Abstract
One of the major obstacles to achieving complete eradication of tumors, even in the presence of circulating tumor-specific immunity, is the tumor-induced immunosuppressive environment, which includes myeloid-derived suppressor cells and regulatory T cells. Attenuated microorganisms have emerged as candidates for a novel anti-cancer approach in which they enhance anti-cancer immunity by boosting the innate immune system. Herein, we will discuss current innate-immunity activating strategies for anti-cancer therapy, with a focus on our recently reported approach involving the use of intratumoral injection of recombinant attenuated Salmonella enterica serovar Typhimurium vaccine; this approach elicits transformation of immunosuppressive myeloid-derived suppressor cells into TNF-α-secreting cells with characteristics of neutrophils, and reduces the generation of regulatory T cells, particularly in the presence of tumor-specific cytotoxic T lymphocytes.
Keywords: Salmonella, tumor, myeloid-derived suppressor cell, regulatory T cell, cytotoxic T lymphocytes
Introduction
Ever since William B. Coley introduced “Coley’s toxins” using the heat-killed bacteria Streptococcus pyogenes and Serratia marcescens for tumor therapy over a century ago,1 many anti-tumor therapeutic approaches using attenuated microorganisms have been investigated. These include Bacillus Calmette-Guerin (BCG), Listeria monocytogenes, Salmonella spp, Clostridium spp and Toxoplasma gondii. These approaches are summarized in Table 1.
Table 1. Cancer immunotherapy based on killing of bacteria-infected tumor cells.
| Microorgamisms | Injection route | Model | Target cancer | Mechanism | Reference |
|---|---|---|---|---|---|
| Salmonella Typhimurium A1 (Leu and Arg auxotroph) | Intravenous or Intratumoral | Nu/nu mice | Human PC-3 prostate cancer cells | Apoptosis induction | 2 |
| Primary orthotopic pancreatic tumor | 3 | ||||
| Orthotopic human breast tumor | 4 | ||||
| Salmonella Typhimurium 14028 strain | Intraperitoneal | C57BL/6 mice | B16F1 melanoma cells | Downregulation of CD44high and CD4+CD25+Tregs | 5 |
| Salmonella Typhimurium SL3261AT InvA | Intratumoral | C57BL/6J mice | B16F10 and EG-7 cells | Cytotoxic T cells and intratumoral recruitment of Gr1hi granulocytes | 6 |
| Salmonella Typhi CVD915 | Intratumoral and peritumoral | BALB/c mice | LM3 mammary adenocarcinoma | IFN-γ-secreting CD4+ and CD8+ T cells Reduction of Tregs TNF-α-secreting neutrophils |
