Abstract
This open-label, multicenter, randomized, comparative study evaluated immunogenicity, safety and tolerability of concomitant (Group 1; n = 330) vs. non-concomitant (Group 2; n = 323) VAQTA™ (25U/0.5 mL) (hepatitis A vaccine; HAV) with ProQuad™ (measles/mumps/rubella/varicella; MMRV) and Prevnar™ (7-valent pneumococcal; PCV-7) in healthy, 12–23 mo old children. Group 1 received HAV/MMRV/PCV-7 concomitantly on Day 1 and second doses of HAV/MMRV at Week 24. Group 2 received MMRV/PCV-7 on Day 1, HAV at Weeks 6 and 30 and MMRV at Week 34. Hepatitis A seropositivity rate (SPR: ≥10 mIU/mL; 4 weeks postdose 2), varicella zoster-virus (VZV) SPR (≥5 gpELISA units/mL) and geometric mean titers (GMT) to S. pneumoniae were examined. Injection-site and systemic adverse experiences (AEs) and daily temperatures were collected. Hepatitis A SPR were 100% for Group 1 and 99.4% for Group 2 after two HAV doses; risk difference = 0.7 (95%CI: −1.4,3.8, non-inferior) regardless of initial serostatus. VZV SPR was 93.3% for Group 1 and 98.3% for Group 2; risk difference = −5.1 (95%CI: −9.3,−1.4; non-inferior). S. pneumoniae GMT fold-difference (7 serotypes) ranged from 0.9 to 1.1; non-inferior. No statistically significant differences in the incidence of individual AEs were seen when HAV was administered concomitantly vs. non-concomitantly. Three (all Group 2 post-administration of MMRV/PCV-7) of 11 serious AEs were considered possibly vaccine-related: dehydration and gastroenteritis (same subject) on Day 52; febrile seizure on Day 9. No deaths were reported. Antibody responses to each vaccine given concomitantly were non-inferior to HAV given non-concomitantly with MMRV and PCV-7. Administration of HAV with PCV-7 and MMRV had an acceptable safety profile in 12- to 23-mo-old children.
Keywords: safety, immunogenicity, hepatitis A, measles, mumps, rubella, varicella, pneumococcal, vaccine, concomitant use
Introduction
VAQTA™ [hepatitis A vaccine, inactivated (HAV); Merck Sharp and Dohme Corp.] is licensed for immunization against disease caused by hepatitis A virus in persons 12 mo of age and older.1 Two (2) doses of the vaccine, administered 6 to 18 mo apart, are recommended.2 Clinical trials conducted worldwide have demonstrated that HAV is efficacious, immunogenic and well tolerated.1,3-7
ProQuad™ [measles, mumps, rubella and varicella vaccine live (MMRV vaccine); Merck Sharp and Dohme Corp.] is a quadrivalent vaccine licensed for immunization against measles, mumps, rubella and varicella.8 The vaccine has been found to be well tolerated and highly immunogenic against measles, mumps, rubella and varicella-zoster virus (VZV). A 2-dose regimen of varicella vaccine has been shown to reduce the incidence of varicella breakthrough illnesses when compared with administration of a single dose.9
Prevnar™ [pneumococcal 7-valent conjugate vaccine, Diphtheria CRM197 Protein (PCV-7 vaccine); Pfizer Inc.] is indicated for active immunization of infants and toddlers against invasive disease caused by Streptococcus pneumoniae,10 which remains a leading cause of serious illness among young children worldwide and is the most frequent cause of pneumonia, bacteremia, sinusitis and acute otitis media.11 PCV-7 and the more recently approved PCV-13 (pneumococcal 13-valent conjugate vaccine; Pfizer, Inc.) are recommended as a 4-dose series at 2, 4 and 6 y and 12–15 mo of age.11
The Advisory Committee on Immunization Practices, the American Academy of Family Physicians, and the American Academy of Pediatrics recommend that children between the ages of 12 and 23 mo receive vaccines with antigens including hepatitis B, diphtheria, tetanus, pertussis, Hemophilus influenzae b (Hib), polio, S. pneumoniae, measles, mumps, rubella and varicella.12 HAV administered to children 12 to 23 mo of age would likely be given concomitantly with one or more of the vaccines recommended by these recommending bodies.
This study was conducted to evaluate the immunogenicity and safety of HAV and concomitantly administered MMRV and pneumococcal conjugate (PCV-7) vaccines in children 12 to 23 mo of age.
