Supplemental Digital Content is available in the text.
Keywords: auditory hair cells, auditory progenitors, p27kip1, PTEN/PI3K/Akt-signaling pathway, PTEN
Abstract
The organ of Corti, which is the sensory organ of hearing, consists of a single row of inner hair cells and three rows of outer hair cells in mice. The auditory hair cells develop from auditory progenitors. Hair cell development is related to several genes, including PTEN. Homozygous null mutant (PTEN−/−) mice die at around embryonic day 9, when hair cells are extremely immature. Moreover, in heterozygous PTEN knockout mice, it was found that PTEN regulates the proliferation of auditory progenitors. However, little is known about the molecular mechanism underlying this regulation. In the present study, we generated PTEN conditional knockout in the inner ear of mice and studied the aforementioned molecular mechanisms. Our results showed that PTEN knockout resulted in supernumerary hair cells, increased p-Akt level, and decreased p27kip1 level. Furthermore, the presence of supernumerary hair cells could be explained by the delayed withdrawal of auditory progenitors from the cell cycle. The increased p-Akt level correlates with p27kip1 downregulation in the cochlea in the Pax2-PTEN−/− mice. The reduced p27kip1 could not maintain the auditory progenitors in the nonproliferative state and some progenitors continued to divide. Consequently, additional progenitors differentiated into supernumerary hair cells. We suggest that PTEN regulates p27kip1 through p-Akt, thereby regulating the proliferation and differentiation of auditory progenitors.
Introduction
The inner ear is the innermost part of the vertebrate ear, and it is mainly responsible for balance and sound detection. The inner ear develops from the otic placode, which is a thickening of the surface ectoderm next to the hindbrain at embryonic day 8 (E8). Over time, the otic placode invaginates to form the otocyst at E9. By E12, the otocyst begins to develop morphologically into the cochlea and the vestibule in mice 1. The cochlea is the auditory portion of the mammalian inner ear 2. Its core component is the organ of Corti, which is the sensory organ of hearing. The organ of Corti in mice consists of a single row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs) 3. The auditory hair cells (HCs) are the sensory receptors that transform sound vibrations into electrical signals. The electrical signals then travel through auditory nerve fibers to the brain. HCs develop from auditory progenitors and several genes coordinate to regulate this process 4,5. All otocyst cells in mice continue to divide from E9 to E12 2,6. The auditory progenitors withdraw from the cell cycle and establish a zone of nonproliferating cells from E12.5 to E14.5. P27kip1 activity maintains zone of nonproliferating cells in a nonmitotic state. At E15, p27kip1 expression is selectively downregulated, and auditory progenitors differentiate into HCs and supporting cells 7. The upstream regulator of p27kip1 in the inner ear remains unknown. Using cell culture systems of 293T and COS-7 cells, p27kip1 was found to be the substrate of Akt. Akt was converted into its activated form (p-Akt) through phosphorylation at specific serine and threonine residues. Subsequently, p-Akt promoted p27kip1 degradation 8–10.
Mammalian HCs cannot regenerate spontaneously 11. The HC loss caused by noise trauma and ototoxic drugs is permanent and often leads to hearing impairment. Thus, study of HC regeneration in mammals is a hot topic in hearing research. Several genes, such as retinoblastoma (Rb), p27kip1, and phosphatase and tensin homolog deleted on chromosome 10 (PTEN), are associated with HC regeneration 7,12–14.
PTEN is a tumor suppressor gene frequently mutated in tumors such as endometrial carcinomas and glioblastomas 15. PTEN expression in the HCs and cochlear–vestibular ganglion in the inner ear of mice shows a transient, specific pattern 16,17. PTEN null mice (PTEN−/−) die at around E9.5, long before the HCs mature 4,18. By contrast, the number of HCs is increased in heterozygous PTEN knockout mice. Withdrawal of auditory progenitors from the cell cycle is delayed 14. These results indicate that PTEN is involved in the proliferation of auditory progenitors. However, little is known about the molecular mechanism of PTEN in regulating the proliferation and differentiation of auditory progenitors. Thus, we conditionally knocked out PTEN in the inner ear of mice. Using this mouse model, we studied the role of PTEN in regulating the proliferation and differentiation of auditory progenitors.