7 |
| C57BL/6 mice | EL4 T cell lymphoma | 8 | |||
| Salmonella choleraesuis | Intraperitoneal | C3H/HeN and C3H/HeJ mice | Murine K1735 melanoma cells | TLR4-dependent TH1 response | 9 |
| Propionibacterium acnes | Intratumoral | C57BL/6 mice | B16 melanoma cells | TH1 immune responses and secretion of IL-12, IFN-γ, and TNF-α | 10 |
| Toxoplasma gondii (cps, uracil auxotroph) | Intratumoral | C57BL/6 mice | B16F10 melanoma cells |
CD8+ T cells and NK cells | 11 |
| Bacillus Calmette–Guérin | Intravesical | Human patients | Bladder cancer | TNF-α, TRAIL and neutrophils | 12,13 |
| Listeria monocytogenes-LLO | Intraperitoneal | BALB/c mice | 4T1 mammary carcinoma | CD8+ T cells | 14 |
| Clostridium novyi non-toxic (NT) spore | Intravenous | C57BL/6N mice | Pancreatic tumor Panc02 cells | NK cells and innate immunity | 46 |
Our recently reported approach showed a potential therapeutic anti-tumor effect of intratumoral delivery of attenuated Salmonella enterica serovar Typhimurium.15 Inflammatory responses were induced within the tumor microenvironment, consequently promoting conversion of immunosuppressive myeloid-derived suppressor cells (MDSCs) into TNF-α-secreting myeloid cells.15 Similarly, others have recently reported that an attenuated but still invasive Salmonella spp preferentially invaded the tumor area, exerting both direct and indirect antitumor effects via recruitment of inflammatory cells and cross-presentation of the tumor antigen.6 Interestingly, intratumoral administration of attenuated Salmonella typhi CVD915 elicited antitumor effects by recruitment of activated TNF-α-secreting neutrophils to the tumor site, and reducing regulatory T cells (Tregs) in tumor-draining lymph nodes (LNs).7 In addition, a critical role of TNF-α in the anti-cancer effects of BCG-stimulated neutrophils in the immunotherapeutic treatment of bladder cancer has been suggested.12
Despite the immunostimulating effect of various immunotherapeutic approaches against cancer, the immunosuppressive environment produced by the tumor can restrict the antitumor potential of these approaches.16 Thus, there is an urgent need to develop effective ways to subvert tumor-driven immune escape mechanisms, while potentiating tumoricidal effects. In this regard, Salmonella-based anti-tumor immunotherapies shed light on the development of effective ways to treat tumor patients, in that they can specifically target and colonize the tumor site, promote an inflammatory response by inducing infiltration of neutrophils, induce tumor-specific T-cell responses and importantly, reduce immunosuppressive cells including MDSCs and Tregs.