Results
Participant accounting and demographics
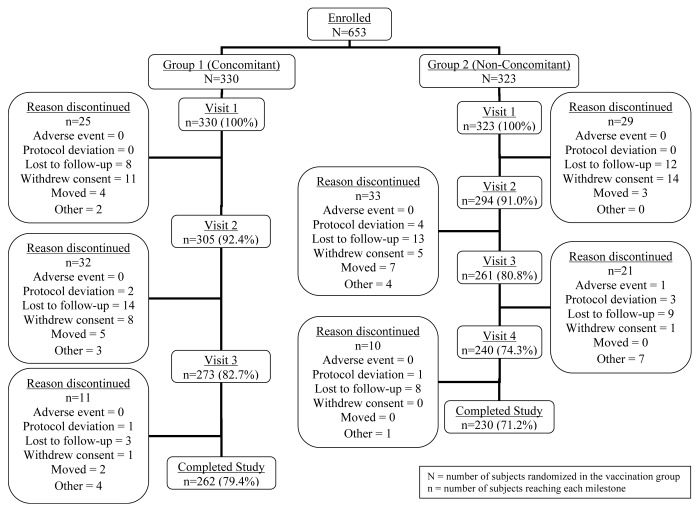
Subjects in both vaccination groups were generally similar across baseline characteristics (Table 1). The mean age at enrollment was just over 12 mo for both groups. The study groups were balanced in race/ethnicity; the largest percentage of participants overall was reported as Caucasian (60.3%). Similar proportions of subjects in Group 1 and Group 2 completed the study (Fig. 1). The most common categories for subjects discontinued were lost to follow-up (66 subjects, 10.1%) and withdrawal of consent (40 subjects, 6.1%).
Table 1. Demographics of study subjects.
|
Group 1 (Concomitant) |
Group 2 (Non-Concomitant) |
|||
| (n = 330) | (n = 323) | |||
| n | (%) | n | (%) | |
| Gender | ||||
| Male | 174 | (52.7) | 157 | (48.6) |
| Female | 156 | (47.3) | 166 | (51.4) |
| Age (months) | ||||
| Mean [SD] | 12.4 [0.80] | 12.5 [0.86] | ||
| Median | 12 | 12 | ||
| Range | 12 to 15 | 12 to 16 | ||
| Race | ||||
| White | 206 | (62.4) | 188 | (58.2) |
| Black | 69 | (20.9) | 72 | (22.3) |
| Hispanic | 33 | (10.0) | 29 | (9.0) |
| Other | 22 | (6.7) | 34 | (10.5) |
N, number of subjects randomized in the vaccination group; n, number of subjects in each category.
Figure 1. Subject accounting.
Immunogenicity
In the per-protocol population, the observed SPR to hepatitis A rates were 100% (182/182) for Group 1 and 99.4% (158/159) for Group 2 (Table 2), with a risk difference between groups of 0.7 [95% confidence interval (CI): −1.4, 3.8] meeting the pre-specified criteria for demonstrating non-inferiority (lower bound of 95% CI > −10.0) (Table 3). Also, for the full analysis set the observed SPR in Group 1 was 99.6% [250/251, 95% CI (98.5%, 100.0%)], and 99.1% [224/226, 95% CI (97.5%, 100.0%)] in Group 2. The results of the full analysis population were comparable to those of the per-protocol population.
Table 2. Hepatitis A, VZV, and S. pneumoniae antibody responses (per-protocol population) among study subjects.
| Parameter | Group 1 | Group 2 | Estimated risk difference or fold differencea | P valueb | |||||
|---|---|---|---|---|---|---|---|---|---|
| (Concomitant) | (Non-concomitant) | ||||||||
| n = 330 | n = 323 | ||||||||
| n | Observed response | (95% CI) | n | Observed response | (95% CI) | (95% CI) | |||
| Hepatitis A SPR and GMT at 4 weeks postdose 2 with HAV | |||||||||
| SPR (≥10 mIU/mL) | 182 | 100% | (98.0, 100) | 159 | 99.40% | (96.5, 100) | 0.7 (−1.4, 3.8) | <0.001 | |
| GMT | 182 | 4976.6 | (4067.6, 6088.6) | 159 | 6123.2 | (4825.6, 7769.7) | not calculated | ||
| VZV antibody response rate and GMT at 6 weeks postdose 1 with MMRV | |||||||||
| ≥5 gpELISA units/mL | 225 | 93.30% | (89.2, 96.2) | 232 | 98.30% | (95.6, 99.5) | −5.1 (−9.3, −1.4) | 0.013 | |
| GMT | 225 | 14.6 | (13.3, 16.1) | 232 | 16.4 | (15.1, 17.7) | not calculated | ||
| S. pneumoniae antibody responses and GMT at 6 weeks post PCV-7 | |||||||||
| Type 4 | GMT | 246 | 1.8 | (1.6, 2.1) | 247 | 1.6 | (1.4, 1.8) | 1.1 (0.9, 1.3) | <0.001 |
| Type 6B | GMT | 246 | 9.5 | (8.3, 11.1) | 246 | 9.6 | (8.5, 10.8) | 1.0 (0.8, 1.2) | <0.001 |
| Type 9V | GMT | 247 | 3.7 | (3.2, 4.2) | 247 | 4.1 | (3.7, 4.7) | 0.9 (0.8, 1.0) | <0.001 |
| Type 14 | GMT | 248 | 7.7 | (6.8, 8.8) | 247 | 7.4 | (6.5, 8.4) | 1.0 (0.9, 1.2) | <0.001 |
| Type 18C | GMT | 247 | 2.9 | (2.6, 3.3) | 247 | 2.6 | (2.3, 3.0) | 1.1 (0.9, 1.3) | <0.001 |
| Type 19F | GMT | 248 | 4 | (3.5, 4.5) | 248 | 3.8 | (3.3, 4.3) | 1.1 (0.9, 1.2) | <0.001 |
| Type 23F | GMT | 247 | 4.9 | (4.3, 5.6) | 247 | 4.5 | (3.9, 5.1) | 1.1 (1.0, 1.3) | <0.001 |
