virus.13 By contrast, we showed that inactivation by heat was rapid, simple, and maintained the integrity and preserved the antigenicity of virus particles.14 We further demonstrated that CDC-9 IRV when adjuvanted with AlPO4 and administered intramuscularly was highly immunogenic and protected piglets from oral challenge with a virulent homotypic human strain.15
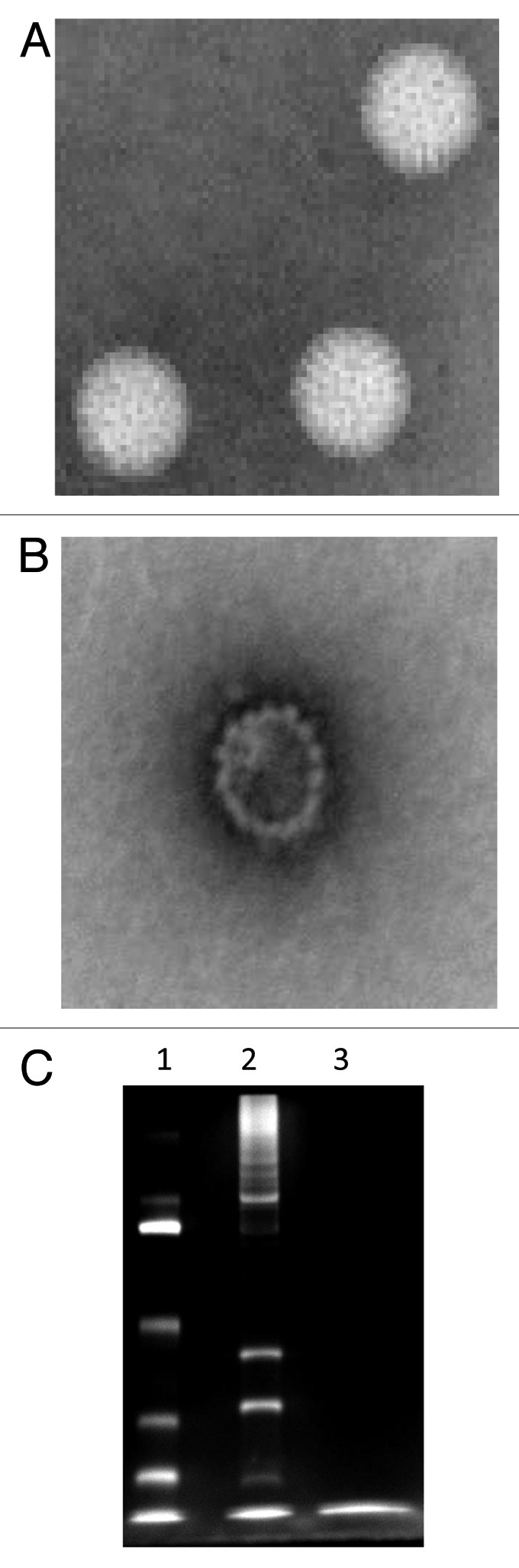
Figure 1. Inactivation of rotavirus using β-propiolactone. Purified live (A) and β-propiolactone inactivated (B) rotavirus particles were stained with phosphatungstic acid and examined with an electron microscope. Live and inactivated rotavirus particles were analyzed on a 12% polyacrylamide gel followed by silver staining (C). Lanes 1, molecular mass markers and 2 and 3, live and killed rotavirus, respectively. Note: major structural rotavirus proteins are seen in lane 2 but are no longer observed in lane 3.
To answer the question whether or not a single strain would induce cross-reactive immunity to different rotavirus genotypes or if a vaccine would need to include multiple strains, we analyzed sera from piglets vaccinated with G1P8 IRV to assess heterotypic neutralizing activity against non-G1P8 human strains, including a G8P4 MW333 strain with a short RNA electropherotype (Fig. 2). Six piglets that received three doses of IRV developed high neutralizing titers (GMT = 403) against the homotypic Wa strain. These vaccinated piglets also developed low to moderate levels of neutralizing titer against the semi-homotypic WI61 strain (GMT = 28) and the heterotypic MW333 strain (GMT = 90).
