Abstract
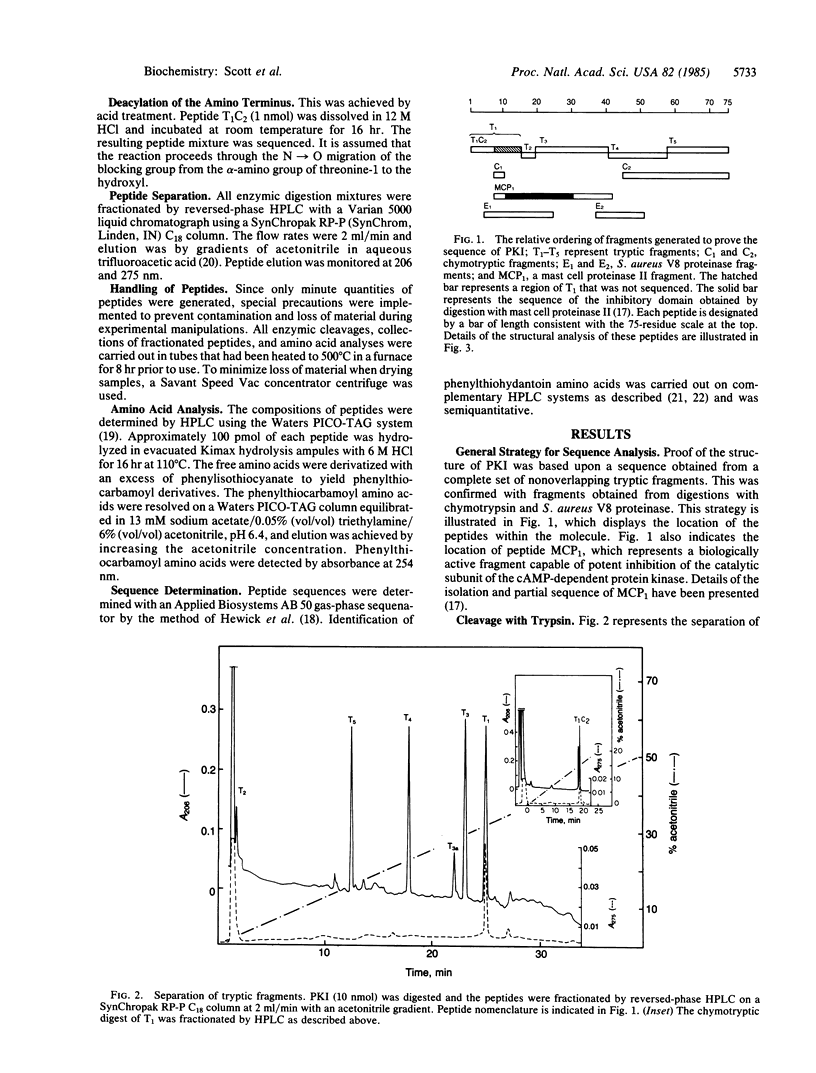
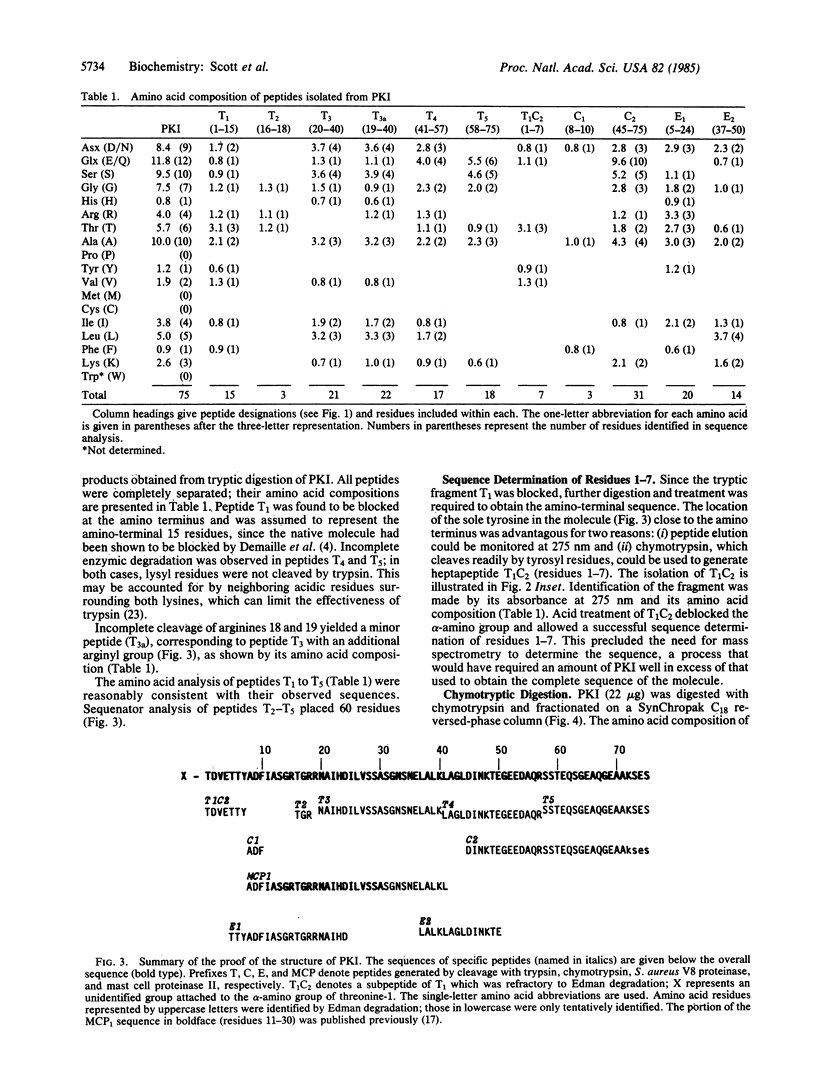
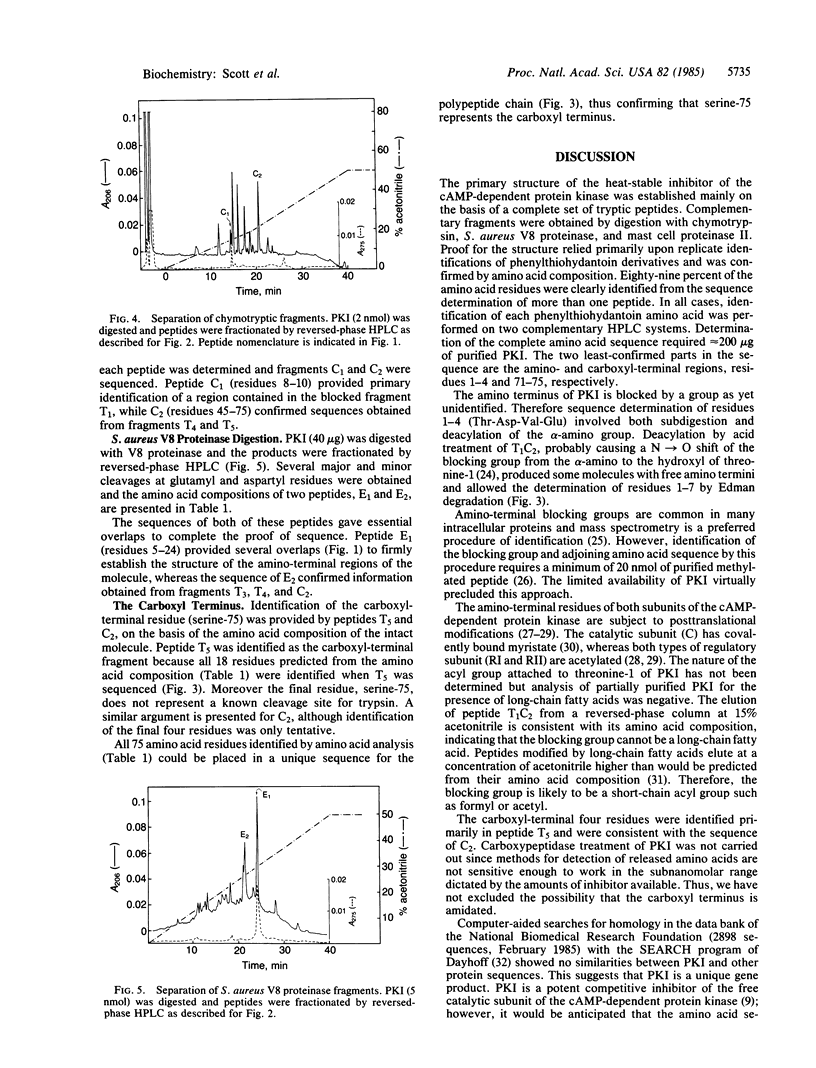
The amino acid sequence of rabbit skeletal muscle heat-stable inhibitor of the cAMP-dependent protein kinase has been determined by microsequencing techniques. Proof of the structure involved a series of nonoverlapping tryptic fragments for primary identification of 86% of the amino acids. Complementary fragments generated by cleavage with chymotrypsin, Staphylococcus aureus V8 proteinase, and mast cell proteinase II contributed to proof of the structure. The inhibitor is a single polypeptide chain of 75 residues and has a molecular weight of 7829. It lacks tryptophan, proline, and sulfur-containing amino acids. The amino terminus of the inhibitor is blocked by an unidentified group. The amino-terminal region of the molecule contains the kinase inhibitory domain, and synthetic peptides based on the sequence of residues 11-30 are potent competitive inhibitors of the cAMP-dependent protein kinase [Scott, J. D., Fischer, E. H., Demaille, J. G. & Krebs, E. G. (1985) Proc. Natl. Acad. Sci. USA 82, 4379-4383]. Residues 14-22 show considerable homology to the "hinge-regions" of the regulatory subunits of the cAMP-dependent protein kinase. The remainder of the molecule shows no similarity to the known amino acid sequence of any protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby C. D., Walsh D. A. Characterization of the interaction of a protein inhibitor with adenosine 3',5'-monophosphate-dependent protein kinases. II. Mechanism of action with the holoenzyme. J Biol Chem. 1973 Feb 25;248(4):1255–1261. [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bridgen P. J., Cross G. A., Bridgen J. N-terminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature. 1976 Oct 14;263(5578):613–614. doi: 10.1038/263613a0. [DOI] [PubMed] [Google Scholar]