Materials and methods
Animals
Mice homozygous for floxed PTEN exon 5 (PTENLoxP/LoxP) 19 were crossed with Pax2-Cre mice 20. Pax2-Cre recombinase was expressed in the developing otocyst, kidneys, and the midbrain–hindbrain boundary 20. Mice for timed mating were placed together at 6:00 p.m. The day the vaginal plugs were established was considered embryonic day 0.5 (E0.5).
All experiments were conducted in accordance with the standards of the Shandong University Ethics Committee.
Genotyping
The following PCR primers were designed to detect the genotypes: the PTENLoxP/LoxP left primer, located upstream of exon 5, was 5′-gaccctgaactcaatgtttagc-3′, whereas the PTENLoxP/LoxP right primer, located within exon 5, was 5′-gccccgatgcaataaatatg-3′. The PCR cycling conditions were as follows: 33 cycles of 94°C for 10 min, 94°C for 30 s, 57°C for 1 min, 72°C for 30 s, and 72°C for 7 min. The Pax2-Cre left primer was 5′-tgcaacgagtgatgaggttc-3′, whereas the Pax2-Cre right primer was 5′-acgaacctggtcgaaatcag-3′. The PCR cycling conditions were 30 cycles of 94°C for 10 min, 94°C for 30 s, 55°C for 30 s, 72°C for 30 s, and 72°C for 7 min.
Immunostaining of cryosections
Inner ears dissected from the heads of mice were fixed overnight in 4% paraformaldehyde at 4°C. Subsequently, the samples were placed in 15% sucrose for 8 h at room temperature for the first dehydration and in 30% sucrose at 4°C overnight for the second dehydration. Finally, the samples were embedded in OCT compound (Sakura Finetek, Torrance, California, USA) and frozen at −20°C until sectioning.
The sections were washed with 10 mM PBS and blocked (10% goat serum) for 1 h at room temperature. Primary antibodies in 5% goat serum were then introduced and the samples were incubated overnight at 4°C. The primary antibodies included anti-myosin VIIa (myo 7a) rabbit polyclonal antibodies (Proteus-Bioscience, Ramona, California, USA; 1 : 200), anti-p27kip rabbit polyclonal antibody (Abcam, Cambridge, Massachusetts, USA; 1 : 200), and anti-p-Akt rabbit monoclonal antibodies (Millipore, Billerica, Massachusetts, USA; 1 : 200). The samples were washed with PBS and incubated at room temperature for 2 h in secondary goat anti-rabbit IgG (H+L) Alexa Fluor 488 (Invitrogen, Carlsbad, California, USA; 1 : 300) diluted in the same solution as the primary antibodies. A laser scanning confocal microscope (LSM700; Carl Zeiss AG, Pentacon, Germany) was used to analyze the samples.
Whole-mount immunostaining
Inner ears dissected from the heads of mice were fixed overnight in 4% paraformaldehyde at 4°C. The cochlear were dissected into basal, middle, and apical sections. The resulting whole mounts were washed with 10 mM PBS and stained with rhodamine phalloidin (Sigma, Santa Clara, California, USA; 1 : 500) for 30 min at room temperature, followed by a final washing in PBS. The samples were analyzed under a laser scanning confocal microscope (LSM700). We counted the rhodamine–phalloidin-stained HCs.
Western blot analysis
Cochlea proteins were incubated in cell lysis buffer (10 mM Tris, pH=7.4, 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 0.2 mM PMSF) and extracted using a homogenizer. The proteins from the samples (50 μg) were subjected to SDS-polyacrylamide gel electrophoresis and blotted onto a polyvinylidene difluoride membrane. The primary antibodies used were anti-p-Akt rabbit monoclonal antibodies (Millipore; 1 : 200), anti-p27kip rabbit polyclonal antibodies (Abcam; 1 : 200), and anti-GAPDH mouse monoclonal antibodies (Millipore; 1 : 3333). Subsequently, the membrane was incubated for 1 h at room temperature with the appropriate secondary antibodies coupled with horseradish peroxidase. Finally, the immunoreactive bands were visualized using ECL detection reagents.
5-Bromodeoxyuridine labeling
Timed pregnant female mice at E13.5, E14.5, or E15.5 were injected three times a day with 5 μg/μl 5-bromodeoxyuridine (BrdU; Sigma) in PBS. At E18.5, the pregnant female mice were killed by cervical dislocation and their embryos were fixed overnight in 4% paraformaldehyde in PBS at 4°C.