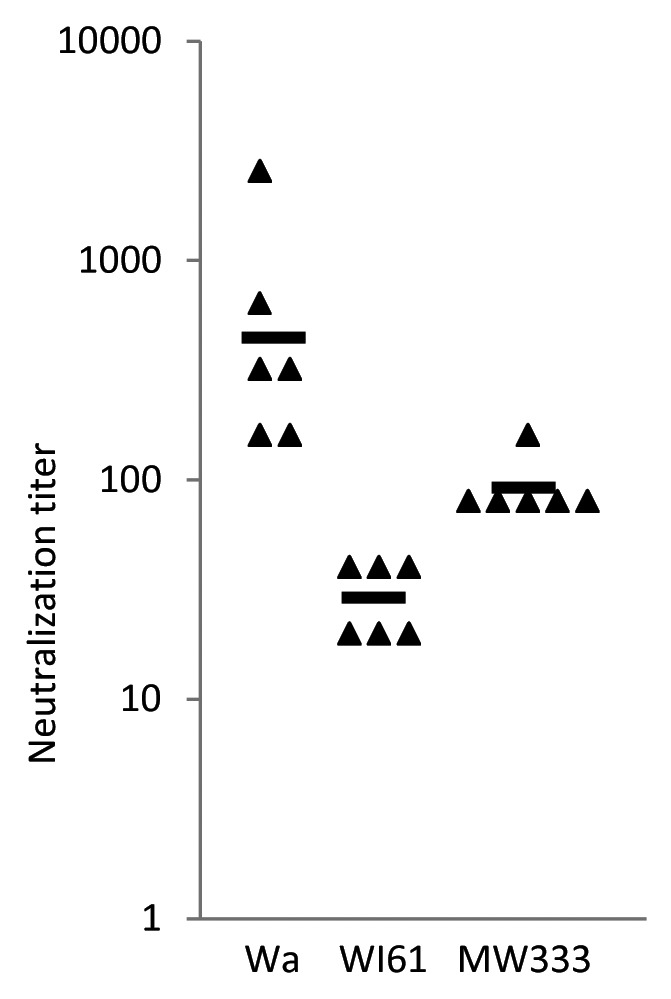
Figure 2. Rotavirus-specific neutralizing activity in sera of gnotobiotic piglets vaccinated with heat-inactivated CDC-9 strain. Six animals were intramuscularly immunized three times with IRV and neutralizing activity in serum was measured using a microneutralization assay.15 Individual neutralization titers and GMT of each group are shown by the characters “▲” and “▬,” respectively. In control, four piglets that received three doses of placebo vaccine all had undetectable titers (<20) of neutralizing activity against the three strains (data not shown).
We further investigated homotypic and heterotypic immunity by examining the kinetics of antibody and neutralizing activity responses to our IRV in a second mammalian species, guinea pigs (Table 1). All animals had undetectable or very low levels of IgG and neutralizing activity in pre-bled and post-dose 1 sera. Animals that received two doses of IRV developed significantly higher titers of IgG (mean = 3,040) and neutralizing activity against the two human strains—homotypic Wa (mean = 320) and heterotypic MW333 (mean = 176). By contrast, animals that received two doses of IRV developed only slightly elevated neutralizing activity against the bovine WC3-human WI79 (G1P5) reassortant (mean = 28) and undetectable or low levels of neutralizing activity against the bovine-human WC3-WI79 (G6P8) reassortant (mean < 20) and the parent WC3 bovine strain (mean = 20). A third dose of IRV further significantly boosted IgG (mean = 23,040) and neutralizing activity titers against the strains Wa (mean = 2048) and MW333 (mean = 704) as well as the G1P5 reassortant (mean = 176). A third dose of IRV only slightly increased neutralizing titer against the WC3 strain (mean = 44) but evoked no detectable neutralizing titer against the G6P8 reassortant virus (mean < 20).
