Abstract
The WHO recommends the administration of sulfadoxine-pyrimethamine (SP) to all pregnant women living in areas of moderate (stable) to high malaria transmission during scheduled antenatal visits, beginning in the second trimester and continuing to delivery. Malaria parasites have lost sensitivity to SP in many endemic areas, prompting the investigation of alternatives that include azithromycin-based combination (ABC) therapies. Use of ABC therapies may also confer protection against curable sexually transmitted infections and reproductive tract infections (STIs/RTIs). The magnitude of protection at the population level would depend on the efficacy of the azithromycin-based regimen used and the underlying prevalence of curable STIs/RTIs among pregnant women who receive preventive treatment. This systematic review summarizes the efficacy data of azithromycin against curable STIs/RTIs.
Keywords: azithromycin, bacterial vaginosis, Chlamydia, gonorrhea, malaria, pregnancy, reproductive tract infections, sexually transmitted infections, sub-Saharan Africa, syphilis, trichomoniasis
The WHO recommends the administration of sulfadoxine-pyrimethamine (SP) to all pregnant women who live in areas of moderate (stable) to high malaria transmission during scheduled antenatal care (ANC) visits, beginning in the second trimester and continuing to delivery [1]. This intervention, known as intermittent preventive treatment of malaria in pregnancy (IPTp), is national policy in 36 countries worldwide, 35 of which are in sub-Saharan Africa [2]. The objective of IPTp-SP is to reduce the incidence of low birthweight and maternal anemia attributable to malaria. In recent years, however, malaria parasites have developed resistance to SP such that IPTp no longer reduces the incidence of low birthweight in some epidemiological settings, particularly in East Africa [3]. Evidence suggests that in areas where parasites express the 581G dhps mutation that is associated with SP resistance, the administration of IPTp-SP may even harm fetal growth [4–6]. Thus, the urgency to replace SP has never been greater and azithromycin-based combination (ABC) therapies are among leading candidates to do so.
Azithromycin is a slow-acting analog of erythromycin in the macrolide (azalide) class of drugs, which targets the ribosomal subunit of susceptible microorganisms and causes cellular death by inhibiting protein synthesis [7]. It has in vitro and in vivo antimalarial properties [8] and can be safely administered during pregnancy [9]. Two human challenge studies have published results of azithromycin monotherapy treatment against Plasmodium falciparum infection. The first study reported a protective effect of 40% (n = 10; 95% CI: 12–74%) among immunologically naïve patients who received 250 mg azithromycin daily for 2 weeks prior to inoculation and for 1 week more following exposure [10]. When the same regimen was used for one additional week post-inoculation, treatment efficacy was 100% (n = 10) [11]. Despite this finding, comparable results have not been replicated in endemic settings where patients often have mixed and multiple infections. However, in vitro evidence suggests that azithromycin may be combined with antimalarial partner drugs to prevent or to cure P. falciparum infection [12], the malaria species most prevalent in sub-Saharan Africa and which uniquely adhere to the placenta of pregnant women. In addition to reducing the burden of malaria infection, ABC therapies may also protect against adverse birth outcomes attributable to curable sexually transmitted and reproductive tract infections (STIs/RTIs). This could offer considerable public health impact. A recent meta-analysis suggests that curable STIs/RTIs are as prevalent as malaria parasitemia, if not more so, among pregnant women who attend ANC facilities in sub-Saharan Africa [13]. Five curable STIs/RTIs – Treponema pallidum, Neisseria gonorrheae, Chlamydia trachomatis, Trichomonas vaginalis and bacterial vaginosis – are associated with adverse birth outcomes that include spontaneous abortion [14–18], stillbirth [19–21], intrauterine growth retardation [20,22,23], premature rupture of membranes [24–26], preterm birth [17,22,23,26–33] and low birthweight (Table 1) [20,23,24,28,29,33–35]. This paper summarizes azithromycin efficacy and sensitivity against these curable STIs/RTIs and highlights important issues for policymakers to consider while determining the potential use of ABC therapies in IPTp.
Table 1.