Results
PTEN knockout in the inner ear of mice
PTENLoxP/LoxP mice, with the PTEN exon 5 flanked by LoxP sites 19, were crossed with Pax2-Cre mice to generate Pax2-PTEN−/− mice 20. The PTEN exon 5 flanked by LoxP sites were removed by Cre upon Cre expression. The genotypes of the pups were determined by PCR analysis (Fig. 1). The Pax2-PTEN−/− mice died perinatally. Although some of the Pax2-PTEN−/− mice remained alive after birth, others were stillborn. We dissected 10 pregnant female mice at E18.5 and all the Pax2-PTEN−/− embryos survived. The Pax2-PTEN−/− mice at P0.5 were physically similar to wild-type mice. No statistically significant differences in weight and length were observed between the Pax2-PTEN−/− and the wild-type mice.
Fig. 1.
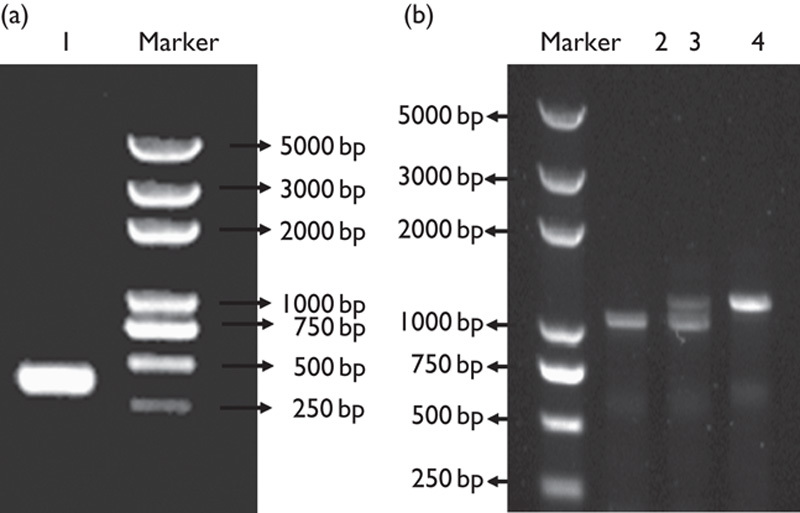
Genotyping through PCR analysis. (a) The PCR product of Pax2-Cre recombinase gene was 319 bp (1). (b) The product of the wild-type allele was 1200 bp and that of the LoxP allele was 1300 bp. A 1200 bp band was detected in the wild-type mice (2). A 1200 bp band and a 1300 bp band were detected in the heterozygous mice (3). Only the 1300 bp band was observed in the homozygous mice (4).
Supernumerary auditory HCs in the cochlea of the Pax2-PTEN−/− mice
We labeled cryosections of wild type and knockout mice with MyoVIIa to identify HCs. In the wild-type mice, we observed the usual one row of IHCs and three rows of OHCs at P0.5 (Fig. 2a). However, supernumerary IHCs and OHCs were noted in sections of the Pax2-PTEN−/− mice at the same age (Fig. 2b). The supernumerary HCs were cylindrical and were similar to the HCs in wild-type mice (Fig. 2a and b). Whole-mount staining using rhodamine phalloidin was performed to determine the distribution of supernumerary HCs in the cochlea of the Pax2-PTEN−/− mice at P0.5. Most of the supernumerary HCs were distributed in the middle and basal regions of the cochlea of the Pax2-PTEN−/− mice (Fig. 3d and f). The Pax2-PTEN−/− mice had 67.8% more OHCs than the wild-type mice. Furthermore, the IHCs were 22.6% greater in Pax2-PTEN−/− mice than those in wild-type mice (n=10; *P<0.05).
Fig. 2.