Ways to Subvert the Immunosuppressive Tumor Microenvironment
In the tumor microenvironment, there are various tumor-infiltrating immune cells, including immune effectors and immune suppressors.17 Although some tumors are potentially immunogenic, immune suppressors present an obstacle to tumor rejection.17 MDSCs are one of the critical immune suppressors.16 The numbers of MDSCs increase in various inflammatory diseases, including cancer.18 While the definition of MDSCs has been based on their immunosuppressive nature, MDSCs are a heterogeneous population and have diverse immunosuppressive mechanisms, including arginase 1, nitric oxide, reactive oxygen species and membrane-bounded TGF-β.19-21 In a recent study by our group, two major subsets of MDSCs, Ly6-GhighLy6-Cinter cells (granulocytic MDSCs) and Ly6-GinterLy6-Chigh cells (monocytic MDSCs) were detected, but only the Ly6-GhighLy6-Cinter subset increased by intratumoral injection of recombinant attenuated Salmonella enterica serovar Typhimurium vaccine (RASV)15 (Fig. 1). These data suggest that each subset of MDSCs may constitute a separate population, induced under distinct circumstances.
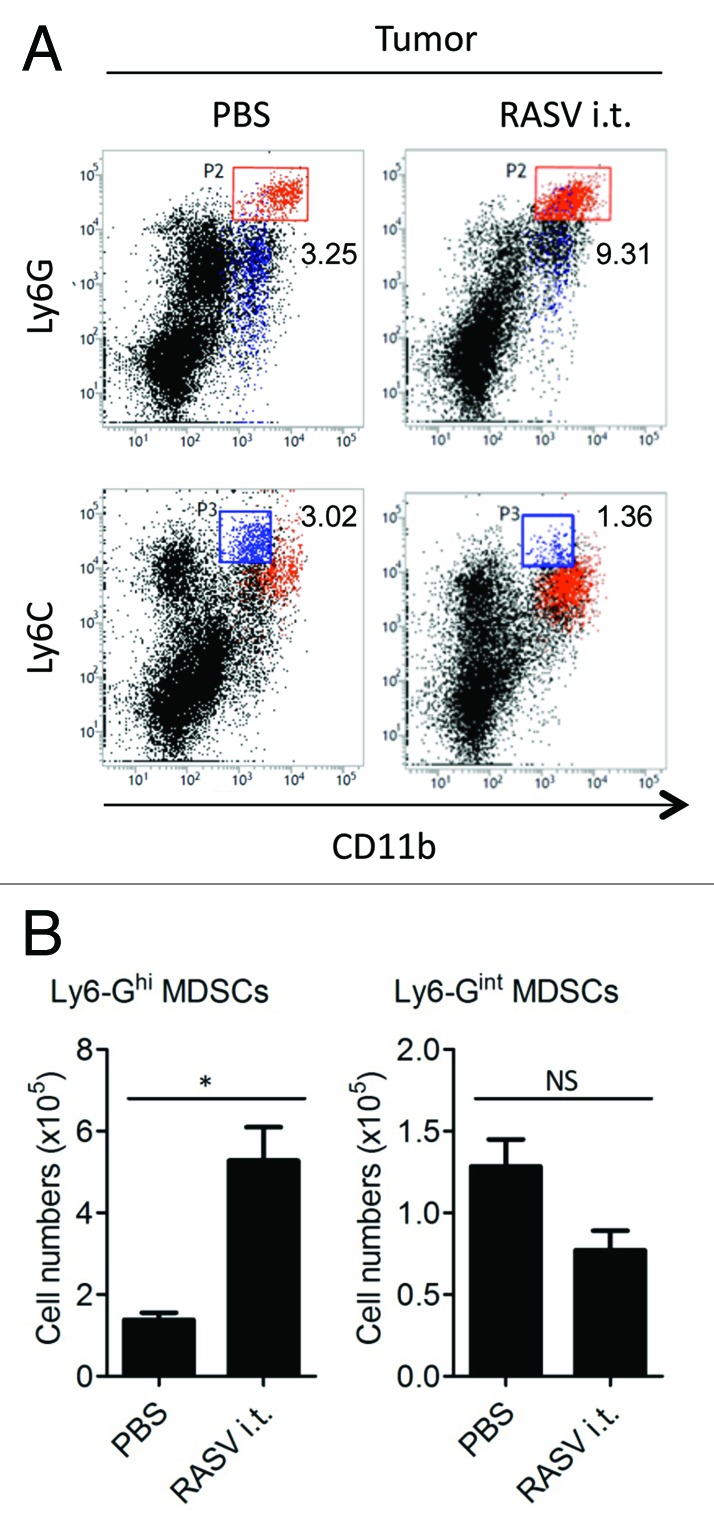
Figure 1. RASV increased Ly6-Ghigh MDSCs in the tumor. Two subsets of MDSCs were evident, Ly6-GhighLy6-Cinter cells (upper panel) and Ly6-G interLy6-Chigh cells (lower panel). (A) FACS plot percentages and (B) absolute number of each MDSC subset in the tumor. *p < 0.05. Adapted from Hong et al.15
Interestingly, there is plasticity in both the phenotype and suppressive function of MDSCs.22 Tumor-derived factors induce the generation of an immunosuppressive subset of MDSCs.23 On the other hand, several pharmacological approaches are competent in regulating MDSC-mediated immune suppression. Treatments with drugs that regulate myelopoiesis reduce the number of MDSCs, and some cytotoxic chemotherapeutic agents have the capacity to eliminate MDSCs selectively. To decrease the number of MDSCs at the tumor site, blockade of MDSC recruitment is one potential strategy. Lastly, various agents for neutralization of the immunosuppressive function of MDSCs have been reported (Table 2).
Table 2. Approaches to overcome the immune suppression mediated by MDSCs.