- Brostrom M. A., Reimann E. M., Walsh D. A., Krebs E. G. A cyclic 3',5'-amp-stimulated protein kinase from cardiac muscle. Adv Enzyme Regul. 1970;8:191–203. doi: 10.1016/0065-2571(70)90017-8. [DOI] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaille J. G., Ferraz C., Fischer E. H. The protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. The NH2-terminal portion of the peptide chain contains the inhibitory site. Biochim Biophys Acta. 1979 Aug 22;586(2):374–383. doi: 10.1016/0304-4165(79)90106-5. [DOI] [PubMed] [Google Scholar]
- Demaille J. G., Peters K. A., Fischer E. H. Isolation and properties of the rabbit skeletal muscle protein inhibitor of adenosine 3',5'-monophosphate dependent protein kinases. Biochemistry. 1977 Jul 12;16(14):3080–3086. doi: 10.1021/bi00633a006. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Glass D. B., Krebs E. G. Optimal spatial requirements for the location of basic residues in peptide substrates for the cyclic AMP-dependent protein kinase. J Biol Chem. 1980 May 10;255(9):4240–4245. [PubMed] [Google Scholar]
- Ferraz C., Demaille J. G., Fisher E. H. The protein inhibitor of adenosine 3':5'-monophosphate-dependent protein kinases. Isolation and characterization of three isoinhibitors. Biochimie. 1979;61(5-6):645–651. doi: 10.1016/s0300-9084(79)80162-5. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Humble E., Berglund L., Titanji V., Ljungström O., Edlund B., Zetterqvist O., Engström L. Non-dependence on native structure of pig liver pyruvate kinase when used as a substrate for cyclic 3',5'-AMP-stimulated protein kinase. Biochem Biophys Res Commun. 