Abstract
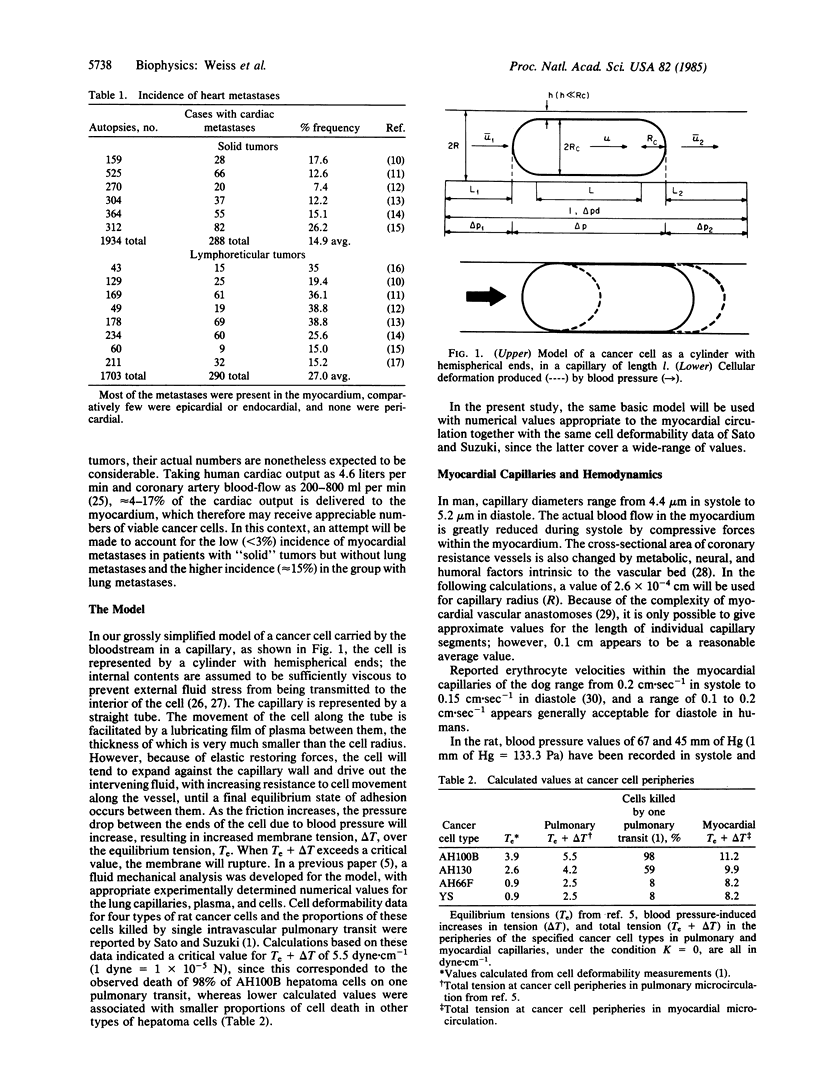
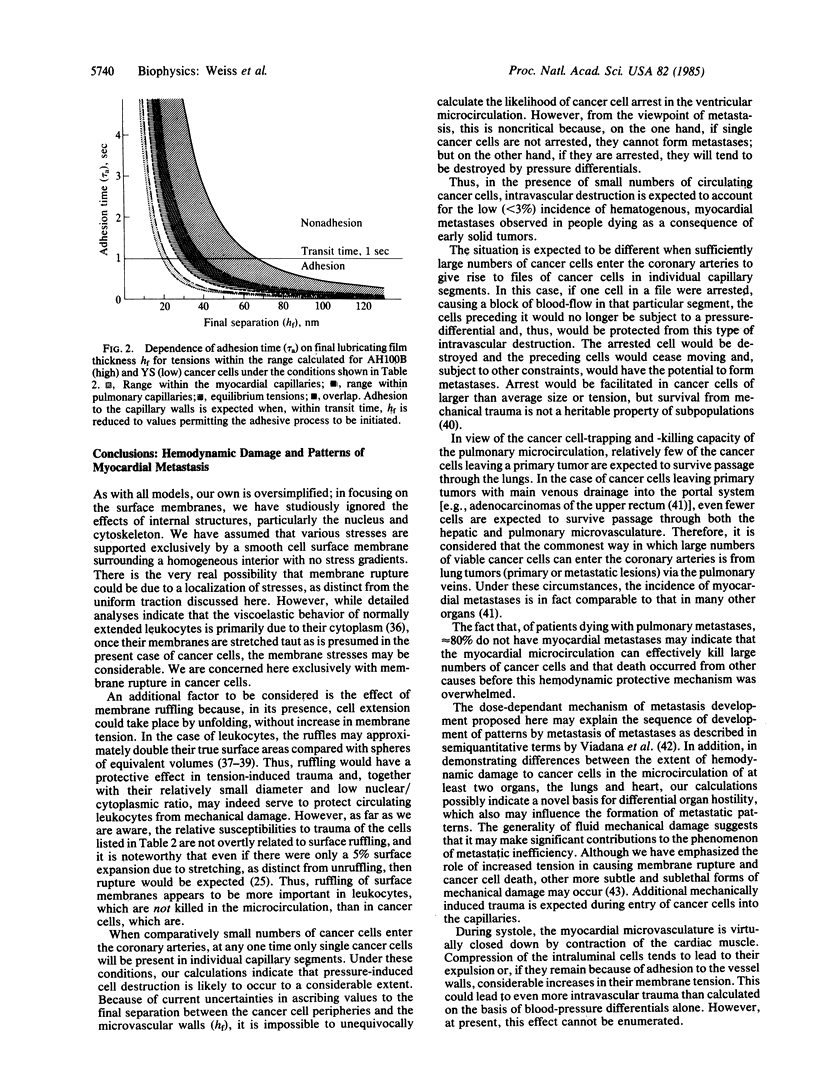
A variety of observations in humans and experimental animals indicate that large numbers of circulating cancer cells are killed in the microvasculature. It is suggested that this occurs when friction or adhesion between individual cancer cells and capillary walls results in an increase of tension in the cancer cell peripheries above a critical level because of (blood) pressure differentials between their free ends. Hemodynamic and anatomic data relating to the myocardial circulation and deformability measurements on four types of rat cancer cells have been reported previously by others. Novel calculations based on these data suggest that the increased tension at the peripheries of cancer cells passing through the myocardial capillaries will exceed the critical levels for rupture. Analysis of autopsy data for solid tumors reveals a low (less than 3%) incidence of myocardial metastases in the absence of lung metastases and a higher (15%) incidence in their presence. One explanation for these observations is that, in the absence of lung metastases, relatively few of the cancer cells enter the coronary arteries from primary tumors with systemic venous drainage because many are retained or destroyed in transit through the pulmonary vasculature, and most of those delivered to the myocardium then suffer hemodynamic destruction. In the presence of pulmonary metastases, large numbers of viable cancer cells are liberated directly into the pulmonary venules and subsequently are delivered to the myocardium without prior exposure to the arterial side of the microcirculation. The combined effects of increased delivery and the protective effects of arrested cells on those preceding them in files along the capillaries account for the higher incidence of myocardial metastases. It is proposed that hemodynamic destruction of circulating cancer cells may be an important underlying cause of metastatic inefficiency, together with other cytocidal mechanisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISEL H. F., WROBLEWSKI F., LADUE J. S. Incidence and clinical manifestations of cardiac metastases. J Am Med Assoc. 1953 Oct 24;153(8):712–715. doi: 10.1001/jama.1953.02940250018005. [DOI] [PubMed] [Google Scholar]