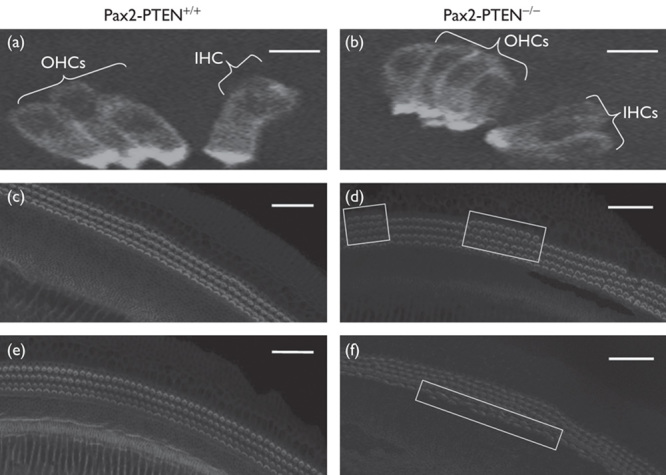
MyoVIIa immunostaining of the cryosections of the cochlea and confocal images of cochlear whole mounts stained with rhodamine phalloidin at P0.5. (a) Three rows of OHCs and a single row of IHCs were found in Pax2-PTEN+/+ mice. The HCs were cylindrical and were stained by MyoVIIa. Scale bar=50 μm. (b) Four rows of OHCs and two rows of IHCs were observed in the Pax2-PTEN−/− mice. The supernumerary HCs were also cylindrical and were stained by MyoVIIa. Scale bar=50 μm. (c) Three rows of OHCs were found in the mid-basal region of the cochlea of the Pax2-PTEN+/+ mice. Scale bar=50 μm. (d) Four rows of OHCs (demarcated by frames) were observed in the mid-basal region of the cochlea of the Pax2-PTEN−/− mice. Scale bar=50 μm. (e) A single row of IHCs was found in the mid-basal region of the cochlea of the Pax2-PTEN+/+ mice. Scale bar=50 μm. (f) Two rows of IHCs (demarcated by frame) were found in the mid-basal region of the cochlea of the Pax2-PTEN−/− mice. Scale bar=50 μm. HC, hair cells; IHCs, inner hair cells; OHCs, outer hair cells.
Fig. 3.
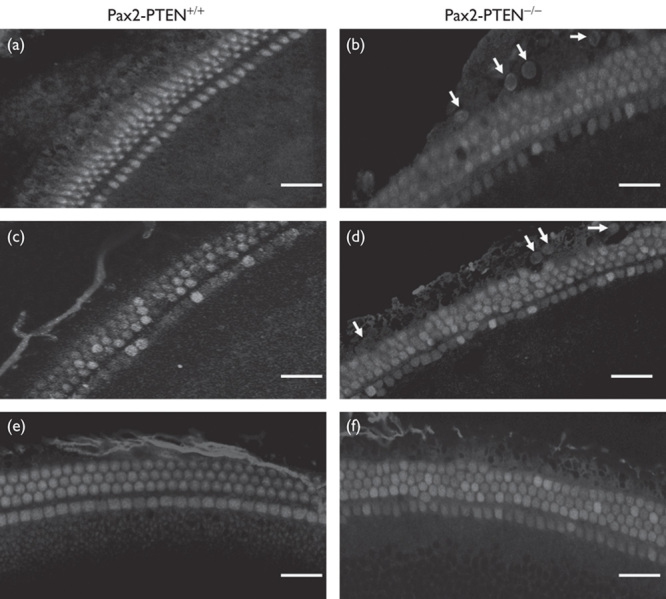
Cochlear whole mounts stained with BrdU at E18.5. Pregnant female mice were injected with BrdU at E13.5, E14.5, and E15.5. BrdU stained the proliferating cells. The HCs were stained with rhodamine phalloidin. (a) In the Pax2-PTEN+/+ mice that were injected with BrdU at E13.5, no BrdU staining was detected at E18.5. Scale bar=30 μm. (b) In the Pax2-PTEN−/− mice that were injected with BrdU at E13.5, BrdU staining (arrows) was detected at E18.5. Scale bar=30 μm. (c) In the Pax2-PTEN+/+ mice that were injected with BrdU at E14.5, no BrdU staining was detected at E18.5. Scale bar=30 μm. (d) In the Pax2-PTEN−/− mice that were injected with BrdU at E14.5, BrdU staining (arrows) was detected at E18.5. Scale bar=30 μm. (e) In the Pax2-PTEN+/+ mice that were injected with BrdU at E15.5, no BrdU staining was detected at E18.5. Scale bar=30 μm. (f) In the Pax2-PTEN−/− mice that were injected with BrdU at E15.5, no BrdU staining was detected at E18.5. Scale bar=30 μm. BrdU, 5-bromodeoxyuridine.
