Abstract
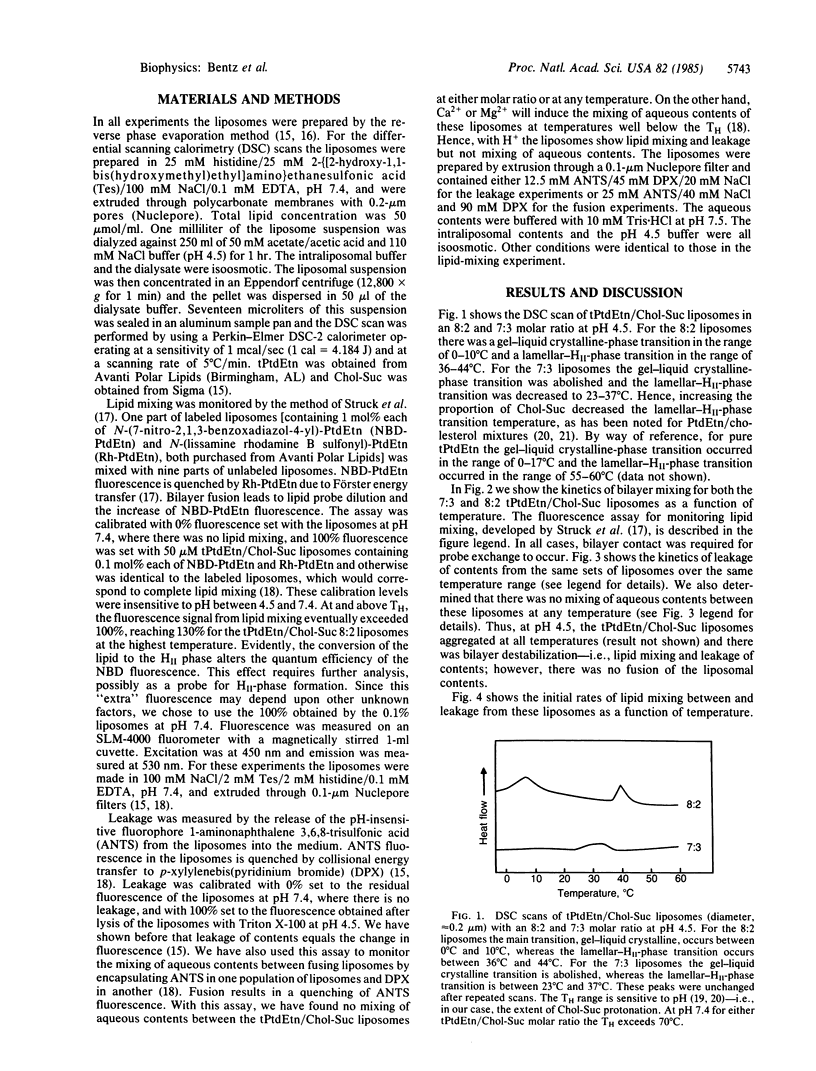
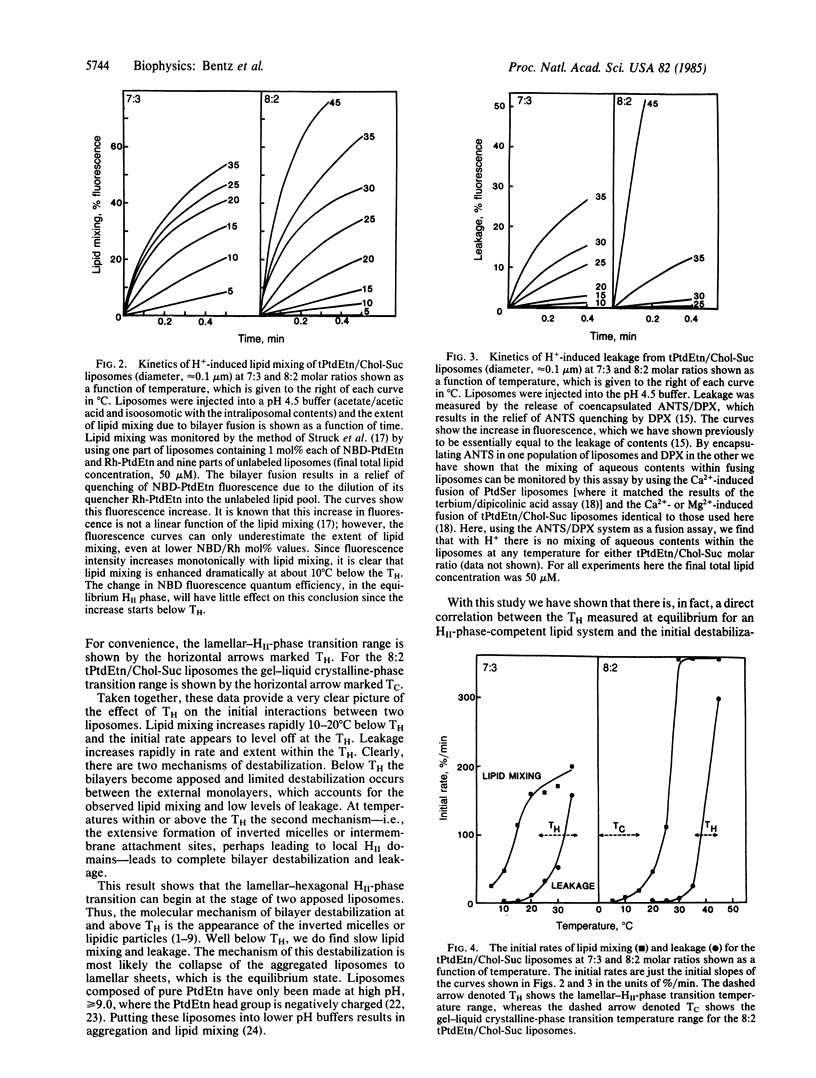
The abundance of phosphatidylethanolamine (PtdEtn) in biological membranes and the capacity of this lipid to sustain nonbilayer structures have been promoted as evidence for a role of PtdEtn in biological fusion processes. To date there has been no direct evidence of a connection between the kinetics of bilayer destabilization and the polymorphism accessible to PtdEtn. We have developed a model system to examine this point directly using the proton-induced destabilization of PtdEtn/cholesterylhemisuccinate unilamellar liposomes. We find that the initial rate of bilayer mixing rapidly increases with temperature and reaches a maximal level just below the HII-phase transition temperature. The leakage from these liposomes rapidly increases, both in rate and extent, within the HII-phase transition temperature range. Of an even greater significance is that at no temperature is there any mixing of aqueous contents within the liposomes. Thus, these lipids can begin to undergo the lamellar- to HII-phase transition at the stage of two apposed liposomes. However, the nonbilayer structures formed do not cause fusion--i.e., the concomitant mixing of aqueous contents.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearer E. L., Düzgünes N., Friend D. S., Papahadjopoulos D. Fusion of phospholipid vesicles arrested by quick-freezing. The question of lipidic particles as intermediates in membrane fusion. Biochim Biophys Acta. 1982 Dec 8;693(1):93–98. doi: 10.1016/0005-2736(82)90474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz J., Düzgüneş N., Nir S. Temperature dependence of divalent cation induced fusion of phosphatidylserine liposomes: evaluation of the kinetic rate constants. Biochemistry. 1985 Feb 12;24(4):1064–1072. doi: 10.1021/bi00325a039. [DOI] [PubMed] [Google Scholar]
