Abstract
Background and aims: Recent studies have shown no detectable antibodies and no response to a challenge dose of vaccine 10–20 y after receiving low doses (2.5–5 µg) of recombinant hepatitis B vaccine during first months of life. Little information is available on long-term persistence of immunity after vaccinating pre-adolescents with low doses of hepatitis B vaccine.
Materials and Methods: This randomized trial was initiated in 1996 with the main objective to assess the persistence of antibodies and immune memory 5, 10 and 15 y after vaccinating 8–10 y-old children with three doses of Recombivax 2.5 µg, as well as the short and long-term effect of a booster dose given at different intervals.
Results: The results of 560 subjects were included in this analysis. All subjects had a seroprotective antibody titer (≥ 10 IU/L) one month post-primary vaccination; 5, 10 and 15 y post-vaccination 95%, 95% and 87% had detectable antibodies, and 82%, 86%, and 68% had a seroprotective antibody titer; GMTs were 73 IU/L, 89 IU/L, and 28 IU/L, respectively. More than 99.4% of subjects had an anamnestic response to a challenge dose of vaccine given 5, 10, or 15 y post-vaccination. Five and ten years post-booster dose 97% and 95% of subjects still have a seroprotective anti-HBs titer with GMTs 16–18-fold higher when compared with those observed 5–10 y post-primary vaccination.
Conclusions: Virtually all children vaccinated at the age of 8–10 y with low doses of hepatitis B vaccine still have an excellent immune memory up to age of 25 y. The results of this study do not support the use of booster doses.
Keywords: hepatitis B, immunity, long term, persistence, vaccination
Introduction
Epidemiological studies have shown that hepatitis B vaccines are highly effective in preventing clinical acute and chronic cases of disease.1 However, during the last years conflicting data were published regarding the duration of protection and the potential need of booster doses after hepatitis B vaccination. Based on results from studies which showed antibody persistence and immune memory presence in the great majority of individuals vaccinated 10–20 y earlier, authors have concluded that booster doses should be unnecessary.2-6 However, based on results from other studies conclusions were made that the decay of immune memory raises concerns about the need for a booster dose.7-10 These divergences could, at least partially, be explained by different vaccines and dosages used, different age at vaccination, and different probability of natural boosters.
A recent meta-analysis of 46 studies looked at determinants of long-term protection after hepatitis B vaccination during infancy.11 Authors concluded that maternal carrier status, interval between the last two doses of the primary series, and vaccine dosage are the main determinants for antibody persistence. A lower dosage given during infancy was also associated with failure to respond to the booster.
Recombivax 2.5 µg had been largely used in the nineties for vaccination of infants and children under 11 y. The minimal recommended dosage of Recombivax has been since changed for 5 µg in the USA but the 2.5 µg dosage is still in use in Canada.
Little is known about the long-term effect of vaccine dosage when vaccinating preadolescents.
In most Canadian provinces, school-based hepatitis B immunization programs have been in place since the mid-nineties. The decision to vaccinate pre-teenagers and teenagers was based on low risk of infection during childhood, uncertainties regarding the duration of protection at the time of program implementation, and higher magnitude of immune response when vaccinating school age children. This approach was also expected to have a faster impact on hepatitis B incidence, which was highest in adolescents and young adults.
This prospective study was initiated in 1996. The main objectives of this final analysis were to assess (1) antibody persistence and immune memory presence 15 y post-primary vaccination of 8–10 y-old children; and (2) short and long-term effects of a booster dose of vaccine when given 5 or 10 y post-primary vaccination.
Results
Study participation
As reported elsewhere12 the strict schedule between the first and the second doses of 28 to 60 d was respected by 94% and the interval of 180–210 d between the first and third dose by 93% of enrolled subjects. Among participants who did not follow the strict schedule, the interval between the first and third dose varied between 151 and 273 d.