Effect of curable STIs/RTIs on pregnancy outcomes.
| Study (year) | Country | Year(s) | Spontaneous abortion | Stillbirth | IUGR | PROM | Preterm | Low birthweight | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Treponema pallidum | |||||||||
| Watson-Jones et al. (2002) | Tanzania | 1998–2000 | NR | 18 (5.5–59.6) RR | 2.1 (1.0–4.2) RR | NR | 6.1 (2.5–15.3) RR | 3.3 (2.0–5.4)† | [21] |
| Temmerman et al. (1995) | Kenya | 1991 | NR | 3.34 RR | NR | NR | NR | 4.01† | [120] |
| McDermott et al. (1993) | Malawi | 1987–1990 | NR | 10.98 | NR | NR | NR | NR | [19] |
| Donders et al. (1993) | South Africa | 1988 | NR | NR | NR | NR | 33%; 5 of 15 cases | NR | [34] |
| Elliott et al. (1990) | Kenya | 1985 | NR | NR | NR | NR | 1.4 (0.5–4.1) | NR | [121] |
| Ratnam et al. (1982) | Zambia | NR | 42% of cases | NR | NR | NR | NR | NR | [15] |
| Williams et al. (1923) | USA | 1923 | 40% of cases | NR | NR | NR | NR | NR | [14] |
| Neisseria gonorrhoeae | |||||||||
| Johnson et al. (2011) | USA | 1996–2002 | NR | NR | NR | NR | 2.0 (1.0–4.0) | 0.8 (0.3–2.3) | [122] |
| Donders et al. (1993) | South Africa | 1988 | NR | NR | NR | NR | 56%; 5 of 9 cases | p < 0.005 | [34] |
| Elliott et al. (1990) | Kenya | 1985 | NR | NR | NR | NR | 3.2 (1.3 to 8.4) | NR | [121] |
| Chlamydia trachomatis | |||||||||
| Rours et al. (2011) | Netherlands | 2003–2005 | NR | NR | NR | NR | 4.4 (1.3–15.2) † ; 2.7 (1.1–6.5) ‡ ; 1.17 (0.6–2.4) § | 1.0 (0.4–2.2) | [32] |
| Silveira et al. (2009) | USA | 2005–2008 | NR | NR | NR | NR | 0.7 (0.4–1.4) | NR | [123] |
| Wilkowska-Trojniel et al. (2009) | Poland | 2003–2006 | 12 versus 2% p = 0.029 | NR | NR | NR | NR | NR | [18] |
| Blas et al. (2007) | USA | 2003 | NR | NR | NR | 1.5 (1.0–2.2) RR | 1.5 (1.1–2.0) RR | 1.1 (0.7–1.7) | [124] |
| Odendaal et al. (2006) | South Africa | 2002–2003 | NR | NR | NR | NR | 22.2%; 8 of 36 cases versus 10.4%; 32 of 307 cases; p = 0.037 | NR | [30] |
| Johnson et al. (2011) | USA | 1996–2002 | NR | NR | NR | NR | 1.0 (0.6–2.0) | 2.1 (1.0–4.2) | [122] |
| Kovacs et al. (1998) | Hungary | 1994–1995 | NR | NR | 7.3 versus 5.8% p > 0.05 | 20 versus 21% p > 0.05 |
NR | 15.5 versus 13.2% p > 0.05 | [125] |
| Donders et al. (1993) | South Africa | 1988 | NR | NR | NR | NR | 27%; 6 of 22 cases | NR | [34] |
| Elliott et al. (1990) | Kenya | 1985 | NR | NR | NR | NR | 0.7 (0.4–1.4) | NR | [121] |
| Johns Hopkins et al. (1989) | USA | 1983–1985 | NR | NR | 2.4 (1.3– 4.2) | NR | 1.6 (1.0–4.2) | NR | [126] |
| Gravett et al. (1986) | USA | 1983 | NR | NR | NR | 2.4 (1.7–5.4) | NR | 2.7 (1.3–5.7) | [24] |
| Trichomonas vaginalis | |||||||||
| Johnson et al. (2011) | USA | 1996–2002 | NR | NR | NR | NR | 1.4 (0.7–2.8) | 1.5 (0.9–2.6) | [122] |
| Meis et al. (1995) | USA | 1992–1994 | NR | NR | NR | NR | 1.5 (0.1–8.1) week 24; 0.9 (0.2–3.6) week 28 | NR | [127] |
| Sutton et al. (1999) | DR Congo | 1989–1990 | NR | NR | NR | NR | NR | 2.1 (1.0–4.7) | [35] |
| Minkoff et al. (1984) | USA | NR | NR | NR | NR | p < 0.03 | NR | NR | [128] |
| Cotch et al. (1997) | USA | 1984–1989 | NR | NR | NR | NR | 1.3 (1.1–1.4) | 1.3 (1.1–1.5) | [28] |
| Bacterial vaginosis | |||||||||
| Johnson et al. (2011) | USA | 1996–2002 | NR | NR | NR | NR | 1.3 (0.9–2.1) | 1.1 (0.6–1.8) | [122] |
| Svare et al. (2006) | Denmark | 1998–2002 | NR | NR | NR | NR | 2.5 (1.6–3.9) | 2.0 (1.3–2.9) | [29] |
| Watson-Jones et al. (2007) | Tanzania | 1997–2000 | NR | NR | NR | NR | 3.0 (1.3–6.6) | NR | [31] |