- Bentz J., Düzgüneş N., Nir S. Temperature dependence of divalent cation induced fusion of phosphatidylserine liposomes: evaluation of the kinetic rate constants. Biochemistry. 1985 Feb 12;24(4):1064–1072. doi: 10.1021/bi00325a039. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., De Kruijff B. Polymorphic phase behaviour of lipid mixtures as detected by 31P NMR. Evidence that cholesterol may destabilize bilayer structure in membrane systems containing phosphatidylethanolamine. Biochim Biophys Acta. 1978 Feb 21;507(2):207–218. doi: 10.1016/0005-2736(78)90417-0. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., Hope M. J. Effects of fusogenic agent on membrane structure of erythrocyte ghosts and the mechanism of membrane fusion. Nature. 1978 Feb 16;271(5646):672–674. doi: 10.1038/271672a0. [DOI] [PubMed] [Google Scholar]
- Düzgünes N., Paiement J., Freeman K. B., Lopez N. G., Wilschut J., Papahadjopoulos D. Modulation of membrane fusion by ionotropic and thermotropic phase transitions. Biochemistry. 1984 Jul 17;23(15):3486–3494. doi: 10.1021/bi00310a016. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Wilschut J., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion. Role of head-group composition in calcium- and magnesium-induced fusion of mixed phospholipid vesicles. Biochim Biophys Acta. 1981 Mar 20;642(1):182–195. doi: 10.1016/0005-2736(81)90148-6. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. H+- and Ca2+-induced fusion and destabilization of liposomes. Biochemistry. 1985 Jun 18;24(13):3099–3106. doi: 10.1021/bi00334a005. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. pH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contact. Biochemistry. 1984 Mar 27;23(7):1532–1538. doi: 10.1021/bi00302a029. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Stewart T. P., Boni L. T., Yeagle P. L. Membrane fusion through point defects in bilayers. Science. 1981 May 22;212(4497):921–923. doi: 10.1126/science.7233185. [DOI] [PubMed] [Google Scholar]
- Kolber M. A., Haynes D. H. Evidence for a role of phosphatidyl ethanolamine as a modulator of membrane-membrane contact. J Membr Biol. 1979 Jun 29;48(1):95–114. doi: 10.1007/BF01869258. [DOI] [PubMed] [Google Scholar]
- Lai M. Z., Vail W. J., Szoka F. C. Acid- and calcium-induced structural changes in phosphatidylethanolamine membranes stabilized by cholesteryl hemisuccinate. Biochemistry. 1985 Mar 26;24(7):1654–1661. doi: 10.1021/bi00328a013. [DOI] [PubMed] [Google Scholar]