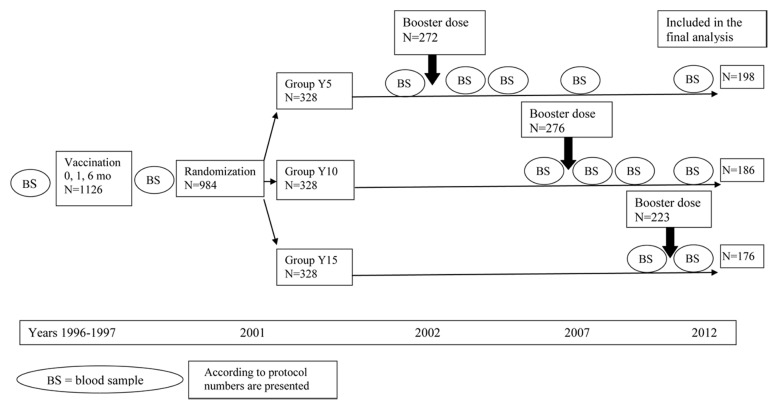
Five hundred seventy subjects participated in the final phase of the study (58% of those randomized). Of them, 560 had all procedures according to the study protocol (Fig. 1). The differences between the Intention To Treat (ITT) and According To Protocol (ATP) analyses were minimal and not significant; we therefore present only the ATP results. The main causes of subject non-participation were as follows: 177 abandoned the study, 102 moved outside the study region, 64 had important protocol violations (e.g., received hepatitis B vaccines outside the study protocol) and were excluded, 60 were lost to follow-up, and 4 passed away. None of non-participation was related to study procedures.
Figure 1. Study Design.
One month post-primary vaccination
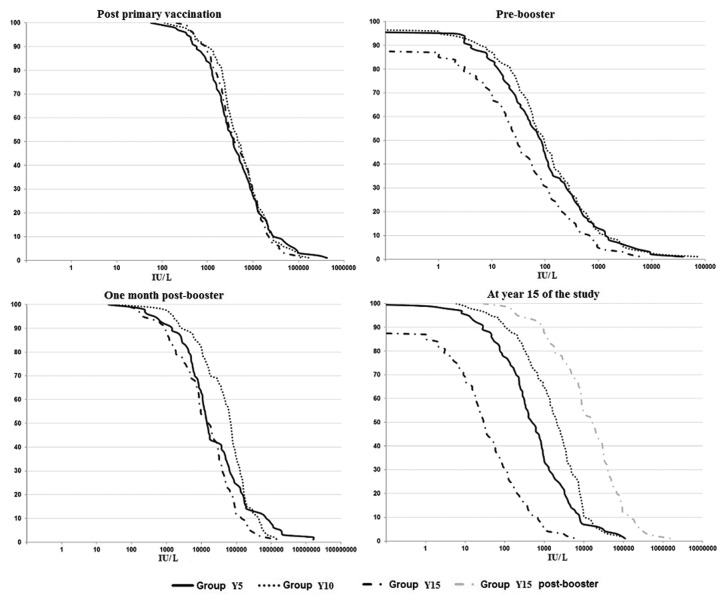
All followed-up subjects had a seroprotective antibody titer and 98.5% - 100% a titer ≥ 100 IU/L. Among the four subjects (1.5%) with an anti-HBs titer < 100 IU/L the titer varied from 34 to 90 IU/L. GMTs were 3 836, 4 740, and 4 171 IU/L in Group Y5, Y10, and Y 15, respectively (Table 1). The distribution of anti-HBs titers was similar in the three study groups (Fig. 2).
Table 1. Proportion of subjects with ≥1 IU/L, ≥10 IU/L, ≥100 IU/L, and anti-HBs GMTs at different study time points.