| Leitich et al. (2003) | Multiple | Multiple | 9.9 (2.0–49.3) | NR | NR | NR | 2.2 (1.5–3.1) | NR | [17] |
| Meis et al. (1995) | USA | 1992–1994 | NR | NR | NR | NR | 1.4 (0.9–2.05) week 24; 1.8 (1.2–3.0) week 28 | NR | [127] |
| McGregor et al. (1995) | USA | 1991–1992 | NR | NR | NR | 3.5 (1.4–8.9) RR | 1.9 (1.2–3.0); 1.5 (0.7–3.0) ¶ RR | NR | [25] |
| Hillier et al. (1995) | USA | 1984–1989 | NR | NR | NR | 1.1 (0.8–1.6) | 1.4 (1.1–1.8) | 1.5 (1.2–1.7) | [129] |
| Hay et al. (1994) | UK | NR | 5.5 (2.3–13.3)†† | NR | NR | NR | 13.1 (4.0–42.6) ‡‡ | NR | [16] |
| Elliott et al. (1990) | Kenya | 1985 | NR | NR | NR | NR | 1.0 (0.6–1.8) | NR | [121] |
| Gravett et al. (1986) | USA | 1983 | NR | NR | NR | 2.0 (1.1–3.7) | NR | 1.5 (0.8–2.0) | [24] |
Results reported as odds ratios unless otherwise noted and 95% confidence intervals in parentheses.
Preterm delivery before 32 weeks.
Preterm delivery before 35 week.
Preterm delivery before 37 weeks.
Bacterial vaginosis at 16–20 weeks.
Bacterial vaginosis at 28–32 weeks.
Intermediate flora (Nugent scores 4–7) and bacterial vaginosis (Nugent scores 7–10).
IUGR: Intrauterine growth retardation; NR: Not reported; PROM: Premature rupture of membranes; RR: Risk ratio.
Methodology
Between April and May 2013, PubMed, MEDLINE and EMBASE were searched using Medical Subject Headings and free-text terms for publications specific to the curable STIs/RTIs noted above. With each query, the infection and causal organism were used together, for example, ‘Syphilis’ AND ‘Treponema pallidum’, and then combined with search terms ‘azithromycin’ OR ‘macrolide’. Because the evidence base is limited with respect to azithromycin and some curable STIs/RTIs, both ‘azithromycin’ and ‘macrolide’ were used as filters. We had particular interest in randomized clinical trials (RCTs) that compared azithromycin against the current first-line treatments for curable STIs/RTIs in pregnancy, noting that azithromycin is the WHO-recommended treatment for pregnant women infected with C. trachomatis. Searches were limited to the English language and strict inclusion and exclusion criteria were applied so as to narrow the number of papers retained. Reference lists were also reviewed for additional documents. Excluded records and full-text articles were in seven categories:
‘Unrelated outcomes’ were studies that reported nonclinical aspects of azithromycin use such as cost-effective analysis, noncommunicable diseases such as heart disease or pharmacological outcomes involving a route of administration that is not applicable to this review (e.g., intravenous);
‘Unrelated organisms’ were papers dedicated to microbes that are not the focus of this review;
‘Not specific to STI/RTI’ were articles on the subject of same genus of interest, for example, Chlamydia, but were not specific to the genital tract, for example, Chlamydia pneumoniae;
‘Not related to azithromycin or close macrolide family’ were papers that did not contain macrolides in their analysis or outcomes, but focused on different antimicrobials against the organisms in question;
‘Sequential observations from same source’ involved surveillance reports from which most recent data set was used;
‘General discussion papers’ contained information pertinent to the search, but failed to provide specific data for STIs/RTIs.
‘Contraindicated in pregnancy’ were papers that reported outcomes of azithromycin combined with antimicrobial compounds that are considered unsafe in pregnancy.