1975 Sep 16;66(2):614–621. doi: 10.1016/0006-291x(75)90554-9. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Benjamini E., Krebs E. G. Synthetic hexapeptide substrates and inhibitors of 3':5'-cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1038–1042. doi: 10.1073/pnas.73.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- McPherson J. M., Whitehouse S., Walsh D. A. Possibility of shape conformers of the protein inhibitor of the cyclic adenosine monophosphate dependent protein kinase. Biochemistry. 1979 Oct 30;18(22):4835–4845. doi: 10.1021/bi00589a011. [DOI] [PubMed] [Google Scholar]
- Potter R. L., Taylor S. S. Correlation of the cAMP binding domain with a site of autophosphorylation on the regulatory subunit of cAMP-dependent protein kinase II from porcine skeletal muscle. J Biol Chem. 1979 Sep 25;254(18):9000–9005. [PubMed] [Google Scholar]
- Scott J. D., Fischer E. H., Demaille J. G., Krebs E. G. Identification of an inhibitory region of the heat-stable protein inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4379–4383. doi: 10.1073/pnas.82.13.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Parmelee D. C., Wade R. D., Kumar S., Ericsson L. H., Walsh K. A., Neurath H., Long G. L., Demaille J. G., Fischer E. H. Complete amino acid sequence of the catalytic subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):848–851. doi: 10.1073/pnas.78.2.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takio K., Smith S. B., Krebs E. G., Walsh K. A., Titani K. Amino acid sequence of the regulatory subunit of bovine type II adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1984 Aug 28;23(18):4200–4206. doi: 10.1021/bi00313a029. [DOI] [PubMed] [Google Scholar]