| Time point | Group | Proportion of subjects with titres ≥1 mIU/ml | Proportion of subjects with titres ≥10 mIU/ml | Proportion of subjects with titres ≥100 mIU/ml | GMT IU/L |
|---|---|---|---|---|---|
| CI95% | CI95% | CI95% | CI95% | ||
| One month post-primary vaccination | Y5 | 100 | 100 | 98.5 | 3836 |
| 98.1-100.0 | 98.1-100.0 | 95.6-99.7 | 3071-4792 | ||
| Y10 | 100 | 100 | 99.5 | 4740 | |
| 98.0-100.0 | 98.0-100.0 | 97.0-100.0 | 3893-5772 | ||
| Y15 | 100 | 100 | 100 | 4171 | |
| 97.9-100.0 | 97.9-100.0 | 97.9-100.0 | 3441-5055 | ||
| Pre-booster | Y5 | 94.9 | 82.3 | 42.4 | 73 |
| 90.9-97.5 | 76.3-87.4 | 35.4-49.6 | 54-99 | ||
| Y10 | 95.2 | 86 | 47.3 | 89 | |
| 91.0-97.8 | 80.2-90.7 | 40.0-54.7 | 66-121 | ||
| Y15 | 86.9 | 68.2 | 29.5 | 28 | |
| 81.0-91.5 | 60.7-75.0 | 22.9-36.9 | 20-39 | ||
| One month post-booster | Y5 | 99.5 | 99.5 | 98.5 | 19544 |
| 97.2-100.0 | 97.2-100.0 | 95.6-99.7 | 13928-27426 | ||
| Y10 | 100 | 99.5 | 98.4 | 40754 | |
| 98.0-100.0 | 97.0-100.0 | 95.4-99.7 | 30853-53831 | ||
| Y15 | 100 | 99.4 | 97.7 | 11232 | |
| 97.9-100.0 | 96.9-100.0 | 94.3-99.4 | 8208-15369 | ||
| One year post-booster | Y5 | 100 | 99 | 91.4 | 3972 |
| 98.1-100.0 | 96.4-99.9 | 86.6-94.9 | 2810-5614 | ||
| Y10 | 100 | 99.5 | 93.5 | 4013 | |
| 98.0-100.0 | 97.0-100.0 | 89.0-96.6 | 3041-5296 | ||
| Five years post booster | Y5 | 100 | 97 | 85.9 | 1297 |
| 98.1-100.0 | 93.5-98.9 | 80.2-90.4 | 952-1768 | ||
| Y10 | 100 | 97.8 | 89.8 | 1423 | |
| 98.0-100.0 | 94.6-99.4 | 84.5-93.7 | 1078-1880 | ||
| Ten years post booster | Y5 | 98.5 | 94.9 | 76.3 | 453 |
| 95.6-99.7 | 90.9-97.5 | 69.7-82.0 | 332-618 |
Note : Y5-received a booster dose 5 years post-primary vaccination; Y10-received a booster dose 10 years post-primary vaccination; Y-15 received a booster dose 15 years post-primary vaccination.
Figure 2. Anti-HBs titers distribution at different study time points.
Pre-booster
Fifteen years post-primary vaccination 87% of subjects still had a detectable level of antibodies and 68% a seroprotective level of antibodies; 30% had an anti-HBs titer ≥ 100 IU/L. The anti-HBs GMTs were 28 IU/L. These results were lower when compared with those observed pre-booster in Group Y5 and Y10 (Table 1).
One month post-booster
All subjects who received a booster dose of vaccine 15 y post-primary vaccination (Y15) had a detectable level of antibodies and all but one a seroprotective anti-HBs titer. The great majority (98%) had an anti-HBs tietr ≥ 100 IU/L. These results were similar to those observed one month post-booster in Group Y5 and Y10. The GMTs in Group Y15 increased by more than 400-fold when compared with pre-booster GMTs (Table 1). All but one subject who did not achieved a seroprotective level of anti-HBs, showed a 4-fold or greater antibody increase when compared with pre-booster titers. The anti-HBs titers distribution at year 15 of the study is shown in Figure 2.
One year post-booster
In both Group Y5 and Y10 99–99.5% of subjects still had a seroprotective anti-HBs titer with > 90% of them presenting an anti-HBs titer ≥ 100 IU/L. During the first year post-booster, a 5-fold and 10-fold GMTs decrease was observed in Group Y5 and Y10, respectively. Despite this dramatic decrease, the observed anti-HBs titers remained close to those observed 1 mo post-primary vaccination (Table 1).