Table 1. Rotavirus-specific IgG and neutralizing activity in IRV-vaccinated guinea pigs.
| Pre | Post dose 1 | Post dose 2 | Post dose 3 | |
|---|---|---|---|---|
| (day 0) | (day 30) | (day 60) | (day 74) | |
| IgG | <100 | <100 | 3,040 ± 960 | 23,040 ± 2,560 |
| Neutralizing activity against | ||||
| Human Wa (G1P8) | 21 ± 12.4 | 20 | 320 ± 88 | 2048 ± 314 |
| Human MW333 (G8P4) | <20 | <20 | 176 ± 39 | 704 ± 157 |
| Bovine WC3-human WI79 (G1P5) | <20 | <20 | 28 ± 5 | 176 ± 39 |
| Bovine WC3-human WI79 (G6P8) | <20 | <20 | <20 | <20 |
| Bovine WC3 (G6P5) | <20 | <20 | <20 | 44 ± 10 |
Five guinea pigs were bled and intramuscularly immunized three times on days 0, 30 and 60 with 50 μg of heat-inactivated CDC-9 formulated in 800 μg of Al(OH)3 (Accurate Chemical and Scientific Corporation), with a 1-mo interval between immunizations and were exsanguinated two weeks after the third injection. Rotavirus-specific IgG and neutralizing activity in sera of IRV-vaccinated guinea pigs were measured by EIA and a microneutralization assay.15 Each serum specimen was tested at an initial dilution of 1:100 and 1:20 for IgG and neutralizing activity, respectively. IgG or neutralizing titers (mean ± 3 standard errors) in a group are shown.
Our findings of IRV testing in piglets and guinea pigs should provide guidance for the development of new parenteral rotavirus vaccines and inform the decision concerning whether a single human strain could protect against diverse human serotypes. First, the choice of strains matters. We demonstrated that a monovalent human IRV induced broad and high-titer cross neutralizing activity against homotypic and heterotypic human strains, but much lower or no detectable neutralizing activity against the bovine-human reassortants or the bovine strain. Second, the number of doses is critical. We showed that two doses of IRV were needed to induce elevated neutralizing activity against homotypic and heterotypic human strains, while three doses of IRV induced low levels of neutralizing activity against the WC3-WI79 G1P5 reassortant or the WC3 virus. Third, we observed a 4-fold higher neutralizing titer against the WC3-WI79 G1P5 reassortant than the WC3 virus in sera of guinea pigs that received three doses of IRV. However, we were not able to detect neutralizing titer against the WC3-WI79 G6P8 reassortant in the same sera, suggesting possible poor expression or antigenicity of the human rotavirus VP4 in the reassortant virus. These findings confirm the importance of rotavirus VP7 in the induction of high-titer cross-reactive neutralizing antibody and suggest that a human strain could mediate better protection against rotavirus infection than an animal strain in humans.
Our findings of heterologous immunity developing from exposure to a single rotavirus strain are similar to those from studies of the natural history of rotavirus and from experience with the monovalent rotavirus vaccine. Following first rotavirus infection, children often are protected or develop less severe disease on subsequent infections with the same or heterologous rotavirus genotypes.16,17 Similarly, infants vaccinated with the monovalent Rotarix are protected from subsequent infections with homologous or heterologous human strains.18 These data from natural infection and Rotarix vaccination studies provide clear evidence for broad cross-reactive immunity and heterotypic protection among human strains in children. Our findings that a human strain could induce broad cross-reactive neutralizing antibody to different human genotypes in animal studies bode well for a single strain approach to develop an IRV, which may lead to an effective and low cost vaccine to protect children against rotavirus disease. However, whether an IRV based on a single animal strain or a more complicated mixture of animal-human reassortant strains would be equally effective in inducing broad cross-reactive immunity and protection in children remains to be determined.