| Major goal | Approach | Result | Reference |
|---|---|---|---|
| Regulation of MDSC generation | Anti-c-kit mAb | Blockade of stem cell factor (SCF)-c-kit signaling and reduction of MDSC number | 24 |
| Tyrosine kinase inhibitor (sunitinib) | Blockade of vascular endothelial growth factor receptors (VEGFR), c-Kit, STAT3, etc., and reduction of MDSC number | 25,26 | |
| Further differentiation of MDSC | All-trans-retinoic acid | MDSC differentiation into mature myeloid cells | 27 |
| Vitamin D3 | CD34+ cell maturation | 28,29 | |
| Depletion of MDSC | Gemcitabine | Elimination of MDSCs | 30,31 |
| 5-fluorouracil | 32 | ||
| Anti-IL-6 receptor mAb | 33 | ||
| Prevention of MDSC recruitment to tumor | COX-2 inhibitor (celecoxib) | Downregulation of CCL2 production and decrease in MDSC recruitment | 34 |
| Inhibitor of CSF1R signaling (GW2580) | Decrease in monocytic MDSC recruitment | 35 | |
| Inhibition of MDSC immunosuppressive function | PDE-5 inhibitor (sildenafil) | Inhibition of iNOS and/or ARG-1 activities | 36 |
| COX-2 inhibitor (celecoxib) | 37 | ||
| Nitroaspirin | 38 | ||
| CpG ODNs | Reduction of suppressive function of Ly6Ghigh MDSC | 39 | |
| Triterpenoid | Inhibition of MDSC immune suppressive effect | 40 | |
| Rapamycin | Downregulation of ARG1, iNOS and Nox2 in MDSC | 41 | |
| α-galactosylceramide | Conversion of MDSC into nonsuppressor cells and increase in immunogenecity of MDSC | 47,48 |
Some conditions that induce MDSC generation cause MDSCs to become immunostimulatory myeloid cells, including tumoricidal neutrophils.12,15,42 Cuenca et al. have reported that in trauma and sepsis, MDSCs play the role of immune effector cells, increasing immune responses.20 In a cancer model, immunogenic MDSCs mediating antitumor immunity were generated in epithelial ovarian cancer-bearing mice.43 In our recently reported study,15 we detected an accumulation of distinct TNF-α-producing Ly6-GhighLy6-Cinter MDSCs in mice treated with intratumoral RASV (Fig. 2), and they exhibited a therapeutic antitumor effect. While activated neutrophils secreting TNF-α can act as direct effector cells in therapeutic anticancer therapy, many cytokines associated with chronic inflammatory status in the tumor microenvironment, including IL-6 and IL-1β, are associated with the accumulation of MDSCs.33,44 Thus, further studies are required to identify the factors that may regulate MDSC conversion into TNF-α-producing neutrophils in the inflammatory tumor microenvironment after intratumoral injection of attenuated Salmonella.
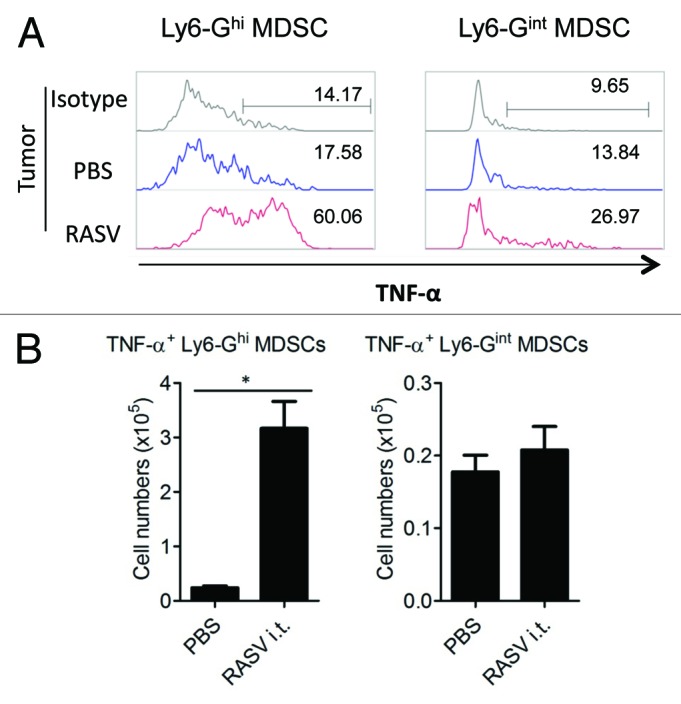
Figure 2. Intratumoral injection of RASV induced Ly6-Ghigh granulocytic MDSCs highly secreting TNF-α in the tumor. Tumor-infiltrating cells were stimulated with 200 ng/ml LPS for 2 h, and then, TNF-α secretion by Ly6-GhighLy6-Cinter and Ly6-GinterLy6-Chigh MDSCs was analyzed by intracellular staining. (A) Percentages of TNF-α+ MDSCs in the tumor. (B) The absolute number of TNF-α+ MDSC subsets in the tumor. *p < 0.05. Adapted from Hong et al.15