N, Number of subjects randomized and vaccinated in each group; n, Number of subjects contributing analysis; CI, Confidence interval; SPR, Seropositivity Rate; SPR, seroprotection rate; GMT, geometric mean titer; gpELISA, glycoprotein enzyme-linked immunosorbent assay; VZV, varicella-zoster virus; HAV, hepatitis A vaccine; aEstimated risk differences and fold differences were calculated based on a statistical analysis model adjusting for study center; bComputed based on a similarity (non-inferiority) test to rule out a decrease of ≥10 percentage points (for risk differences) or 2-fold (for fold-difference).
Table 3. Adverse experience summary of two doses of HAV (days 1–14 after any dose of HAV).
| Group 1 (Concomitant) |
Group 2 (Non-concomitant) |
|||
|---|---|---|---|---|
| (n = 330) | (n = 323) | |||
| n | (%) | n | (%) | |
| Subjects with follow-up | 330 | (100) | 286 | (100) |
| With one or more AE | 231 | (70.0) | 172 | (60.1) |
| Injection-site AEs† | 105 | (31.8) | 95 | (33.2) |
| Systemic AEs | 204 | (61.8) | 133 | (46.5) |
| With vaccine-related AEs | 183 | (55.5) | 122 | (42.7) |
| Injection-site AEs† | 104 | (31.5) | 95 | (33.2) |
| Systemic AE† | 123 | (37.3) | 51 | (17.8) |
| With serious AEs | 2 | (0.6) | 4 | (1.4) |
| Serious vaccine-related AEs | 0 | 0.0 | 1 | (0.3) |
| Who died | 0 | 0.0 | 0 | 0.0 |
| Discontinued due to AE | 0 | 0.0 | 1 | (0.3) |
| Discontinued due to vaccine-related AE | 0 | 0.0 | 0 | 0.0 |
| Discontinued due to serious AE | 0 | 0.0 | 1 | (0.3) |
| Discontinued due to serious vaccine-related AE | 0 | 0.0 | 0 | 0.0 |
N, Number of subjects randomized and vaccinated in the vaccination group; n, Number of subjects in each category; †Determined by the investigator to be possibly, probably, or definitely related to the vaccine. The same subject may appear in different categories, but counted only once in each category.
The observed VZV antibody response rate [percent with titer ≥ 5 glycoprotein enzyme-linked immunosorbent assay (gpELISA) units/mL] in the per-protocol population was 93.3% for Group 1 and 98.3% for Group 2 (Table 2), with a risk difference in the seroprotection rates (SPRs) between groups of −5.1 (95% CI: −9.3, −1.4) meeting the pre-specified criteria for demonstrating non-inferiority (lower bound of CI > −10.0). Also, for the full analysis set the observed rate of subjects with a titer ≥ 5 gpELISA units/mL in Group 1 was 94.0% [252/268, 95% CI (90.5%, 96.5%)] and was 97.7% [254/260, 95% CI (95.0%, 99.1%)] in Group 2. The results of the full analysis population appeared comparable to those of the per-protocol population.
The fold-difference in geometric mean titers (GMTs) between the 2 groups for each of the 7 S. pneumoniae serotypes ranged from 0.9 to 1.1, with the 2-sided 95% CI on each of the fold-differences in GMTs excluded a decrease of ≥2-fold (all lower bounds of CIs >0.5-fold), thereby demonstrating non-inferiority between the 2 groups for all 7 serotypes. Also, the results of subjects in the full analysis population appeared comparable to the results observed for the per-protocol population
Safety
The number and proportion of subjects reporting clinical AEs during the 14 d following any dose of HAV is summarized in Table 3. Group 2 (60.1%) reported a lower proportion of clinical AEs than Group 1 (70.0%); similarly, a numerically lower rate of systemic AEs was observed in Group 2 (46.5%) compared with Group 1 (61.8%). There were no statistically significant differences detected between the vaccination groups for any of the vaccination report card (VRC)-prompted injection-site AEs Postdose 1 or Postdose 2 of HAV, nor between any of the prespecified systemic or injection-site AE parameters during the 42-d combined safety follow-up period following Visits 1 and 2 (data not shown).