- Takio K., Walsh K. A., Neurath H., Smith S. B., Krebs E. G., Titani K. The amino acid sequence of a hinge region in the regulatory subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase II. FEBS Lett. 1980 May 19;114(1):83–88. doi: 10.1016/0014-5793(80)80865-9. [DOI] [PubMed] [Google Scholar]
- Titani K., Sasagawa T., Ericsson L. H., Kumar S., Smith S. B., Krebs E. G., Walsh K. A. Amino acid sequence of the regulatory subunit of bovine type I adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1984 Aug 28;23(18):4193–4199. doi: 10.1021/bi00313a028. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]
- Walsh K. A., Ericsson L. H., Parmelee D. C., Titani K. Advances in protein sequencing. Annu Rev Biochem. 1981;50:261–284. doi: 10.1146/annurev.bi.50.070181.001401. [DOI] [PubMed] [Google Scholar]
- Walsh K. A., Sasagawa T. High-performance liquid chromatography probes for posttranslationally modified amino acids. Methods Enzymol. 1984;106:22–29. doi: 10.1016/0076-6879(84)06005-5. [DOI] [PubMed] [Google Scholar]
- Whitehouse S., McPherson J. M., Walsh D. A. Characterization of multiple charge isomers of the inhibitor protein of the cyclic AMP-dependent protein kinase from bovine heart and rabbit skeletal muscle. Arch Biochem Biophys. 1980 Sep;203(2):734–743. doi: 10.1016/0003-9861(80)90233-7. [DOI] [PubMed] [Google Scholar]
- Whitehouse S., Walsh D. A. Mg X ATP2-dependent interaction of the inhibitor protein of the cAMP-dependent protein kinase with the catalytic subunit. J Biol Chem. 1983 Mar 25;258(6):3682–3692. [PubMed] [Google Scholar]
- Zetterqvist O., Ragnarsson U. The structural requirements of substrates of cyclic AMP-dependent protein kinase. FEBS Lett. 1982 Mar 22;139(2):287–290. doi: 10.1016/0014-5793(82)80872-7. [DOI] [PubMed] [Google Scholar]