Five years post-booster
All subjects had a detectable level of antibodies and more than 97% a seroprotective anti-HBs titer; 86 to 90% had an anti-HBs titer ≥ 100 IU/L (Table 1). When compared with one month post-booster, the GMTs decreased 15-fold and 29-fold in Group Y5 and Y10, respectively (Fig. 2).
Ten years post-booster
The great majority (95%) of subjects in Group Y5 had a seroprotective anti-HBs titer. The anti-HBs GMT was 453 IU/L, which is significantly higher (p < 0.0001) when compared with GMTs observed 10 y post-primary vaccination (Table 1).
Safety
Both primary vaccination and booster doses were well tolerated. No vaccine related serious adverse events were reported during the study period.
Discussion
To our knowledge, this is the first prospective randomized study which assessed the persistence of anti-HBs and immune memory for 15 y post-primary vaccination of preadolescents with low doses of a recombinant vaccine.
The results show that like in previous studies,13-16 the anti-HBs titers decrease with time since vaccination. Indeed, by year 15 of the study an important proportion of subjects (32%) had an anti-HBs titer under the seroprotective level. However, in contrast to studies with low doses of vaccine given beginning at birth,7,17,18 the majority of subjects in our study still had a detectable level of antibodies up to 15 y later and virtually all (99.4%) showed an excellent immune memory capable of an anamnestic response when challenged with a booster dose of vaccine. Interestingly to mention our results are in contrast with the one of a recent study where a booster dose of hepatitis B vaccine given 10–15 y after vaccinating infants induced a seroprotective level of antibodies in 83% of those with an anti-HBs titer 1–9 IU/L pre-booster, but in only 50% of those with no detectable antibodies.19 In our study all those who had a detectable level of antibodies and 96% (22 out of 23) of those with no detectable antibodies pre-booster had a seroprotective level of anti-HBs post-booster. The great majority (98%) of subjects with an anti-HBs titer < 10 IU/L pre-booster achieved an anti-HBs titer ≥ 100 IU/L post-booster.
The magnitude of the observed immune response was notably higher when booster doses were given 5 or 10 y post-primary vaccination than 15 y post-primary vaccination. This observation might be related to a weaker immune memory 15 y post-primary vaccination or to the use of MONOLISA assay instead of AUSAB assay which were no longer available by the year 15 of the study, but we cannot exclude that this difference is due to the age related immune response to hepatitis B vaccine. When they received their booster doses, subjects in the Y5 and Y10 group were around 15 and 20 y-old, respectively, and 25 y-old in the Y15 group. It is well known that compared with 1 to 15 y- old children, hepatitis B vaccines induce lower GMTs when vaccinating infants20-22 or adults above the age of 40 y.1,23 However, little is known regarding the potential differences in the magnitude of immune response when administering hepatitis B vaccines to adolescents or young adults. Recent data reported with HPV vaccines show significantly higher antibody GMTs when vaccinating 9–14 y-old girls when compared with 16–26 y old females.12,24 These results suggest that a relatively small difference in age at vaccination might have an important impact on the magnitude of the immune response25 and potentially different persistence of immunity. In our study, despite the observed lower magnitude of immune response to the booster dose in Y15 group when compared with Y5 and Y10, the observed GMTs were almost 3-fold higher than those observed in the same group one month post-primary vaccination. While the GMTs were higher in our previous preadolescent study with Engerix 10 µg,5 than in this one with Recombivax 2.5 µg (GMTs around 2-fold higher when vaccinating with Engerix) both products induced a strong anamnestic response in virtually all vaccinees at 15 y. These results are consistent with the results from a study24 which assessed the presence of different memory subsets of T cells several years after vaccinating with Hexavac or Infanrix-hexa. The authors concluded that, although the two vaccines generated different serum antibody titers, both vaccines are efficient in generating T cells recall responses. Altogether, these data suggest that excellent priming is obtained in pre-adolescents vaccinated with low doses of a recombinant hepatitis B vaccine. However, these results should not be extrapolated to those immunized in infancy and vaccination of preadolescents might not be suitable in high endemicity areas where the risk of infection is high during early childhood.