The most common systemic AEs following any vaccination were pyrexia (40.0% in Group 1 and 44.0% in Group 2), upper respiratory tract infection (11.8% in Group 1 and 9.0% in Group 2), and otitis media (9.1% in Group 1 and 9.6% in Group 2. The most common vaccine-related systemic AEs following any vaccination were pyrexia (25.8% in Group 1 and 28.8% in Group 2) and irritability (4.5% in Group 1 and 7.1% in Group 2). Based on the 95% CI for the risk difference, there were no statistically significant differences detected between the groups for any of the systemic AEs.
Serious AEs were reported by two subjects (0.6%) in Group 1 (cellulitis and perineal abscess; pneumonia) and six (1.9%) in Group 2 (bronchopneumonia; febrile convulsion; dehydration and gastroenteritis; gastrointestinal hemorrhage; gastroenteritis and dehydration; genital abscess) during the 28 d following any vaccination. Only one subject, in Group 2, discontinued from the study (postdose 2 of HAV) due to a clinical AE: gastrointestinal hemorrhage that the investigator considered to be a serious AE of severe intensity which was definitely not related to the study vaccination, and which subsequently resolved. Three of 11 serious AEs (all Group 2 post-administration of MMRV/PCV-7) were considered possibly vaccine-related: dehydration and gastroenteritis (same subject) on Day 52; febrile seizure on Day 9. No subjects died during the study.
Overall, 155 (52.5%) subjects in Group 1 and 162 (56.6%) subjects in Group 2 reported maximum temperatures of ≥100.4 °F (≥38.0 °C) during the 42-d combined safety follow-up period. Additionally, 65 (22.0%) of subjects in Group 1 and 57 (19.9%) of subjects in Group 2 reported temperatures ≥102.2 °F (≥39.0 °C). Based on the 2.1% risk difference (95% CI: −4.6, 8.7) and the associated 0.534 p-value, there was no statistically significant difference in the incidence of elevated temperatures ≥102.2 °F (≥39.0 °C) between the 2 groups.
Of the vaccine-associated rashes and mumps-like symptoms, reported by study subjects during 42 d of follow-up, measles-like rashes were observed in 8 (2.4%) subjects in Group 1 and 17 (5.3%) subjects in Group 2 [risk difference: −2.8 (95% CI: −6.1, 0.1); p = 0.059], and varicella-like rashes were observed in 6 (1.8%) subjects in Group 1 and 9 (2.8%) subjects in Group 2 [risk difference: −1.0 (95% CI: −3.6, 1.5); p = 0.409]. All other vaccine-associated rashes and mumps-like symptoms occurred in <1% of the population in each vaccination group. There were no reported mumps-like or zoster-like rashes. Based on the 95% CI for the risk difference, there were no statistically significant differences between the 2 groups.
Discussion
Multiple vaccines for children between the ages of 12 mo and two years of age are recommended by public health authorities and professional medical societies.12 Among these recommendations is protection against hepatitis A, measles, mumps, rubella, varicella and S. pneumoniae. To comply with the recommended immunization schedules, it will be necessary to administer many of these vaccines simultaneously at different body sites. Assessing that no untoward vaccine interactions are encountered is a critical element in any vaccine program where more than one vaccine is administered at the same visit. This study was designed to determine if a difference would be detected in the antibody response of individual vaccine components among healthy children 12 to 23 mo of age with a negative clinical history of hepatitis A and varicella/zoster who received HAV administered concomitantly or non-concomitantly with MMRV and PCV-7 vaccines. We found that antibody response to hepatitis A, varicella and all seven components of the pneumococcal conjugate vaccine were similar (non-inferior) whether or not HAV was given alone or concomitant with PCV-7 and MMRV. The hepatitis A SPR rates were nearly identical (99.6% and 100%, respectively, for concomitant and non-concomitant groups). While numerically higher GMTs were noted in the non-concomitant groups, the difference is not clinically relevant because the confidence intervals overlapped and the GMTs observed (4976.6 and 6123.2 mIU/mL) were several orders of magnitude higher than the seropositivity level (10 mIU/mL).