PTEN knockout delayed the withdrawal of auditory progenitors from the cell cycle
Pregnant female mice were injected with BrdU at E13.5, E14.5, or E15.5. The BrdU labeling was checked among the embryos at E18.5. BrdU labeling was detected in the Pax2-PTEN−/− mice injected with BrdU at E13.5 or E14.5 (Fig. 3b and d). However, no BrdU labeling was observed in the Pax2-PTEN−/− mice injected with BrdU at E15.5 or wild-type mice injected with BrdU at E13.5, E14.5, or E15.5 (Fig. 3a, c, e, and f). Thirty-two BrdU-labeled nuclei were observed in one cochlea of Pax2-PTEN−/− mice injected with BrdU at E13.5. Furthermore, 40 BrdU-labeled nuclei were observed in one cochlea of Pax2-PTEN−/− mice injected with BrdU at E14.5.
These results indicate that the auditory progenitors were still dividing at E13.5 and E14.5. They withdrew from the cell cycle in the Pax2-PTEN−/− mice late. The presence of supernumerary HCs can be attributed to the delayed withdrawal of auditory progenitors from the cell cycle in the Pax2-PTEN−/− mice.
PTEN knockout increased the p-Akt level
We checked the levels of Akt and p-Akt in the Pax2-PTEN−/− mice through immunofluorescence and western blot analysis. Immunofluorescence analysis was used under identical imaging conditions at the same cochlear location. The results showed that the Akt level remained unchanged (data not shown), but the p-Akt level increased in the Pax2-PTEN−/− mice compared with wild-type mice at E13.5 and E14.5 (Fig. 4a, b, d and e). Quantitative analysis of relative fluorescence intensity further confirmed these results (Fig. 4c and f). Western blot analysis also confirmed that the Akt level remained unchanged (data not shown) and the p-Akt level increased in the Pax2-PTEN−/− mice compared with wild-type mice (Fig. 4g and h).
Fig. 4.
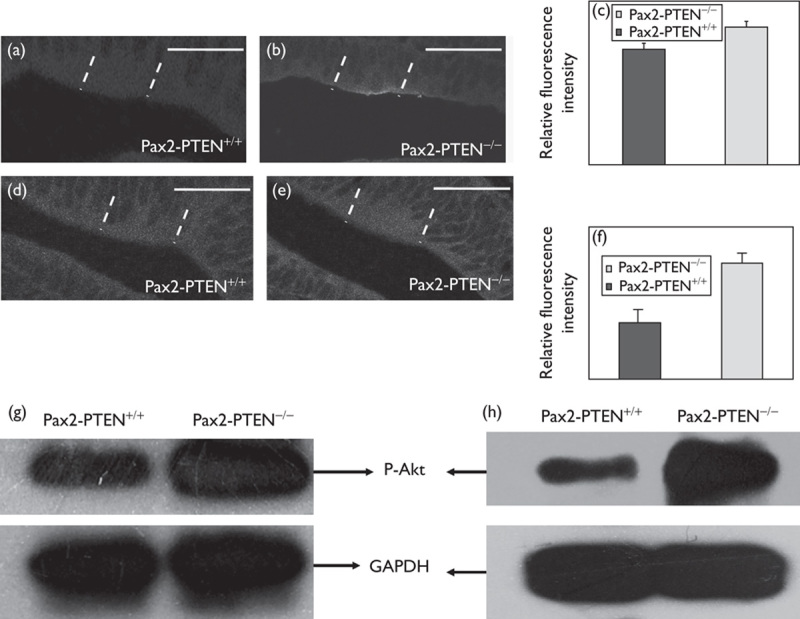
PTEN knockout increased p-Akt level. (a, b) Immunostaining for p-Akt in otocyst at E13.5 (demarcated by dashed lines). The p-Akt staining was more intensive in the Pax2-PTEN−/− mice. Scale bar=50 μm. (c) Quantitative analysis of the relative fluorescence intensity showed that the Pax2-PTEN−/− mice had a higher p-Akt level compared with the Pax2-PTEN+/+ mice at E13.5 (n=10; P<0.05). (d, e) Immunostaining for p-Akt in otocyst at E14.5 (demarcated by dashed lines). The p-Akt staining was more intensive in the Pax2-PTEN−/− mice. Scale bar=50 μm. (f) Quantitative analysis of the relative fluorescence intensity showed that the Pax2-PTEN−/− mice had a higher p-Akt level compared with the Pax2-PTEN+/+ mice at E14.5 (n=10; P<0.05). (g, h) Western blot analysis of the cochlea protein at E13.5 (g) and E14.5 (h) showed that the Pax2-PTEN−/− mice had a higher p-Akt level than the Pax2-PTEN+/+ mice.