The similarity between our results and those where higher vaccine dosages (10–20 µg) were used for primary vaccination,3-5 suggests that lower doses of recombinant hepatitis B vaccines can be used in preadolescents without affecting the long-term antibody or immune memory persistence.
Our study has some limitations. We used for booster the dosage of vaccine recommended for the respective age group in Canada (5 or 10 µg) and we cannot exclude that the results might be different when using lower booster doses. It is to note that in Group Y10 the GMTs one month post-booster dose administration were 2-fold higher when compared with the GMTs observed one month post-booster in Group Y5. It is highly probable that this difference is due to higher booster dosage used in Group Y10 when compared with Y5. Interesting to mention, that despite 2-fold higher anti-HBs titers one month post-booster in Y10 group when compared with Y5 group, one year later similar titers where observed in two study groups. Another limitation is the time between booster administration and blood sampling. According to study protocol, this interval was 28–60 d and in some recent studies a 14 d period was used for the assessment of immune memory.9,17 However, taking into consideration the long incubation period of hepatitis B infection, we think the results observed one–two months post-booster indicates an excellent protection against the disease. Another limitation is the absence of testing blood samples obtained at year 15 of the study for anti-HBc and HBsAg. These tests were performed at study entry for all subjects and in Group Y5 and Y10 at year 5 and year 10 of the study, respectively and all were negative for anti-HBc. For budgetary reasons this was not done in Group Y15. However, all subjects had lower anti-HBs titers at year 15 (pre-booster) than they had one month post-primary vaccination suggesting that no participants had natural infection. In the province of Quebec, the prevalence of chronic carriage of HBsAg is less than 2% and the incidence of acute hepatitis B was low during the study period5 and varied around 0.3 cases per 100,000 person-years between 2007 and 2012. The probability of contact with hepatitis B virus was likely minimal for our subjects and if any, this would not invalidate the overall study results.
Another limitation of this study, like of many other long-term studies, is the relatively high proportion of subjects lost to follow-up. This was anticipated in the study design where the loss to follow-up was expected to be 5% per study year. Based on the similarity of the socio-demographic characteristics and high similarity of the amplitude of the immune response one month post-primary vaccination in subjects who participated in the final phase of the study and those who did not (data not shown), the results are unlikely to be biased.
In summary, the results show the persistence of an excellent immune memory in virtually all vaccinees for at least 15 y post-primary vaccination. The clinical relevance of different antibody titers is not clear and we conclude that preadolescents vaccinated with Recombivax 2.5 µg are well protected up to age of 25 y.
The results of our study do not support the use of booster doses and in concordance with previous studies’ results, suggest that the effect of a booster dose would be negligible if any for protection against clinical infection when vaccinating pre-adolescents with a recombinant hepatitis B vaccine.
Materials and Methods
Population and study design
This phase IV randomized non blinded study was conducted in Quebec City, Canada. Children eligible for provincial school based hepatitis B vaccination program were invited to participate. To be eligible, children could not be younger than 8 y and not older than 10 y, and should have not received any other hepatitis B vaccine. The detailed characteristics of the study sample and study design were presented elsewhere.12,26 Nine subjects (0.8%) out of 1126 who did not reach a seroprotective level of anti-HBs (≥ 10 IU/L) one month after the third dose of vaccine were offered additional doses and were excluded from the study. In 2002, 984 subjects (88.1%) agreed to continue their participation and were randomized 1:1:1 to Group Year 5 (Y5), Group Year 10 (Y10) or Group Year 15 (Y15). To ensure the comparability of the three study groups, subjects were allocated randomly (SAS Institute software) to a study group accordingly to their gender and the anti-HBs titers observed one month post-primary vaccination (five strata: 10–99, 100–199, 200–999, 1000–9 999, ≥ 10 000 IU/L).