Acknowledgments
We thank Linda Saif of the Ohio State University for conducting the initial challenge study of IRV in gnotobiotic piglets, Stan Cryz of PATH for sponsoring the immunogenicity testing of IRV in guinea pigs and Charles Humphrey for performing the analysis of live and inactivated rotavirus particles by electron microscopy.
The finding and conclusions in this report are those of the authors and do not necessarily represent to the views of CDC
Disclosure of Potential Conflicts of Interest
Drs. Jiang and Glass and Ms. Wang hold patents through CDC for their work with inactivated rotavirus vaccine.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/24958
References
- 1.Tate JE, Mutuc JD, Panozzo CA, Payne DC, Cortese MM, Cortes JE, et al. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J. 2011;30:S30–4. doi: 10.1097/INF.0b013e3181ffe3eb. [DOI] [PubMed] [Google Scholar]
- 2.Yen C, Armero Guardado JA, Alberto P, Rodriguez Araujo DS, Mena C, Cuellar E, et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J. 2011;30:S6–10. doi: 10.1097/INF.0b013e3181fefa05. [DOI] [PubMed] [Google Scholar]
- 3.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–98. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 4.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 5.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 6.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis. 2009;200:S39–48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel MM, López-Collada VR, Bulhões MM, De Oliveira LH, Bautista Márquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364:2283–92. doi: 10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- 8.Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. PAEDS/APSU Study Group Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011;29:3061–6. doi: 10.1016/j.vaccine.2011.01.088. [DOI] [PubMed] [Google Scholar]
- 9.Payne DC, Edwards KM, Bowen MD, Keckley E, Peters J, Esona MD, et al. Sibling transmission of vaccine-derived rotavirus (RotaTeq) associated with rotavirus gastroenteritis. Pediatrics. 2010;125:e438–41. doi: 10.1542/peds.2009-1901. [DOI] [PubMed] [Google Scholar]
- 10.Patel NC, Hertel PM, Estes MK, de la Morena M, Petru AM, Noroski LM, et al. Vaccine-acquired rotavirus in infants with severe combined immunodeficiency. N Engl J Med. 2010;362:314–9. doi: 10.1056/NEJMoa0904485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang B, Gentsch JR, Glass RI. Inactivated rotavirus vaccines: a priority for accelerated vaccine development. Vaccine. 2008;26:6754–8. doi: 10.1016/j.vaccine.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Esona MD, Foytich K, Wang Y, Shin G, Wei G, Gentsch JR, et al. Molecular characterization of human rotavirus vaccine strain CDC-9 during sequential passages in Vero cells. Hum Vaccin. 2010;6:247–53. doi: 10.4161/hv.6.3.10409. [DOI] [PubMed] [Google Scholar]
- 13.Offit PA, Dudzik KI. Noninfectious rotavirus (strain RRV) induces an immune response in mice which protects against rotavirus challenge. J Clin Microbiol. 1989;27:885–8. doi: 10.1128/jcm.27.5.885-888.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang B, Wang Y, Saluzzo JF, Bargeron K, Frachette MJ, Glass RI. Immunogenicity of a thermally inactivated rotavirus vaccine in mice. Hum Vaccin. 2008;4:143–7. doi: 10.4161/hv.4.2.5263. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Azevedo M, Saif LJ, Gentsch JR, Glass RI, Jiang B. Inactivated rotavirus vaccine induces protective immunity in gnotobiotic piglets. Vaccine. 2010;28:5432–6. doi: 10.1016/j.vaccine.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Velázquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–8. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 17.Bishop RF, Barnes GL, Cipriani E, Lund JS. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983;309:72–6. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- 18.Steele AD, Neuzil KM, Cunliffe NA, Madhi SA, Bos P, Ngwira B, et al. Human rotavirus vaccine Rotarix™ provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infect Dis. 2012;12:213–20. doi: 10.1186/1471-2334-12-213. [DOI] [PMC free article] [PubMed] [Google Scholar]