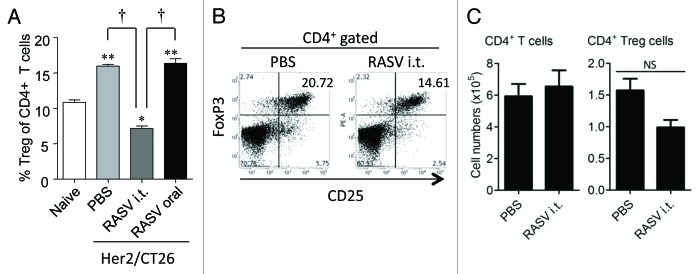
Another type of immune suppressors, which control self-reactive T cells to prevent autoimmunity and are a major obstacle for anti-tumor immunotherapy, is CD4+CD25+ Tregs.19,45 The Treg population is reportedly expanded in some tumor patients, and they are recruited to tumor sites, where they exert a suppressive role against cytotoxic T lymphocytes (CTLs).17 There was a close correlation between the expansion of Tregs and MDSCs,19 and a recent report showed that the suppression of tumor growth by Salmonella enterica serovar Typhimurium was related to down regulation of CD4+CD25+ Tregs.5 Likewise, Salmonella typhi-based immunotherapy reportedly mediated tumor-specific immune responses in tumor-draining LNs, with an associated reduction in the number of Tregs among the CD4+ T cell population.8 In our recently reported study, we also found that the percentage of CD25+FoxP3+ Tregs among the CD4+ T cell population was significantly reduced in tumor-bearing mice intratumorally treated with RASV, compared with PBS-treated controls15 (Fig. 3). However, it is not certain whether Salmonella-based immunotherapy directly inhibits the generation of Tregs in tumor-bearing mice, or whether reduction in MDSCs indirectly affects the expansion of Tregs.
Figure 3. CD4+CD25+FoxP3+ regulatory T-cell levels decreased in tumor-bearing mice after i.t. injection of RASV. (A) The percentages of CD4+CD25+FoxP3+ Tregs among the CD4+ T cell population in splenocytes (n = 6 mice per group). **p < 0.01, ***p < 0.001 compared with naïve control mice. †p < 0.01, Her2/CT26-PBS vs. Her2/CT26-RASV i.t. and Her2/CT26-RASV i.t. vs. Her2/CT26-RASV oral. (B) The percentages of FoxP3+ Tregs among the CD25+ cells are shown after gating the tumor-infiltrating CD4+ T cells. (C) The absolute number of tumor-infiltrating CD4+ T cells and CD4+ CD25+ FoxP3+ Tregs in the tumors. NS, not significant. Adapted from Hong et al.15
Mechanism of Immune Reversion from Immunosuppressive into Anti-Cancer Immunity by Microorganisms
The mechanism underlying the induction of antitumor activity by treatment with attenuated microorganisms could be explained by several factors, including the regulation of Treg generation, conversion of MDSCs into immunostimulatory cells, and generation of IFN-γ-producing TH1 and CTLs. In a RASV treatment model, we investigated the underlying mechanism by analyzing these factors.15
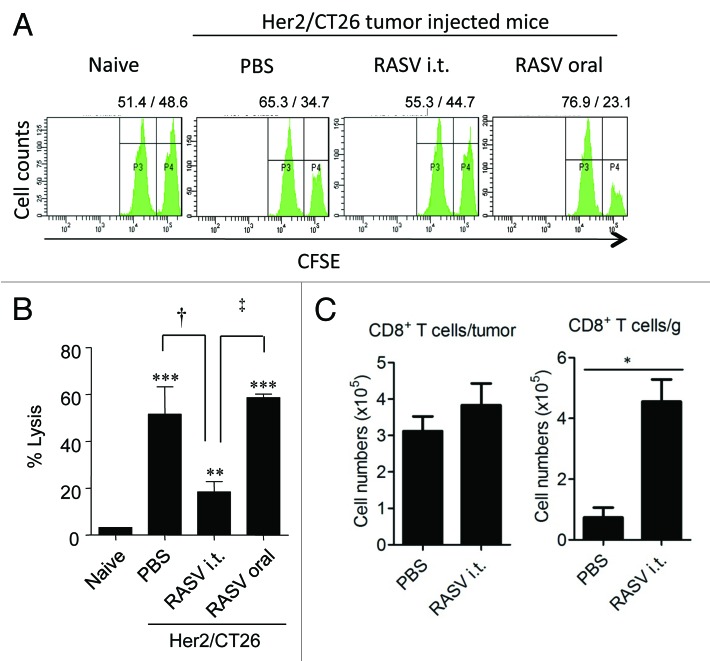
First, CTLs in untreated vs. intratumoral Salmonella-injected tumors were compared. When tumor-infiltrating CD8+ T cells were restimulated with a tumor antigen-specific peptide, tumor antigen-specific IFN-γ secretion by CTLs was significantly lower in RASV-injected tumors compared with that in PBS-treated tumors. These results suggest that the anti-tumor effect of intratumoral RASV injection may not be mediated by circulating tumor antigen-specific CTLs (Fig. 4). However, the absolute number of tumor-infiltrating CD8+ T cells per tumor weight significantly increased by intratumoral RASV administration. Thus, the effector function of tumor-infiltrating CD8+ T cells can be significantly increased by RASV injection, and they exhibit important therapeutic anti-tumor effects, although it is uncertain whether they are reactive to other tumor associated antigens and Salmonella-infected tumors.