We further attempted to determine if concomitant vs. non-concomitant administration of these vaccines would result in a difference in AEs or in a different safety profile. We found no statistically significant differences in the incidence of individual AEs among the children in the two vaccine groups in this study. Administration of two doses of HAV concomitantly or non-concomitantly with MMRV and PCV-7 vaccines displayed an acceptable safety profile. Rates of reported systemic and injection-site AEs and elevated temperatures appeared consistent with prior experience following two doses of HAV. The types of vaccine-related AEs (e.g., pyrexia and irritability) reported following 2 doses of HAV administered alone or concomitantly MMRV and PCV-7 vaccines were consistent with prior studies of these vaccines.1,13
The limitations in this study included (1) this was an open-label study, (2) only Prevnar-7 was available (not Prevnar-13) and (3) immunogenicity results after MMRV dose 1 only are available.
Conclusion
This study has demonstrated that the antibody responses elicited by HAV, MMRV and PCV-7 vaccines given concomitantly were non-inferior to these vaccines given non-concomitantly. Concomitant administration of these vaccines had an acceptable safety profile in 12- to 23-mo-old children.
Materials and Methods
Study design
This study evaluated the immunogenicity, safety and tolerability of the concomitant administration of HAV with MMRV and PCV-7 vaccines vs. the administration of HAV non-concomitantly with MMRV and PCV-7 vaccines in healthy children 12 to 15 mo of age at the time of enrollment. From April 2006 to March 2008, this open-label, multicenter, randomized, comparative clinical trial was conducted across 39 sites in the United States.
Study subjects were randomly assigned to concomitant (Group 1) or non-concomitant (Group 2; control) study groups in a 1:1 ratio (Table 4). Subjects in Group 1 received the first dose of HAV, the first dose of MMRV vaccine and the fourth dose of PCV-7 vaccine concomitantly at separate injection sites on Day 1 (12 to 15 mo of age). Group 1 subjects received the second doses of HAV and MMRV vaccines at Week 24 or later (approximately 18 to 21 mo of age, depending on their age of their first vaccinations). Subjects in Group 2 received the first dose of MMRV vaccine and the fourth dose of PCV-7 vaccine on Day 1 and the first dose of HAV at Week 6. Subjects in Group 2 received a second dose of HAV at Week 30 or later (approximately 19.5 mo of age) and a second dose of MMRV vaccine at Week 34 or later (approximately 20.5 mo of age). Subjects in both study groups received the second dose of HAV at least 24 weeks after the first dose. Group 2 was the control group for the antibody responses to hepatitis A, VZV and the 7 S. pneumoniae types.
Table 4. Vaccination and blood draw timelines.
| Time points | |||||||
|---|---|---|---|---|---|---|---|
| Group | N | Day 1 | Week 6 | Week 24 | Week 28 | Week 30 | Week 34 |
| 1 | 330 | HAV+MMRV+PCV-7a | (blood draw) | HAV+MMRVa | (blood draw)c | NA | NA |
| (blood draw) | |||||||
| 2 | 323 | MMRV+PCV-7a | HAVb | NA | NA | HAVb | MMRVa |
| (blood draw) | (blood draw) | (blood draw) | |||||
Safety Data Collection Schedule: a Temperatures: Days 1 through Day 28; Injection-site AEs: Days 1 through 5; Systemic AEs: Days 1 through 28; b Temperatures: Days 1 through Day 5; Injection-site AEs: Days 1 through 5; Systemic AEs: Days 1 through 14; c Temperatures: Days 1 through Day 5; Injection-site AEs: Days 1 through 5; Systemic AEs: Days 1 through 5; Note: Temperatures were to be recorded 4 to 6 h after vaccination on Day 1 and then daily as indicated.
Study objectives
The study objectives were (1) to demonstrate that the first dose of HAV can be administered concomitantly with MMRV and PCV-7 vaccines and the second dose of HAV can be administered concomitantly with a second dose of MMRV vaccine without impairing the antibody response to hepatitis A; (2) to demonstrate that HAV can be administered concomitantly with MMRV and PCV-7 vaccines without impairing the antibody response to the VZV antigen in MMRV vaccine; (3) to demonstrate that HAV can be administered concomitantly with MMRV and PCV-7 vaccines without impairing the antibody responses to Streptococcus pneumoniae types 4, 6B, 9V, 14, 18C, 19F and 23F; and (4) to demonstrate that HAV administered concomitantly with MMRV and PCV-7 vaccines is generally well tolerated.
Study population
Healthy children between 12 and 15 mo of age with no clinical history of hepatitis A, measles, mumps, rubella, varicella and/or zoster, who received three prior doses of PCV-7 were included, but subjects with prior HAV or measles-, mumps-, rubella- or varicella-containing vaccines were excluded. Other key exclusion criteria included immune impairment or deficiency, neoplastic disease or depressed immunity including those resulting from corticosteroid use, and history of allergy or anaphylactoid reaction to any component of the vaccines. The protocol was conducted in accordance with principles of Good Clinical Practice, approved by the Ethical Review Committee of each participating site, and written informed consent was obtained from each subject’s parent/legal guardian prior to study entry.