- BURNETT R. C., SHIMKIN M. B. Secondary neoplasms of the heart. AMA Arch Intern Med. 1954 Feb;93(2):205–218. doi: 10.1001/archinte.1954.00240260041004. [DOI] [PubMed] [Google Scholar]
- DELOACH J. F., HAYNES J. W. Secondary tumors of heart and pericardium; review of the subject and report of one hundred thirty-seven cases. AMA Arch Intern Med. 1953 Feb;91(2):224–249. doi: 10.1001/archinte.1953.00240140084007. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Jain R. K. Membrane stability. Biochim Biophys Acta. 1984 Dec 4;779(4):437–468. doi: 10.1016/0304-4157(84)90020-0. [DOI] [PubMed] [Google Scholar]
- Gabor H., Weiss L. Mechanically induced trauma suffered by cancer cells in passing through pores in polycarbonate membranes. Invasion Metastasis. 1985;5(2):71–83. [PubMed] [Google Scholar]
- Gabor H., Weiss L. Survival of L1210 and Ehrlich ascites cancer cells after mechanical trauma is a random event. Invasion Metastasis. 1985;5(2):84–95. [PubMed] [Google Scholar]
- Glaves D. Correlation between circulating cancer cells and incidence of metastases. Br J Cancer. 1983 Nov;48(5):665–673. doi: 10.1038/bjc.1983.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaves D., Mayhew E. Selective therapy of metastasis. I. Quantitation of tumorigenic circulating and covert cancer cells disseminated from metastatic and nonmetastatic tumors. Cancer Drug Deliv. 1984 Fall;1(4):293–302. doi: 10.1089/cdd.1984.1.293. [DOI] [PubMed] [Google Scholar]
- HANFLING S. M. Metastatic cancer to the heart. Review of the literature and report of 127 cases. Circulation. 1960 Sep;22:474–483. doi: 10.1161/01.cir.22.3.474. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Koo J., Fung K., Siu K. F., Lee N. W., Lett Z., Ho J., Wong J., Ong G. B. Recovery of malignant tumor cells from the right atrium during hepatic resection for hepatocellular carcinoma. Cancer. 1983 Nov 15;52(10):1952–1956. doi: 10.1002/1097-0142(19831115)52:10<1952::aid-cncr2820521029>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Malaret G. E., Aliaga P. Metastatic disease to the heart. Cancer. 1968 Aug;22(2):457–466. doi: 10.1002/1097-0142(196808)22:2<457::aid-cncr2820220225>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Mayhew E., Glaves D. Quantitation of tumorigenic disseminating and arrested cancer cells. Br J Cancer. 