The study design scheme and the number of participants at each study time point are presented in Figure 1.
To diminish subjects’ loss during follow-up, an annual contact by mail and/or phone was established. The study was approved by the Research Ethics Board of the Laval University Hospital Center. Written informed consents were signed by parents in 1996 and by the subjects themselves starting at the age of 14 y.
Vaccine administration and blood sampling
During primary vaccination, three doses of Recombivax 2.5 µg were administered according to a 0, 1 and 6 mo schedule. A strict vaccination schedule was defined as an interval between dose 1 and dose 2 of 28–60 d and an interval between dose 1 and dose 3 of 180–210 d. Booster doses of the same vaccine were administered 5, 10 or 15 y post-primary vaccination to subjects randomized to group Y5, Y10 and Y15, respectively. The dosage recommended for the vaccination of respective age groups in Canada was used for boosters (5 µg in Y5 and 10 µg in Y10 and Y15). All vaccines were administered intramuscularly in the deltoid. Blood samples were collected 28–60 d after the third vaccine dose, immediately before each booster dose and 28–60 d after the booster dose. In group Y5 and Y10, blood samples were also collected 1 y and 5 y post-booster administration. In group Y5, a blood sample was also collected 10 y post-booster (Fig. 1).
Laboratory procedures
Titers at time of the primary vaccination and 5 or 10 y later were measured by AUSAB EIA and AUSAB Quantification Panel (Abbott Laboratories Diagnostic Division). At the end of the 15 y follow-up in 2012, antibodies were measured by MONOLISA Anti-HBs EIA and MONOLISA Anti-HBs Calibrator Kit (Bio-Rad Laboratories). Samples with results beyond the linearity of the standard curves of assay kits were diluted into specimen dilution buffer and results were multiplied by the dilution factor to obtain the final concentration. An internal control serum was tested on each run to monitor assay performance. Assays were performed using the protocol recommended by the manufacturers.
Data analysis
We assessed the proportion of participants with ≥ 1 IU/L, ≥ 10 IU/L, ≥ 100 IU/L of anti-HBs in the samples collected in the three study groups at the different time points with 95% Clopper-Pearson confidence intervals. An anti-HBs titer equal or greater than 10 IU/L was considered seroprotective.27 There is no consensus about a definition of an anamnestic response for hepatitis B. We defined a priori an anamnestic response as an anti-HBs titer ≥ 4-fold greater post- than pre-booster. For subjects with pre-booster titers under 10 IU/L, a post-booster level above this threshold was required. A log transformed titers were used for geometrical mean titers (GMTs) calculation. GMTs variance was analyzed by Fisher’s test. To allow GMT calculation, samples with undetectable anti-HBs were assigned the arbitrary value of 1 IU/L. Fisher’s exact test was used for the comparison of anti-HBs titers distribution. The Spearman correlation coefficient was calculated for anti-HBs titers observed post-vaccination, pre-booster and post-booster. All statistics were two-tailed. P values of 0.05 or less were considered significant. SAS Institute software version 9.2 (Cary, NC, USA) was used for statistical analysis.