Vaccine descriptions
HAV is indicated for immunization against disease caused by hepatitis A virus in persons 1 y of age or older. Each 0.5 mL dose of HAV contains approximately 25 U of hepatitis A virus antigen adsorbed onto approximately 0.225 mg of aluminum, provided as amorphous aluminum hydroxyphosphate sulfate, and 35 μg of sodium borate as a pH stabilizer, in 0.9% sodium chloride. HAV was used as supplied; no reconstitution was necessary.
MMRV vaccine is indicated for vaccination against measles, mumps, rubella, and varicella in children 12 mo to 12 y of age. MMRV vaccine is a sterile, lyophilized, preservative-free, frozen, live virus vaccine supplied in 3.0-mL single-dose glass vials containing the required dosage for subcutaneous injection. Sterile diluent was supplied to reconstitute MMRV vaccine.
PCV-7 vaccine is indicated for active immunization of infants and toddlers against invasive pneumococcal disease. PCV-7 vaccine is a heptavalent, conjugated vaccine targeting S. pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F and 23F. The vaccine is administered as a 4-dose series at 2, 4 and 6 y and 12–15 mo of age and was supplied as one 0.5-mL dose per vial. PCV-7 vaccine was used as supplied; no reconstitution was necessary.
Measures
Immunogenicity
A 5-mL blood sample was drawn from all subjects immediately prior to vaccination on Day 1 and at Week 6 (Table 4). A 5-mL blood sample was drawn from subjects at ≥Week 28 for Group 1 and ≥Week 34 for Group 2. Day 1 blood samples were mandatory for enrollment. Serum samples from the Day 1 blood draws were tested for antibody to hepatitis A in Group 1, and antibodies to all 7 S. pneumoniae serotypes and VZV in Groups 1 and 2. Postvaccination serum samples at Week 6 were tested for antibody to hepatitis A in Group 2, and antibodies to S. pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F and 23F and VZV for Groups 1 and 2. Varicella was the only component of MMRV vaccine that was tested, since the concomitant administration of M-M-R™II (Measles, Mumps and Rubella Virus Vaccine Live; Merck Sharp and Dohme Corp.) and HAV had been previously demonstrated.1,13,14 Postvaccination serum samples at ≥Week 28 and ≥Week 34 were tested for antibody to hepatitis A following the administration of 2 doses of HAV for Groups 1 and 2, respectively. Antibody responses to all antigens of interest were evaluated by an appropriately sensitive and reliable method [HAVK enzyme immunoassay (EIA) for anti-HAV, gpELISA for anti-VZV and an enzyme-linked immunosorbent assay (ELISA) for antibodies to the 7 serotypes of S. pneumoniae].
Safety
Temperatures were recorded from Day 1 (the day of vaccination) through Day 28 following vaccination with MMRV vaccine (Day 1 and Week 24 study visits for Group 1; Day 1 and Week 34 study visits for Group 2) and through Day 5 following vaccination with HAV alone (Week 6 study visit for Group 1; Week 6 and Week 30 study visits for Group 2) consistent with standard follow-up periods for live virus and inactivated vaccines, respectively. Injection-site adverse experiences (AEs) were recorded Day 1 through Day 5 following each vaccination. Systemic AEs were recorded Day 1 through Day 28 following vaccination with MMRV vaccine, and Day 1 through Day 14 following vaccination with HAV alone. The parent/legal guardian was instructed to contact study personnel as soon as possible if the subject developed a measles-, rubella-, varicella- or zoster-like rash, or other symptoms resembling measles, mumps, rubella, varicella or zoster during the 28-d safety follow-up periods following Day 1 and Week 24 study visits for Group 1 and Day 1 and Week 34 study visits for Group 2.
Statistical analysis
Immunogenicity
For the first primary immunogenicity hypothesis regarding the non-inferiority of the SPR of hepatitis A, the method of Miettinen and Nurminen was used to conduct the non-inferiority test.15 The statistical criterion for demonstrating non-inferiority of the SPR to hepatitis A corresponded to the lower bound of the 2-sided 95% CI on the difference in SPRs (Group 1 minus Group 2) excluding a decrease of 10 percentage points or more.
In the second primary immunogenicity hypothesis (the non-inferiority of the VZV antibody response rate), the VZV antibody response rate was defined as the percent of subjects with VZV antibody titer ≥5 gpELISA units/mL in subjects whose baseline VZV antibody titer was < 1.25 gpELISA units/mL. The statistical criterion for demonstrating non-inferiority of the antibody response rate for VZV corresponded to the lower bound of the 2-sided multiplicity adjusted 95% CI on the difference (Group 1 minus Group 2) excluding a decrease of 10 percentage points or more.