Figure 4. Tumor antigen-specific CTL activity and tumor-infiltrating CD8+ T cells. (A) Spleens from RASV-treated mice were obtained, and specific lysis of hP63 (TYLPTNASL) peptide-loaded target cells was estimated by in vivo CTL levels. (B) Results are expressed as the mean cytotoxicity ± SEM from in vivo CTL assays. (C) The absolute number of tumor-infiltrating CD8+ T cells per tumor (left) and per tumor weight (right). *p < 0.05. Adapted from Hong et al.15
With regard to the involvement of NK cells and TH1 cells in anti-tumor activity after RASV treatment, the percentages and absolute numbers of NK1.1+CD3− NK cells in the draining LNs were significantly increased but they did not secrete IFN-γ at all, whereas IFN-γ-secreting CD4+ T cells (TH1) marginally increased.15 Thus, NK cells and TH1 cells could participate in the anti-tumor activity of RASV treatment, but may only play a minor role.
The absolute number of tumor-infiltrating Tregs reduced slightly in the spleen, draining LNs, and tumors.15 In the draining LNs and spleen, the numbers of CD4+ T cells increased consistently with enlarged LNs and the spleen after RASV treatment. Therefore, the absolute number of Tregs in these tissues was similar (the spleen) or rather increased (draining LNs) after RASV injection, although the percentages of Tregs reduced. Collectively, RASV treatment could reduce the percentages of Tregs among CD4+ T cells by increasing effector CD4+ T cells.
With regard to MDSC modulation, of the two major subsets of MDSCs, Ly6-GinterLy6-Chigh (monocytic) MDSCs and Ly6-GhighLy6-Cinter (granulocytic) MDSCs, intratumoral RASV injection significantly increased only the latter in the spleen and tumor as compared with PBS-injected tumor-bearing mice15 (Fig. 1). In particular, Ly6-GhighLy6-Cinter MDSCs are significantly increased in the tumor by RASV injection, and they become a major population. Thus, we postulated that the increased MDSC populations in RASV-injected mice may not be immunosuppressive, but instead may help stimulate antitumor immune activity.
Upon assessing the characteristics of MDSCs, we found that tumor-infiltrating Ly6-Ghigh populations secreted more TNF-α than that secreted by Ly6-Ginter populations, and over 60% of tumor-infiltrating Ly6-Ghigh populations expressed TNF-α after lipopolysaccharide (LPS) restimulation15 (Fig. 2). These data suggested that intratumoral injection of RASV can induce TNF-α-secreting Gr-1highLy6-GhighLy6-Cinter populations, which have neutrophil-like characteristics. Intratumoral injection of RASV increased sub-populations of CD11b+Gr-1+ cells, which are distinct from classical suppressive MDSCs because they secrete TNF-α, and consequently resulted in tumor regression.