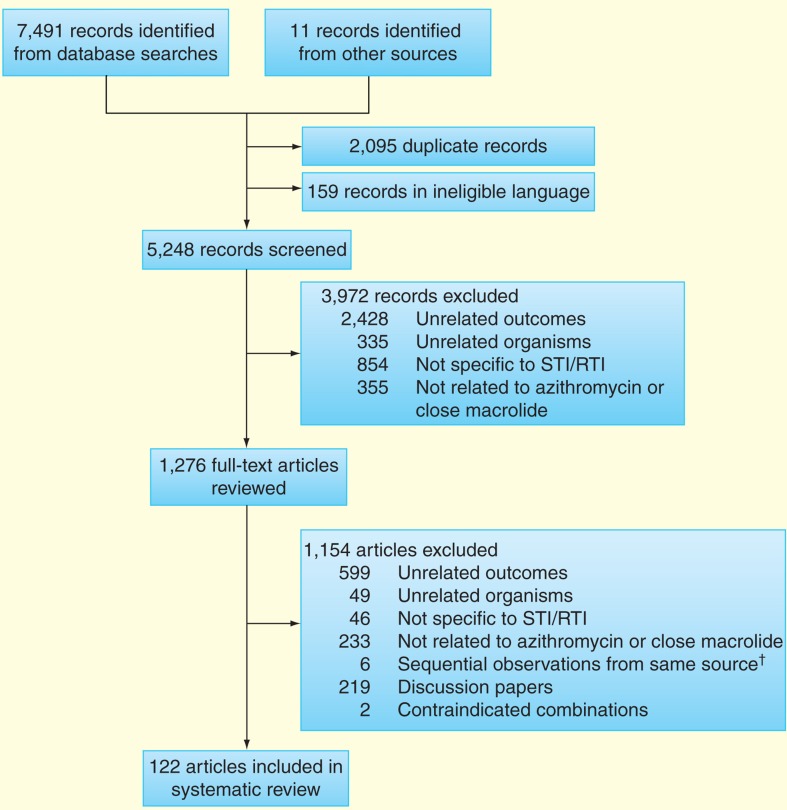
A total of 122 articles met our primary inclusion criteria (Figure 1).
Figure 1.
Identification, screening and eligibility of studies included in systematic review.
†Surveillance reports from which the most recent data set was used.
STI/RTI: Sexually transmitted infection and reproductive tract infection.
Results
Treponema pallidum
In vivo evidence
The WHO recommends treating pregnant women with syphilis infection using 2.4 million units of benzanthine penicillin G (BPG) administered by intramuscular injection [36]. Thus, we summarize the results of the six clinical trials that reported outcomes among nonpregnant adults following treatment with BPG, azithromycin or a combination of BPG and azithromycin (Table 2). The oldest data are from a trial in the USA (1993–1997) in which individuals who discovered they had been exposed to infectious stage syphilis through sexual intercourse in the preceding 30 days were given either 1 g azithromycin (n = 40) or BPG (n = 23). Three months post-treatment, rapid plasma reagin (RPR) and fluorescent treponemal antibody absorption tests (FTA-ABS) were negative for all participants in both treatment groups [37]. Another trial in the USA during the same time period was designed to measure treatment outcomes in a population at high risk of contracting STIs/RTIs. Although diagnostic methods were not reported, the trial was suspended after two of the first 12 patients were provided 1 g azithromycin failed their test of cure while all 13 participants were cured using BPG (p = 0.18) [38]. A three-arm trial of early syphilis in the USA then compared treatment outcomes among patients given BPG, or 2 g azithromycin once or 2 g azithromycin two-times with 1 week in between doses. RPR and FTA-ABS testing showed that cure was achieved in 85.7% (n = 14; 95% CI: 60.0–95.7%) of patients given BPG, 94.1% (n = 17; 95% CI: 72.7–98.6%) among recipients of 2 g azithromycin once and 82.8% (n = 29; 95% CI: 65.3–92.3%) in participants who twice received 2 g azithromycin [39].
Table 2.