For the third primary immunogenicity hypothesis regarding the non-inferiority of the antibody responses to S. pneumoniae types 4, 6B, 9V, 14, 18C, 19F and 23F, the comparison of GMTs between the investigational group and the control group was based on an Analysis of Covariance (ANCOVA) model using the natural logarithm of the baseline titer and combined study center as covariates and the natural logarithm of the postvaccination titer as the dependent variable. The statistical criterion for demonstrating non-inferiority of GMTs for S. pneumoniae types 4, 6B, 9V, 14, 18C, 19F and 23F (7 tests in total) required the Group1/Group 2 ratio of the lower bound of the 2-sided multiplicity adjusted 95% CI for the ratio of GMTs to be > 0.5 for each antigen (i.e., excluding a decrease of 2-fold or more).
The primary analysis population for immunogenicity is the per-protocol population, which included all subjects who received vaccinations within the specified day ranges, completed appropriate follow-up, and were without any prespecified protocol violations. Immunogenicity results for the full analysis population (all subjects with a serology measurement, regardless of initial serostatus or protocol violations) are also provided as a supportive analysis. This study was to consist of ~600 healthy children 12 to 15 mo of age upon receipt of the first study vaccine. Assuming an evaluability rate of 85%, a true SPR of 90% for hepatitis A, a true response rate of 88% for VZV, a standard deviation ranging from 1.1 to 1.5 for the components of PCV-7 vaccine, independence among all 9 immunogenicity comparisons, and no underlying difference in the vaccination groups, the overall power for the study was ~92% for an α of 0.025 (1-sided) for each hypothesis.
Safety
The primary safety hypothesis proposed that HAV given concomitantly with MMRV and PCV-7 vaccines would be well tolerated when compared with HAV given separately from these vaccines. All subjects who were vaccinated with at least one dose and had safety follow-up data were evaluated for safety. The primary safety evaluation with respect to concomitant use was based on Dose 1 of HAV, Dose 1 of MMRV vaccine, and the 4th dose of PCV-7 vaccine. Safety data were first combined over the first two visits for each group (28 d after the first visit and 14 d after the second, for a total of 42 d of follow-up) to compare the concomitant administration of HAV, MMRV vaccine, and PCV-7 vaccine (Group 1) with the concomitant administration of MMRV vaccine and PCV-7 vaccine followed by HAV six weeks later (Group 2). Analysis of safety data included the computation of risk differences, 95% CIs for the risk difference, and p-values for the comparisons of the groups for injection-site AEs (including redness, swelling and pain/tenderness/soreness) solicited on the VRC during Days 1 to 5 and rashes solicited on the VRC Days 1 to 28. Analysis of safety data also included a comparison between vaccination groups for body temperatures collected within Days 1 to 5. Power analyses estimated that if a particular safety signal was not observed in ~300 subjects who received HAV concomitantly with MMRV and PCV-7 vaccines, the study would provide 95% confidence that the true rate was <1.3%. With 262 and 230 subjects completing the study in the concomitant and non-concomitant groups, respectively, the study actually provides 95% confidence that the true rate of an unobserved safety signal is <1.4% and <1.6%, respectively.
Acknowledgments
The authors would like to thank all the subjects who participated in this study. VAQTA® Protocol 067 Study Group: WP Andrews (GA), E Barranco (PR), MJ Benbow (TX), HH Bernstein (NH), HR Bertrand (SC), CC Chang (CA), T Crum (WA), AL Duke (AR), ER Franklin (NC), IP Godoy (CA), E Goldblatt (AL), U Goswami (IL), SE Grogg (OK), E Hammel (CA), B Harvey (AR), CD Jackson (AR), NB Klein (CA), W Johnston (AL), J Kratzer (CA), KH Lee (CA), E Maddela (CA), LK Nassri (AR), A Naz (CA), CD Nelms (TX), LO Sass (VA), TJ Schechtman (FL), SD Senders (OH), OS Shaikh (OH), JS Shepard (OH), PE Silas (UT), M Sperling (CA), RA Stanford (AR), BJ Sullivan (WI), RJ Yetman (TX).
Study identification: V251–067
CLINICALTRIALS.GOV identifier: NCT00312858
Data from this manuscript were presented in poster format at the 2011 European Society for Paediatric Infectious Diseases (abstract #371).
Yetman, Shepard, Duke, Shaikh: enrollment of subjects and/or data collection, analysis and interpretation of data and preparation of manuscript. Stek, Petrecz: analysis and interpretation of data and preparation of manuscript. Klopfer, Kuter, Schödel, Lee: study concept and design, analysis and interpretation of data and preparation of manuscript.