Conclusions and Future Prospects
Attenuated Salmonella can be used as a therapeutic anti-tumor vaccine, mediating conversion of immunosuppressive MDSCs into TNF-α-secreting neutrophil-like myeloid cells. Intratumoral administration of attenuated Salmonella induced CD8+ T cell-dependent tumor regression. Thus, intratumoral injection of attenuated Salmonella vaccine can be a successful therapeutic anti-tumor regimen, inducing anti-tumor effectors including CTL and TNF-α-secreting neutrophils, as well as overcoming aspects of the immunosuppressive tumor environment including MDSCs and Tregs.
Acknowledgments
This work was supported by the Ministry for Health, Welfare and Family Affairs, Republic of Korea (C1007254), and by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2011-0009018, No. 2011-0006965).
Glossary
Abbreviations:
- RASV
recombinant attenuated Salmonella enterica serovar Typhimurium vaccine
- MDSC
myeloid-derived suppressor cell
- CTL
cytotoxic T lymphocyte
- Treg
regulatory T cells
- LN
lymph node
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/24917
References
- 1.Starnes CO. Coley’s toxins in perspective. Nature. 1992;357:11–2. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- 2.Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A. 2005;102:755–60. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, et al. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 2009;29:1873–8. [PubMed] [Google Scholar]
- 4.Zhang Y, Tome Y, Suetsugu A, Zhang L, Zhang N, Hoffman RM, et al. Determination of the optimal route of administration of Salmonella typhimurium A1-R to target breast cancer in nude mice. Anticancer Res. 2012;32:2501–8. [PubMed] [Google Scholar]
- 5.Liu T, Chopra AK. An enteric pathogen Salmonella enterica serovar Typhimurium suppresses tumor growth by downregulating CD44high and CD4T regulatory (Treg) cell expression in mice: the critical role of lipopolysaccharide and Braun lipoprotein in modulating tumor growth. Cancer Gene Ther. 2010;17:97–108. doi: 10.1038/cgt.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avogadri F, Martinoli C, Petrovska L, Chiodoni C, Transidico P, Bronte V, et al. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005;65:3920–7. doi: 10.1158/0008-5472.CAN-04-3002. [DOI] [PubMed] [Google Scholar]
- 7.Vendrell A, Gravisaco MJ, Pasetti MF, Croci M, Colombo L, Rodríguez C, et al. A novel Salmonella Typhi-based immunotherapy promotes tumor killing via an antitumor Th1-type cellular immune response and neutrophil activation in a mouse model of breast cancer. Vaccine. 2011;29:728–36. doi: 10.1016/j.vaccine.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Vendrell A, Gravisaco MJ, Goin JC, Pasetti MF, Herschllik L, De Toro J, et al. Therapeutic effects of Salmonella typhi in a mouse model of T-cell lymphoma. J Immunother. 2013;36:171–80. doi: 10.1097/CJI.0b013e3182886d95. [DOI] [PubMed] [Google Scholar]
- 9.Lee CH, Wu CL, Shiau AL. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis. Clin Cancer Res. 2008;14:1905–12. doi: 10.1158/1078-0432.CCR-07-2050. [DOI] [PubMed] [Google Scholar]
- 10.Tsuda K, Yamanaka K, Linan W, Miyahara Y, Akeda T, Nakanishi T, et al. Intratumoral injection of Propionibacterium acnes suppresses malignant melanoma by enhancing Th1 immune responses. PLoS One. 2011;6:e29020. doi: 10.1371/journal.pone.0029020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baird JR, Byrne KT, Lizotte PH, Toraya-Brown S, Scarlett UK, Alexander MP, et al. Immune-mediated regression of established B16F10 melanoma by intratumoral injection of attenuated Toxoplasma gondii protects against rechallenge. J Immunol. 2013;190:469–78. doi: 10.4049/jimmunol.1201209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jinesh G G, Chunduru S, Kamat AM. Smac mimetic enables the anticancer action of BCG-stimulated neutrophils through TNF-α but not through TRAIL and FasL. J Leukoc Biol. 2012;92:233–44. doi: 10.1189/jlb.1211623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp TJ, Ludwig AT, Earel JK, Moore JM, Vanoosten RL, Moses B, et al. Neutrophil stimulation with Mycobacterium bovis bacillus Calmette-Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood. 2005;106:3474–82. doi: 10.1182/blood-2005-03-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SH, Castro F, Paterson Y, Gravekamp C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009;69:5860–6. doi: 10.1158/0008-5472.CAN-08-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong EH, Chang SY, Lee BR, Pyun AR, Kim JW, Kweon MN, et al. Intratumoral injection of attenuated Salmonella vaccine can induce tumor microenvironmental shift from immune suppressive to immunogenic. Vaccine. 