Randomized clinical trials of azithromycin versus benzathine penicillin G for the treatment of Treponema pallidum
| Study (year) | Country | Year(s) | Regimen | Number cured of treated | Percent cured | 95% CI | Diagnostic methods used | Follow up | Stage of infection | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Specified low-risk populations | ||||||||||
| Hook et al. (2010) | Madagascar, USA | 2000–2007 | 2 gazithromycin 2.4 mu BPG | 180/232 186/237 | 77.6% 78.5% | 71.8–82.5% 72.8–83.2% | RPR and FTA-ABS | 6 months | Primary, secondary, early latent syphilis | [42] |
| Kiddugavu et al. (2005) |
Uganda | 1994–1998 | 1 g azithromycin 2.4 mu BPG 1 g azithromycin plus 2.4 mu BPG |
55/94 66/93 221/313 | 58.5% 71.0% 70.6% | 48.4–68.0% 61.0–79.2% 65.3–75.4% | TRUST and TPHA (initial TRUST titers ≤ 1:2) | 10 months | Serologic syphilis | [130] |
| 1 g azithromycin 2.4 mu BPG1 g azithromycin plus 2.4 mu BPG | 38/71 31/75 169/309 | 53.3% 41.3% 54.7% | 42.0–64.7% 30.9–52.7% 49.1–60.2% | TRUST and TPHA (initial TRUST titers ≥ 1:4) | 10 months | Serologic syphilis | ||||
| Unspecified low-risk populations | ||||||||||
| Klausner et al.(2006) | USA | 2004 | 1 g azithromycin2.4 mu BPG | 10/12 13/13 | 83.3% 100% | 54.6–95.0% NA | NR | NR | Exposed to infectious syphilis | [38] |
| Hook et al. (2002) | USA | 1995–1997 | 2 g azithromycin 2 g azithromycin (two-times) week apart 2.4 mu BPG | 14/14 19/22 | 100% 86.4% | NA 66.4–95.0% | RPR and MHA-TP or FTA-ABS | 12 months | Primary, secondary, early latent syphilis | [39] |
| Hook et al. (1999) | USA | 1995–1997 | 1 g azithromycin2.4 mu BPG | 40/40 23/23 | 100% 100% | NA NA | RPR and FTA-ABS | 3 months | Exposed to infectious syphilis | [131] |
| High-risk population | ||||||||||
| Riedner et al. (2005) | Tanzania | 2000–2003 | 2 g azithromycin 2.4 mu BPG | 159/163 157/165 | 97.5% 95.2% | 93.9–99.0% 90.7–97.5% | RPR and PCR | 9 months | Primary, secondary, higher titer latent syphilis | [132] |
2.4 mu BPG: 2.4 million units benzathine penicillin G; FTA-ABS: Fluorescent treponemal antibody absorption; MHA-TP: Microhemagglutination assay-Treponema pallidum; NA: Not applicable; NR: Not reported; RPR: Rapid plasma regain; TPHA: Treponema pallidum hemagglutination; TRUST: Toluidine red unheated serum test.
In sub-Saharan Africa, three trials have investigated BPG versus azithromycin, the first being a community-randomized trial in Uganda (1994–1998) among nonpregnant adults with serological syphilis. Diagnosis and test of cure were based on toluidine red unheated serum tests (TRUSTs) and Treponema pallidum hemagglutination assays. Treatment efficacy varied across regimens depending on TRUST titers at enrolment. Among patients with initial titers <1:2, BPG cured 71.0% (n = 93; 95% CI: 61.0–79.2%) of cases compared with 58.5% (n = 94; 95% CI: 48.4–68.0%) among recipients of 1 g azithromycin and 70.6% (n = 313; 95% CI: 65.3–75.4%) of participants given 1 g azithromycin plus BPG. If titers at enrolment were >1:4, the efficacy of BPG was reduced to 41.3% (n = 75; 95% CI: 30.9–52.7%). Treatment efficacy was also lower among groups given azithromycin but higher than BPG alone. Recipients of 1 g azithromycin alone had a cure rate of 53.3% (n = 71; 95% CI: 42.0–64.7%), whereas 1 g azithromycin plus BPG cured 54.7% of cases (n = 309; 95% CI: 49.1–60.2%) [40].
These results were followed by a trial carried out in Tanzania (2000–2003) among patients who were recruited by screening high-risk populations. All 328 subjects had a titer of at least 1:8 on RPR test; 106 had baseline titers of >1:64, levels indicative of active syphilitic lesions. Confirmed by RPR test and T. pallidum particle agglutination assay, serological cure was observed in 97.5% (n = 163; 95% CI: 93.9–99.0%) of participants given 2 g azithromycin versus 95.2% (n = 165; 95% CI: 90.7–97.5%) in the BPG group [41].
The most recent study comparing the efficacy of azithromycin versus BPG is a multicenter trial (2000–2007) in Madagascar (n = 421) and North America (n = 94) among HIV-negative patients with early syphilis. Based on RPR testing, serological cure was reported in 77.6% of subjects given 2 g azithromycin (n = 232; 95% CI: 71.8–82.5%) and 78.5% (n = 237; 95% CI: 72.8–83.3%) in the BPG group. Nonserious adverse events were reported by 61.5% (n = 174; 95% CI: 55.7–67.0%) of individuals treated with 2 g azithromycin, most of whom had self-limiting gastrointestinal discomfort, whereas 46.1% (95% CI: 40.6–52.1%) of BPG recipients reported nonserious adverse events [42].