Acknowledgments
This study was initiated by Dr. Bernard Duval who prematurely passed away. The study was financially supported by Quebec Ministry of Health and Social Services. We are grateful to all study participants, to our research coordinators and research nurses, as well as to France Lavoie and Colette Couture for their dedication to this study realization.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25015
References
- 1.Plotkin SA, Orenstein WA, Offit PA. Vaccines. 6th ed. Philadelphia: Saunders Elsevier; 2012. [Google Scholar]
- 2.Wu Q, Zhuang GH, Wang XL, Wang LR, Li N, Zhang M. Antibody levels and immune memory 23 years after primary plasma-derived hepatitis B vaccination: results of a randomized placebo-controlled trial cohort from China where endemicity is high. Vaccine. 2011;29:2302–7. doi: 10.1016/j.vaccine.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 3.Zanetti AR, Mariano A, Romanò L, D’Amelio R, Chironna M, Coppola RC, et al. Study Group Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366:1379–84. doi: 10.1016/S0140-6736(05)67568-X. [DOI] [PubMed] [Google Scholar]
- 4.Gabbuti A, Romanò L, Blanc P, Meacci F, Amendola A, Mele A, et al. Long-term immunogenicity of hepatitis B vaccination in a cohort of Italian healthy adolescents. Vaccine. 2007;25:3129–32. doi: 10.1016/j.vaccine.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Gilca V, De Serres G, Boulianne N, Murphy D, De Wals P, Ouakki M, et al. Antibody persistence and the effect of a booster dose given 5, 10 or 15 years after vaccinating preadolescents with a recombinant hepatitis B vaccine. Vaccine. 2013;31:448–51. doi: 10.1016/j.vaccine.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Floreani A, Baldo V, Cristofoletti M, Renzulli G, Valeri A, Zanetti C, et al. Long-term persistence of anti-HBs after vaccination against HBV: an 18 year experience in health care workers. Vaccine. 2004;22:607–10. doi: 10.1016/j.vaccine.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Bialek SR, Bower WA, Novak R, Helgenberger L, Auerbach SB, Williams IT, et al. Persistence of protection against hepatitis B virus infection among adolescents vaccinated with recombinant hepatitis B vaccine beginning at birth: a 15-year follow-up study. Pediatr Infect Dis J. 2008;27:881–5. doi: 10.1097/INF.0b013e31817702ba. [DOI] [PubMed] [Google Scholar]
- 8.Jan CF, Huang KC, Chien YC, Greydanus DE, Davies HD, Chiu TY, et al. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology. 2010;51:1547–54. doi: 10.1002/hep.23543. [DOI] [PubMed] [Google Scholar]
- 9.Hammitt LL, Hennessy TW, Fiore AE, Zanis C, Hummel KB, Dunaway E, et al. Hepatitis B immunity in children vaccinated with recombinant hepatitis B vaccine beginning at birth: a follow-up study at 15 years. Vaccine. 2007;25:6958–64. doi: 10.1016/j.vaccine.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 10.Petersen KM, Bulkow LR, McMahon BJ, Zanis C, Getty M, Peters H, et al. Duration of hepatitis B immunity in low risk children receiving hepatitis B vaccinations from birth. Pediatr Infect Dis J. 2004;23:650–5. doi: 10.1097/01.inf.0000130952.96259.fd. [DOI] [PubMed] [Google Scholar]
- 11.Schonberger K, Riedel C, Ruckinger S, Mansmann U, Jilg W, Kries RV. Determinants of Long Term Protection after Hepatitis B Vaccination in Infancy: A Meta-Analysis. Pediatr Infect Dis J. 2013;32:307–13. doi: 10.1097/INF.0b013e31827bd1b0. [DOI] [PubMed] [Google Scholar]
- 12.Duval B, Boulianne N, De Serres G, Laflamme N, De Wals P, Massé R, et al. Comparative immunogenicity under field conditions of two recombinant hepatitis B vaccines in 8-10-year-old children. Vaccine. 2000;18:1467–72. doi: 10.1016/S0264-410X(99)00422-3. [DOI] [PubMed] [Google Scholar]