Glossary
Abbreviations:
- AE
adverse experience
- CI
confidence interval
- GMT
geometric mean titer
- gpELISA
glycoprotein enzyme-linked immunosorbent assay
- HAV
VAQTA™ (hepatitis A vaccine, inactivated)
- Hib
Haemophilus influenzae b
- MMRV vaccine
ProQuad™ (measles, mumps, rubella and varicella vaccine live)
- PCV-7 vaccine
Prevnar™ (pneumococcal 7-valent conjugate vaccine)
- SPR
seropositivity rate
- VZV
varicella-zoster virus
Disclosure of Potential Conflicts of Interest
Other than employees of Merck Sharp and Dohme Corp. (as indicated on the title page), all authors have been investigators for the sponsor. Employees may hold stock and/or stock options in the company.
This study was funded by Merck Sharp and Dohme Corp. (sponsor). In conjunction with the external investigators, this study was designed, executed, and analyzed by the sponsor. The sponsor formally reviewed a penultimate draft. All co-authors approved the final version of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/24873
References
- 1.U.S. Package Circular. VAQTA™ (Hepatitis A Vaccine, Inactivated): February 2003. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM110049.pdf. Accessed: 03-Apr-2013.
- 2.Fiore AE, Wasley A, Bell BP, Advisory Committee on Immunization Practices (ACIP) Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR07):1–23. [PubMed] [Google Scholar]
- 3.Werzberger A, Kuter B, Shouval D, Mensch B, Brown L, Wiens B, et al. Anatomy of a trial: a historical view of the Monroe inactivated hepatitis A protective efficacy trial. J Hepatol. 1993;18(Suppl 2):S46–50. doi: 10.1016/S0168-8278(05)80378-2. [DOI] [PubMed] [Google Scholar]
- 4.Linglöf T, van Hattum J, Kaplan KM, Corrigan J, Duval I, Jensen E, et al. An open study of subcutaneous administration of inactivated hepatitis A vaccine (VAQTA) in adults: safety, tolerability, and immunogenicity. Vaccine. 2001;19:3968–71. doi: 10.1016/S0264-410X(01)00134-7. [DOI] [PubMed] [Google Scholar]
- 5.Hornick R, Tucker R, Kaplan KM, Eves KA, Banerjee D, Jensen E, et al. A randomized study of a flexible booster dosing regimen of VAQTA in adults: safety, tolerability, and immunogenicity. Vaccine. 2001;19:4727–31. doi: 10.1016/S0264-410X(01)00224-9. [DOI] [PubMed] [Google Scholar]
- 6.Black S, Shinefield H, Hansen J, Lewis E, Su L, Coplan P. A post-licensure evaluation of the safety of inactivated hepatitis A vaccine (VAQTA, Merck) in children and adults. Vaccine. 2004;22:766–72. doi: 10.1016/j.vaccine.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Wallace MR, Brandt CJ, Earhart KC, Kuter BJ, Grosso AD, Lakkis H, et al. Safety and immunogenicity of an inactivated hepatitis A vaccine among HIV-infected subjects. Clin Infect Dis. 2004;39:1207–13. doi: 10.1086/424666. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Package Circular. ProQuad™ (Measles, Mumps, Rubella and Varicella [Oka/Merk] Virus Vaccine Live): October 2007. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm123793.pdf. Accessed: 03-Apr-2013.
- 9.Marin M, Broder KR, Temte JL, Snider DE, Seward JF, Centers for Disease Control and Prevention (CDC) Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59(RR03):1–12. [PubMed] [Google Scholar]
- 10.U.S. Package Circular. Prevnar™ (Pneumococcal 7-Valent Conjugate Vaccine [Diphtheria CRM197 Protein]): 2005. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM137038.pdf. Accessed:03-Apr-2013.
- 11.Advisory Committee on Immunization Practices Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49(RR09):1–35. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Child, Adolescent & “Catch-up” Immunization Schedules. Available at: http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed: August 22, 2012.
- 13.Guerra FA, Gress J, Werzberger A, Reisinger K, Walter E, Lakkis H, et al. Pediatric Study Group for VAQTA Safety, tolerability and immunogenicity of VAQTA given concomitantly versus nonconcomitantly with other pediatric vaccines in healthy 12-month-old children. Pediatr Infect Dis J. 2006;25:912–9. doi: 10.1097/01.inf.0000238135.01287.b9. [DOI] [PubMed] [Google Scholar]
- 14.Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L, Centers for Disease Control and Prevention Measles, mumps, and rubella--vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1998;47(RR-8):1–57. [PubMed] [Google Scholar]
- 15.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–26. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]