In vitro evidence
Fourteen in vitro studies met our inclusion criteria, seven with isolates from low-risk populations (Table 3) and seven from high-risk or mixed-risk groups (Table 4). A report from San Francisco in 2001 was the first to associate azithromycin treatment failure with A→G mutations at the 2,058 position of the 23S rRNA gene of T. pallidum [43]. Retrospective analysis of samples revealed that 4.0% (n = 25; 95% CI: 0.9–19.6%) of isolates had A→G mutations between 1999 and 2002. In 2003, the proportion of isolates with A→G mutations increased to 36.7% (n = 30; 95% CI: 21.9–54.6%) [44]; by 2004, 56.1% (n = 66; 95% CI: 44.0–67.3%) had selected for resistance [43]. However, in Dublin, 88.2% (n = 17; 95% CI: 65.3–96.4%) of isolates already had A→G mutations by 2002 [44].
Table 3.
Sensitivity testing of T. pallidum isolates to azithromycin and other macrolides.
| Study (year) | Country | Year(s) | Specimen source | Sample size | Resistant mutation | Number resistant | Percent resistant | 95% CI | Additional details | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Low-risk populations | ||||||||||
| Chen et al. (2013) | USA | 2007-2009 | Surveillance | 129 | 2058A 2058G 2059G | 83 67 17 | 64.3% 51.9% 13.2% |
55.8–72.1% 43.4–60.4% 8.4–20.1% | From patients with primary of secondary syphilis attending STI clinics | [133] |
| Van Damme et al. (2009) | Madagascar | NR | Randomized clinical trial | 186 | 23S rRNA | 0 | 0.0% | NA | DNA of T. pallidum was detected in 141 samples; 61% of patients were male; 98% were heterosexual | [49] |
| Unspecified low-risk populations | ||||||||||
| Mϋller et al. (2012) | South Africa Lesotho |
2005–2010 | Surveillance | 100 | A2058G A2059G | 1 0 | 1.0% 0.0% | 0.0–5.4% | 117 ulcer specimens collected of which 100 were positive for T. pallidum | [134] |
| A2058G Prevalence Workgroup (2012) | USA | 2007–2009 | Surveillance | 141 | A2058G | 75 | 53.1% | 45.0–61.2% | From patients with primary of secondary syphilis attending STI clinics; MSM were nearly 6 times more likely to have resistant syphilis compared with heterosexual women and men | [135] |
| Matejková et al. (2009) | Czech Republic | 2005–2008 | Passive case detection | 22 | 23S rRNA A2058G A2059G | 8 4 4 | 36.4% 18.2% 18.2% | 19.7–57.3% 7.5–38.8% 7.5–38.8% |
|
[136] |
| Martin et al. (2009) | China | 2007–2008 | Passive case detection | 38 | A2058G | 38 | 100% | NA | Patients presenting to STI clinic with symptoms compatible with primary syphilis | [137] |
| Lukehart et al. (2004) |
USA | PCR to detect 23S rRNA gene mutations; confirmation of azithromycin resistance was conducted through intradermal rabbit inoculation | [44] | |||||||
| San Francisco San Francisco Seattle Baltimore |
1999–2002 2003 2001–2003 1998–2000 |
Surveillance |
25 30 23 19 |
23S rRNA | 1 11 3 2 |
4.0% 36.7% 13.0% 10.5% |
1.0–19.6% 21.9–54.6% 4.7–32.3% 3.2–31.7% |
|||
| Ireland | ||||||||||
| Dublin | 2002 | Surveillance | 17 | 23S rRNA | 15 | 88.2% | 65.3–96.4% | |||
| Multiple locations | 1912–1987 | Historical | 18 | 23S rRNA | 1 | 5.6% | 1.3–2.6% | |||
DNA: Deoxyribonucleic acid; MSM: Men who has sex with men; NA: Not applicable; NR: Not reported; STI: Sexually transmitted infection.
Table 4.