2013;31:1377–84. doi: 10.1016/j.vaccine.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 18.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17:281–92. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425–, author reply 426. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sevko A, Umansky V. Myeloid-derived suppressor cells interact with tumors in terms of myelopoiesis, tumorigenesis and immunosuppression: thick as thieves. J Cancer. 2013;4:3–11. doi: 10.7150/jca.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–28. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 26.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67:11021–8. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 28.Garrity T, Pandit R, Wright MA, Benefield J, Keni S, Young MR. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into dendritic cells. Int J Cancer. 1997;73:663–9. doi: 10.1002/(SICI)1097-0215(19971127)73:5<663::AID-IJC9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 29.Kulbersh JS, Day TA, Gillespie MB, Young MR. 1alpha,25-Dihydroxyvitamin D(3) to skew intratumoral levels of immune inhibitory CD34(+) progenitor cells into dendritic cells. Otolaryngol Head Neck Surg. 2009;140:235–40. doi: 10.1016/j.otohns.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 31.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9:900–9. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 33.Sumida K, Wakita D, Narita Y, Masuko K, Terada S, Watanabe K, et al. Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T-cell responses. Eur J Immunol. 2012;42:2060–72. doi: 10.1002/eji.201142335. [DOI] [PubMed] [Google Scholar]
- 34.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–74. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–71. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, et al. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. doi: 10.1186/1471-2407-10-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A. 2005;102:4185–90. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zoglmeier C, Bauer H, Nörenberg D, Wedekind G, Bittner P, Sandholzer N, et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17:1765–75. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- 40.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–23. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YS, Kim YJ, Lee JM, Kim EK, Park YJ, Choe SK, et al. Functional changes in myeloid-derived suppressor cells (MDSCs) during tumor growth: FKBP51 contributes to the regulation of the immunosuppressive function of MDSCs. J Immunol. 2012;188:4226–34. doi: 10.4049/jimmunol.1103040. [DOI] [PubMed] [Google Scholar]
- 42.Johansson C, Ingman M, Jo Wick M. Elevated neutrophil, macrophage and dendritic cell numbers characterize immune cell populations in mice chronically infected with Salmonella. Microb Pathog. 2006;41:49–58. doi: 10.1016/j.micpath.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Tomihara K, Guo M, Shin T, Sun X, Ludwig SM, Brumlik MJ, et al. Antigen-specific immunity and cross-priming by epithelial ovarian carcinoma-induced CD11b(+)Gr-1(+) cells. J Immunol. 2010;184:6151–60. doi: 10.4049/jimmunol.0903519. [DOI] [PubMed] [Google Scholar]
- 44.Elkabets M, Ribeiro VS, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN, et al. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010;40:3347–57. doi: 10.1002/eji.201041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–41. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 46.Maletzki C, Gock M, Klier U, Klar E, Linnebacher M. Bacteriolytic therapy of experimental pancreatic carcinoma. World J Gastroenterol. 2010;16:3546–52. doi: 10.3748/wjg.v16.i28.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182:1818–28. doi: 10.4049/jimmunol.0802430. [DOI] [PubMed] [Google Scholar]
- 48.Lee JM, Seo JH, Kim YJ, Kim YS, Ko HJ, Kang CY. The restoration of myeloid-derived suppressor cells as functional antigen-presenting cells by NKT cell help and all-trans-retinoic acid treatment. Int J Cancer. 2012;131:741–51. doi: 10.1002/ijc.26411. [DOI] [PubMed] [Google Scholar]