- 13.van der Sande MA, Waight PA, Mendy M, Zaman S, Kaye S, Sam O, et al. Long-term protection against HBV chronic carriage of Gambian adolescents vaccinated in infancy and immune response in HBV booster trial in adolescence. PLoS One. 2007;2:e753. doi: 10.1371/journal.pone.0000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aypak C, Yüce A, Yıkılkan H, Görpelioğlu S. Persistence of protection of hepatitis B vaccine and response to booster immunization in 2- to 12-year-old children. Eur J Pediatr. 2012;171:1761–6. doi: 10.1007/s00431-012-1815-4. [DOI] [PubMed] [Google Scholar]
- 15.Teoharov P, Kevorkyan A, Petrova N, Baltadzhiev I, Van Damme P. Immune Memory and Immune Response in Children from Bulgaria 5-15 Years after Primary Hepatitis B Vaccination. Pediatr Infect Dis J. 2013;32:51–3. doi: 10.1097/INF.0b013e31826f354e. [DOI] [PubMed] [Google Scholar]
- 16.Zanetti AR, Romanò L, Giambi C, Pavan A, Carnelli V, Baitelli G, et al. study group Hepatitis B immune memory in children primed with hexavalent vaccines and given monovalent booster vaccines: an open-label, randomised, controlled, multicentre study. Lancet Infect Dis. 2010;10:755–61. doi: 10.1016/S1473-3099(10)70195-X. [DOI] [PubMed] [Google Scholar]
- 17.Chaves SS, Fischer G, Groeger J, Patel PR, Thompson ND, Teshale EH, et al. Persistence of long-term immunity to hepatitis B among adolescents immunized at birth. Vaccine. 2012;30:1644–9. doi: 10.1016/j.vaccine.2011.12.106. [DOI] [PubMed] [Google Scholar]
- 18.Hammitt LL, Bulkow L, Hennessy TW, Zanis C, Snowball M, Williams JL, et al. Persistence of antibody to hepatitis A virus 10 years after vaccination among children and adults. J Infect Dis. 2008;198:1776–82. doi: 10.1086/593335. [DOI] [PubMed] [Google Scholar]
- 19.Spradling PR, Xing J, Williams R, et al. Immunity to hepatitis B virus infection two decades after implementation of universal infant hepatitis B vaccination: the association of detectable residual antibody and response to a single hepatitis B vaccine challenge dose. Clin Vaccine Immunol. 2013:13. doi: 10.1128/CVI.00694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Serres G, Gilca V, Boulianne N, Sauvageau C, De Wals P. Hepatitis B vaccine: what is the best protection and the most relevant age? Another view of timing. Can Ass Med J. 2009;180:196–202. [Google Scholar]
- 21.Vryheid RE, Kane MA, Muller N, Schatz GC, Bezabeh S. Infant and adolescent hepatitis B immunization up to 1999: a global overview. Vaccine. 2000;19:1026–37. doi: 10.1016/S0264-410X(00)00239-5. [DOI] [PubMed] [Google Scholar]
- 22.Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15-18 years after neonatal immunization. J Infect Dis. 2008;197:1419–26. doi: 10.1086/587695. [DOI] [PubMed] [Google Scholar]
- 23.Chlibek R, von Sonnenburg F, Van Damme P, Smetana J, Tichy P, Gunapalaiah B, et al. Antibody persistence and immune memory 4 years post-vaccination with combined hepatitis A and B vaccine in adults aged over 40 years. J Travel Med. 2011;18:145–8. doi: 10.1111/j.1708-8305.2010.00499.x. [DOI] [PubMed] [Google Scholar]
- 24.Carollo M, Palazzo R, Bianco M, Pandolfi E, Chionne P, Fedele G, et al. Hepatitis B specific T cell immunity induced by primary vaccination persists independently of the protective serum antibody level. Vaccine. 2013;31:506–13. doi: 10.1016/j.vaccine.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 25.Dobson S, Dawar M, Money D, et al. Two dose vaccine trial of Q-HPV: results at 36 months. Oral presention. 27th International Papillomavirus Conference and Clinical Workshop, Berlin, Germany, September 17-22, 2011. [Google Scholar]
- 26.Duval B, Gîlca V, Boulianne N, De Wals P, Massé R, Trudeau G, et al. Comparative long term immunogenicity of two recombinant hepatitis B vaccines and the effect of a booster dose given after five years in a low endemicity country. Pediatr Infect Dis J. 2005;24:213–8. doi: 10.1097/01.inf.0000154329.00361.39. [DOI] [PubMed] [Google Scholar]
- 27.European, Consensus, Group. Are booster immunisations needed for lifelong hepatitis B immunity European? Consensus Group on Hepatitis B Immunity. Lancet. 2000;355:561–5. doi: 10.1016/S0140-6736(99)07239-6. [DOI] [PubMed] [Google Scholar]