PTEN knockout decreased the p27kip level because of the loss of PTEN
P27kip1 is one of the substrates of Akt and is phosphorylated by p-Akt 8. We used immunofluorescence and western blot analysis to determine p27kip1 levels. Immunofluorescence analysis was used under identical imaging conditions at the same cochlear location. P27kip1 immunostaining was lower in the inner ear of the Pax2-PTEN−/− mice compared with wild-type mice at E13.5 and E14.5 (Fig. 5a, b, d and e). Quantitative analysis of relative fluorescence intensity further confirmed these results (Fig. 5c and f). Western blot analysis also showed that the p27kip1 level was lower in the Pax2-PTEN−/− mice compared with wild-type mice (Fig. 5g and h).
Fig. 5.
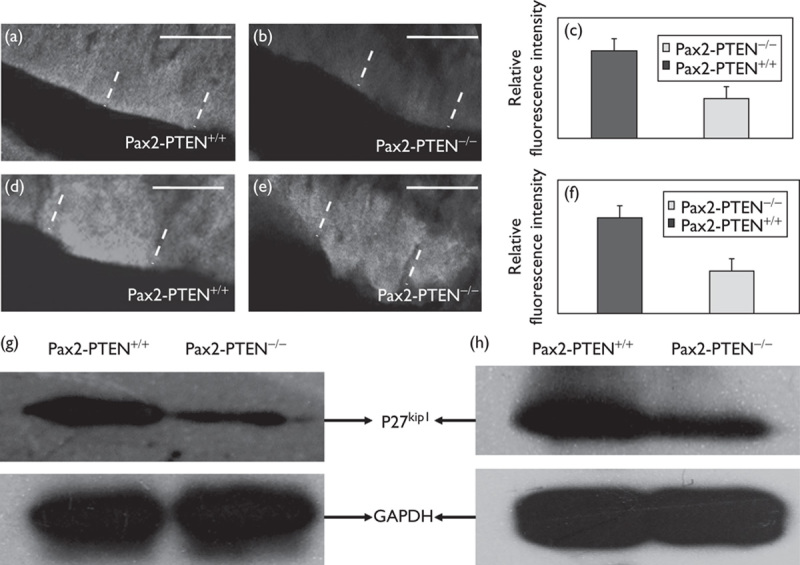
PTEN knockout decreased the p27kip1 level. (a, b) P27kip1 immunolabeling in the otocyst at E13.5 (demarcated by dashed lines) showed that the p27kip1 level was lower in the Pax2-PTEN−/− mice compared with the Pax2-PTEN+/+mice. Scale bar=50 μm. (c) Quantitative analysis of the relative fluorescence intensity showed that the p27kip1 level was lower in the Pax2-PTEN−/− mice compared with the Pax2-PTEN+/+ mice at E13.5 (n=10; P<0.05). (d, e) P27kip1 immunolabeling in the otocyst at E14.5 (demarcated by dashed lines) showed that the p27kip1 level was lower in the Pax2-PTEN−/− mice compared with the Pax2-PTEN+/+ mice. Scale bar=50 μm. (f) Quantitative analysis of the relative fluorescence intensity showed that the p27kip1 level was lower in the Pax2-PTEN−/− mice compared with the Pax2-PTEN+/+ mice at E14.5 (n=10; P<0.05). (g, h) Western blot analysis of the cochlea protein at E13.5 (g) and E14.5 (h) showed a lower p27kip1 level in the Pax2-PTEN−/− mice compared with the Pax2-PTEN+/+ mice.
Discussion
PTEN plays a crucial role during embryonic development 21. The heterozygous PTEN knockout mice showed that PTEN regulates the proliferation of auditory progenitors. However, the molecular mechanism by which PTEN regulates the proliferation and differentiation of auditory progenitors remains unknown. Thus, to investigate the molecular mechanism underlying PTEN regulation, we created PTEN conditional knockout in the inner ear of mice. Our results show supernumerary HCs in the Pax2-PTEN−/− mice. We evaluated the expression of genes that may be related to the proliferation and differentiation of auditory progenitors. The results show that the Pax2-PTEN−/− mice have higher p-Akt level and lower p27kip1 level compared with wild-type mice at around E13.5 and E14.5.