Sensitivity testing of T. pallidum isolates to azithromycin and other macrolides.
| Study (year) | Country | Year(s) | Specimen source | Sample size | Resistant mutation | Number resistant | Percent resistant | 95% CI | Additional details | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| High-risk populations | ||||||||||
| Tipple et al. (2011) | UK | 2006–2008 | Cross-sectional survey | 18 | 23S rRNA A2058G | 12 11 | 66.6% 61.1% | 43.4–83.7% 38.4–79.7% | Specimens from men, 94.1% were MSM | [138] |
| Rekart et al. (2003) | Canada | 2000 | Mass drug administration | 25 | 23S rRNA | 0 | 0.0% | NA | 1.8 g azithromycin given to sex workers and clients (n = 4,384) | [139] |
| Mixed-risk populations | ||||||||||
| Chen et al. (2012) | China | 2008–2011 | Cross-sectional survey | 211 | A2058G | 194 | 91.9% | 87.2–95.1% | 391 samples collected; 6.1% from FSW, 71.8% reported sex with FSW and 1.4% were MSM | [47] |
| Muldoon et al. (2012) | Ireland | 2009–2010 | Cross-sectional survey | 29 | A2058G | 27 | 93.1% | 77.9–97.9% | 34.6% (36/104) of samples were positive for T. pallidum by PCR; 29 sequenced | [140] |
| Martin et al. (2010) | Canada | 2007–2009 | Cross-sectional survey | 43 | A2058G | 7 | 16.3% | 8.2–30.1% | 449 samples collected from 374 patients; 43 were positive for T. pallidum and sequenced | [141] |
| Mitchell et al. (2006) | USA | 2000–2002 | Retrospective study | 25 | 23S rRNA | 1 | 4.0% | 1.0–19.6% | Patients (n = 1,308) diagnosed primary or secondary syphilis; all treatment failure and resistance in MSM/bisexual patients | [43] |
| 2003 | 32 | 13 | 40.6% | 25.5–57.9% | ||||||
| 2004 | 66 | 37 | 56.1% | 44.0–67.4% | ||||||
| Morshed et al. (2006) | Canada | 2000–2003 | Retrospective study | 47 | 23S rRNA | 1 | 2.1% | 0.5–11.1% | MSM patients presenting to STI clinic with primary or secondary syphilis | [142] |
| 2004 | 9 | 4 | 44.4% | 18.7–73.8% | ||||||
FSW: Female sex worker; MSM: Men who have sex with men; NA: Not applicable; STI: sexually transmitted infection.
Macrolide resistance is strongly associated with use by an individual in the previous year. Isolates from Seattle (2001–2005) were two-times more likely to be resistant if patients had been treated with macrolides in the past 12 months (RR: 2.2; 95% CI: 1.1–4.4; p = 0.02) [45]. This relationship persisted over the decade. A2058G and A2059G mutations, which are associated with clinical failures of azithromycin, were found in 88.9% (n = 36; 95% CI: 74.6–95.5%) of isolates from 2001 to 2010 among patients exposed to macrolides in the preceding 12 months, whereas 61.2% (n = 98; 95% CI: 51.3–70.4%) of isolates from patients who had not received prior macrolide treatment contained the same mutations [46]. Similar mutations were found among strains of T. pallidum in eight cities across China (2008–2011). A2058G was present in 97.0% individuals who had taken macrolides in the previous 12 months versus 62.5% of patients who had not (n = 211; OR: 19.65; 95% CI: 5.8–66.9) [47]. The opposite was found in Taiwan (2009–2011) where no single A2058G or A2059G mutation was seen among 211 isolates tested from a population where only one person had been given macrolide therapy in the previous year [48]. Similarly, there was no evidence of resistance among 141 amplified samples from HIV-negative heterosexual patients in Madagascar [49]. Although use of macrolides in the previous year was not reported, the Essential Drugs List of the Malagasy Ministry of Health does not include macrolides [301].
Neisseria gonorrhoeae
In vivo evidence
The WHO recommends treating pregnant women with Neisseria gonorrhoeae infection using 400 mg cefixime as a single dose or 125 mg ceftriaxone by intramuscular injection [50]. However, azithromycin has been used for the treatment of gonorrhea among nonpregnant adults during the past two decades. Eleven trials were identified through our review (Table 5). Nine trials conducted between the late 1980s and 1999 investigated the use of 1 g azithromycin among individuals attending sexually transmitted infection (STI) clinics. Of these, three were open label without comparators [51–53] and six were two-arm trials that compared azithromycin to ciprofloxacin and/or doxycycline [54–59]. The pooled efficacy of azithromycin against N. gonorrhoea, estimated using random effects models [60], was 97.0% (n = 539; 95% CI: 95.5–98.5%). This is slightly higher than 96.5% (n = 539; 95% CI: 94.3%–97.6%) reported in a 2010 review [61] that added numerators and divided the sum of denominators among the same nine trials. Such an approach does not account for heterogeneity across study populations and gives equal weight to all trials regardless of their precision. Regardless of pooling methods, it is unlikely that the same efficacy would be observed today using 1 g azithromycin in high-income countries following 25 years of cumulative drug pressure. However, the epidemiological context in sub-Saharan Africa is likely different where azithromycin use has been almost exclusively limited to trachoma eradication campaigns [62].