1984 Aug;50(2):159–166. doi: 10.1038/bjc.1984.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NADEL E. M. PROSPICE--TUMOR CELLS IN CIRCULATING BLOOD. Acta Cytol. 1965 Mar-Apr;9:185–188. [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Sung K. L., Tözeren H., Skalak R., Chien S. Passive mechanical properties of human leukocytes. Biophys J. 1981 Oct;36(1):243–256. doi: 10.1016/S0006-3495(81)84726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmanns H., Ikeda S., Hansen H., Sarma J. S., Fauvel J. M., Bing R. J. Microcirculation in the ventricle of the dog and turtle. Circ Res. 1974 Apr;34(4):561–569. doi: 10.1161/01.res.34.4.561. [DOI] [PubMed] [Google Scholar]
- Tillmanns H., Steinhausen M., Leinberger H., Thederan H., Kübler W. Pressure measurements in the terminal vascular bed of the epimyocardium of rats and cats. Circ Res. 1981 Nov;49(5):1202–1211. doi: 10.1161/01.res.49.5.1202. [DOI] [PubMed] [Google Scholar]
- Weiss L. Cancer cell traffic from the lungs to the liver: an example of metastatic inefficiency. Int J Cancer. 1980 Mar 15;25(3):385–392. doi: 10.1002/ijc.2910250313. [DOI] [PubMed] [Google Scholar]
- Weiss L., Dimitrov D. S. A fluid mechanical analysis of the velocity, adhesion, and destruction of cancer cells in capillaries during metastasis. Cell Biophys. 1984 Mar;6(1):9–22. doi: 10.1007/BF02788577. [DOI] [PubMed] [Google Scholar]
- Weiss L., Glaves D. Cancer cell damage at the vascular endothelium. Ann N Y Acad Sci. 1983;416:681–692. doi: 10.1111/j.1749-6632.1983.tb35220.x. [DOI] [PubMed] [Google Scholar]
- Weiss L., Voit A., Lane W. W. Metastatic patterns in patients with carcinomas of the lower esophagus and upper rectum. Invasion Metastasis. 1984;4(1):47–60. [PubMed] [Google Scholar]
- Weiss L., Ward P. M., Harlos J. P., Holmes J. C. Target organ patterns of tumors in mice following the arterial dissemination of B16 melanoma cells. Int J Cancer. 1984 Jun 15;33(6):825–830. doi: 10.1002/ijc.2910330618. [DOI] [PubMed] [Google Scholar]
- YOUNG J. M., GOLDMAN I. R. Tumor metastasis to the heart. Circulation. 1954 Feb;9(2):220–229. doi: 10.1161/01.cir.9.2.220. [DOI] [PubMed] [Google Scholar]