The p27kip1 level is correlated with the proliferation and differentiation of auditory progenitors. From E9 to E12, little p27kip1 expression occurs and all cells in the otocyst proliferate in mice 2,6. From E12 to E14, with p27kip1 expression, the auditory progenitors in the otocyst withdraw from the cell cycle, and the persistence of p27kip1 maintains the progenitors in a nonproliferative state. Subsequently, the p27kip1 level is downregulated during HC differentiation 7. Nevertheless, little is known about p27kip1 upstream regulators during inner ear development. Our results suggest that p27kip1 is a downstream target of the PTEN/PI3K/Akt-signaling pathway, and it is regulated by Akt in the cochlea. In the PTEN/PI3K/Akt-signaling pathway, PIP2 is converted into PIP3 through phosphorylation by phosphatidylinositol 3-kinase (PI3K). Subsequently, Akt is converted into its activated form (p-Akt) through phosphorylation by PIP3. PIP3 can be dephosphorylated back into PIP2 by PTEN 9. The PTEN spatiotemporal expression pattern is similar to that of p27kip1 in the inner ear 16. Loss of PTEN in the inner ear prevented the dephosphorylation of PIP3, which then phosphorylated more Akt and caused p-Akt accumulation. P-Akt was found to phosphorylate p27kip1 and promote p27kip1 degradation in cell culture 8. Thus, p-Akt may function in a similar manner and downregulate p27kip1 in the cochlea. Consequently, the p27kip1 level was lower in the PTEN knockout mice (Fig. 6). The decreased p27kip1 delayed the withdrawal of the auditory progenitors from the cell cycle. Some progenitors continued to divide. Therefore, more progenitors differentiated into supernumerary HCs.
Fig. 6.
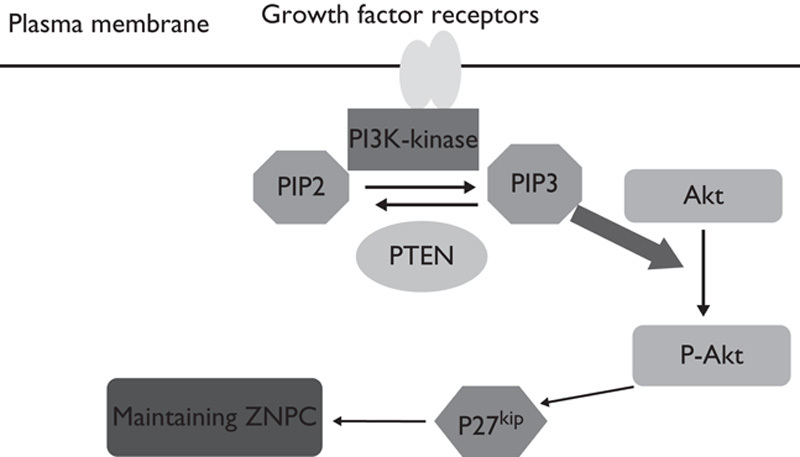
Model of the PTEN/PI3K/Akt-signaling pathway for regulating the proliferation and differentiation of auditory progenitors. PI3K phosphorylates PIP2, converting PIP2 into PIP3. Conversely, PTEN dephosphorylates PIP3, converting PIP3 back into PIP2. PIP3 directly phosphorylates Akt to generate p-Akt. P-Akt phosphorylates p27kip1 and triggers its degradation. The p27kip1 level maintains ZNPCs in the nonproliferative state. Downregulation of p27kip1 induces the differentiation of progenitors at ZNPC into HCs. Thus, PTEN may regulate the proliferation and differentiation of auditory progenitors through p-Akt and p27kip1. HCs, hair cells; ZNPCs, zone of nonproliferating cells.
Conclusion
P27kip1 is expressed specifically and transiently in the inner ear of mice. p27kip1 plays an important role in the proliferation and differentiation of auditory progenitors. This study suggests that PTEN regulates p27kip1 through p-Akt, thereby regulating the proliferation and differentiation of auditory progenitors. PTEN knockout in the inner ear resulted in supernumerary HCs. Therefore, PTEN manipulation provides a potential strategy for regenerating HCs and treating hearing loss in mammals. (Supplementary video, Supplemental digital content 1, http://links.lww.com/WNR/A267).
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (Nos 30871436, 30973297, and 31171194), independent Development Foundation of Shandong University (2012JC019, 2012ZD030) and Shandong Provincial Science and Technology Key Program (No. 2009GG10003039), China.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.neuroreport.com).