- Lucy J. A. Do hydrophobic sequences cleaved from cellular polypeptides induce membrane fusion reactions in vivo? FEBS Lett. 1984 Jan 30;166(2):223–231. doi: 10.1016/0014-5793(84)80085-x. [DOI] [PubMed] [Google Scholar]
- Miller R. G. Do 'lipidic particles' represent intermembrane attachment sites? Nature. 1980 Sep 11;287(5778):166–167. doi: 10.1038/287166a0. [DOI] [PubMed] [Google Scholar]
- Pryor C., Bridge M., Loew L. M. Aggregation, lipid exchange, and metastable phases of dimyristoylphosphatidylethanolamine vesicles. Biochemistry. 1985 Apr 23;24(9):2203–2209. doi: 10.1021/bi00330a014. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Reese T. S., Miller R. G. Phospholipid bilayer deformations associated with interbilayer contact and fusion. Nature. 1981 Sep 17;293(5829):237–238. doi: 10.1038/293237a0. [DOI] [PubMed] [Google Scholar]
- Sen A., Williams W. P., Brain A. P., Dickens M. J., Quinn P. J. Formation of inverted micelles in dispersions of mixed galactolipids. Nature. 1981 Oct 8;293(5832):488–490. doi: 10.1038/293488a0. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar structures in bilayer membranes. Formation rates and half-lives. Biophys J. 1984 Feb;45(2):399–420. doi: 10.1016/S0006-3495(84)84164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollery J. G., Vail W. J. Interactions of divalent cations or basic proteins with phosphatidylethanolamine vesicles. Biochim Biophys Acta. 1977 Dec 15;471(3):372–390. doi: 10.1016/0005-2736(77)90043-8. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Hoekstra D., Pagano R. E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981 Jul 7;20(14):4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij A. J. Lipidic intramembranous particles. Biochim Biophys Acta. 1984 Jan 27;779(1):43–63. doi: 10.1016/0304-4157(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Momvers C., Leunissen-Bijvelt J., Ververgaert P. H. Lipidic intramembranous particles. Nature. 1979 May 10;279(5709):162–163. doi: 10.1038/279162a0. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Papahadjopoulos D. Ca2+-induced fusion of phospholipid vesicles monitored by mixing of aqueous contents. Nature. 1979 Oct 25;281(5733):690–692. doi: 10.1038/281690a0. [DOI] [PubMed] [Google Scholar]