Correspondence to Jiangang Gao, PhD, Key Laboratory of the Ministry of Education for Experimental Teratology and School of Life Science, Shandong University, Jinan 250100, China Tel: +86 531 88365399; fax: +86 531 88565610; e-mail: jggao@sdu.edu.cn or Huashun Li, PhD, Advanced Institute of Translational Medicine, Tongji University School of Medicine, Shanghai 200123, China Tel: +86 216 1569673; fax: +86 215 8798999; e-mail: huashunli@tongji.edu.cn
References
- 1.Ruben RJ.Development of the inner ear of the mouse: a radioautographic study of terminal mitoses.Acta Otolaryngol 1967;220:221–244 [PubMed] [Google Scholar]
- 2.Gilroy AM, MacPherson BR, Ross LM.Atlas of anatomy 2008New York, NY:Thieme [Google Scholar]
- 3.Holley MC.Keynote review: the auditory system, hearing loss and potential targets for drug development.Drug Discov Today 2005;10:1269–1282 [DOI] [PubMed] [Google Scholar]
- 4.Fekete DM, Wu DK.Revisiting cell fate specification in the inner ear.Curr Opin Neurobiol 2002;12:35–42 [DOI] [PubMed] [Google Scholar]
- 5.Kelley MW, Driver EC, Puligilla C.Regulation of cell fate and patterning in the developing mammalian cochlea.Curr Opin Otolaryngol Head Neck Surg 2009;17:381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barald KF, Kelley MW.From placode to polarization: new tunes in inner ear development.Development 2004;131:4119–4130 [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Segil N.P27Kip1 links cell proliferation to morphogenesis in the developing organ of orti.Development 1999;26:1581–1590 [DOI] [PubMed] [Google Scholar]
- 8.Fujita N, Sato S, Katayama K, Tsuruo T.Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization.J Biol Chem 2002;277:28706–28713 [DOI] [PubMed] [Google Scholar]
- 9.Toker A, Cantley LC.Signalling through the lipid products of phosphoinositide-3-OH kinase.Nature 1997;7:673–676 [DOI] [PubMed] [Google Scholar]
- 10.Stephens L, Anderson K, Stoke D, Erdjument-Bromage H, Painter GF, Holmes AB, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B.Science 1998;9:710–714 [DOI] [PubMed] [Google Scholar]
- 11.Forge A, Li L, Corwin JT, Nevill G.Ultrastructural evidence for hair cell regeneration in the mammalian inner ear.Science 1993;259:1616–1619 [DOI] [PubMed] [Google Scholar]
- 12.Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, et al. Proliferation of functional hair cells in vivo in the absence of the Retinoblastoma protein.Science 2005;307:1114–1118 [DOI] [PubMed] [Google Scholar]
- 13.Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of Corti.Proc Natl Acad Sci USA 1999;96:4084–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Y, Sui L, Yamaguchi F, Kamitori K, Hirata Y, Hossain MA, et al. Phosophatase and tensin homolog deleted on chromosome 10 regulates sensory cell proliferation and differentiation of hair bundles in the mammalian cochlea.Neuroscience 2010;170:1304–1313 [DOI] [PubMed] [Google Scholar]
- 15.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer.Science 1997;275:1943–1947 [DOI] [PubMed] [Google Scholar]
- 16.Dong Y, Sui L, Yamaguchi F, Kamitori K, Hirata Y, Suzuki A, et al. Role of phosphatase and tensin homolog in the development of the mammalian auditory system.NeuroReport 2010;21:731–735 [DOI] [PubMed] [Google Scholar]
- 17.Sha SH, Chen FQ, Schacht J.PTEN attenuates PIP3/Akt signaling in the cochlea of the aging CBA/J mouse.Hear Res 2010;264:86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posdsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems.Proc Natl Acad Sci USA 1999;96:1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene.Genesis 2002;32:148–149 [DOI] [PubMed] [Google Scholar]
- 20.Takahiro O, Andrew KG.Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome.Genesis 2004;38:195–199 [DOI] [PubMed] [Google Scholar]
- 21.Di CA, Pesce B, Cordon-Cardo C, Pandolfi PP.Pten is essential for embryonic development and tumour suppression.Nat Genet 1998;19:348–355 [DOI] [PubMed] [Google Scholar]