Table 5.
Randomized clinical trials of azithromycin for the treatment of Neisseria gonorrhoeae
| Study (year) | Country | Year(s) | Azithromycin dose | Number cured of evaluated | Percent cured | 95% CI | Diagnostic methods | Additional details | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Dose of 2 g azithromycin | |||||||||
| Khaki et al. (2007) | India | 2005–2006 | 2 g | 22/22 | 100% | NA | Gram stain TOC days 5–7 |
[143] | |
| Handsfield et al. (1994) | USA | 1991–1992 | 2 g | 370/374 | 98.9% | 97.3–99.6% | [63] | ||
| Dose of 1 g azithromycin | |||||||||
| Rustomjee et al. (2002) | South Africa | 1999 | 1 g | 30/31 |
96.8% | 83.8–99.2% | LCR for NG/CT TOC day 14 |
100% (n = 21) NG infections cured 100% (n = 14) CT infections cured 90.0% (n = 10; 95% CI: 58.7–97.8%) co-infections cured |
[60] |
| Swanston et al. (2001) | Trinidad and Tobago | 1996 | 1 gram | 125/127 |
98.4% | 94.5–99.5% | ELISA for NG culture for CT; TOC days 7–10 | 100% (n = 115) NG infections cured 95.7% (n = 23; 95% CI: 78.9–99.0%) CT infections cured 88.3% (n = 12; 95% CI: 54.6–95.0%) co-infections cured |
[53] |
| Gruber et al. (1997) | Croatia | 1994–1995 | 1 g | 48/50 | 96.0% | 86.5–98.8% | Culture and gram stain for NG; TOC day 14 | [58] | |
| Gruber et al. (1995) | Croatia | 1991–1993 | 1 g | 24/24 | 96.0% | 80.4–99.1% | Culture and gram stain for NG; TOC day 14 | [57] | |
| Steingrimsson et al. (1994) | Iceland | NR | 1 g | 27/28 | 96.4% | 82.4–99.2% | Culture for NG w/ DFA; Culture for CT; TOC day 28 | 92.4% (n = 79; 84.4–96.4%) CT infections cured | [56] |
| Waugh et al. (1993) | UK | 1990–1991 | 1 g | 85/89 | 95.5% | 89.0–98.2% | Culture and gram stain for NG; culture for CT; TOC day 10 |
100% (n = 22) NG/CT co-infections cured |
[52] |
| Odugbemi et al. (1993) | Nigeria | 1989–1990 | 1 g | 114/120 | 95.0% | 89.5–97.6% | Culture for NG; TOC day 14 | [51] | |
| Steingrimsson et al. (1990) | Iceland | NR | 1 g (day 0) | 11/12 | 91.7% | 64.0–98.1% | Culture for NG/CT; TOC day 28 |
97.7% (n = 44; 88.2–99.5%) CT infections cured | [55] |
| 500 mg (day 0 x 2) | 7/8 | 87.5% | 51.8–97.2% | 96.3% (n = 27; 81.7–99.1%) CT infections cured | |||||
| 500 mg (day 0) 250 mg (days 1 and 2) | 7/7 | 100% | NA | 88.0% (n = 25; 69.8–95.6%) CT infections cured | |||||
| Lassus et al. (1990) | Finland | NR | 1 g (day 0) | 15/15 | 100% | NA | Culture and gram stain for NG/CT w/ DFA; TOC day 14 | 100% (n = 12) CT infections cured 100% (n = 5) co-infections cured |
[54] |
| 500 mg (day 0) 250 mg (days 1 and 2) |
14/14 | 100% | NA | 100% (n = 9) CT infections cured 83.3% (n = 6; 95% CI: 42.1–96.3%) co-infections cured |
|||||
CT: Chlamydia trachomatis; DFA: Direct fluorescent antibody; ELISA: Enzyme-linked immunosorbent assay; LCR: Ligase chain reaction; mg: milligrams; NA: Not applicable NG: Neisseria gonorrhoeae; NR: Not reported; TOC: Test of cure.
We identified two RCTs that investigated the use of 2 g azithromycin among patients at STI clinics. The first was a multicenter trial in the USA (1991–1992) in which 98.9% (n = 374; 95% CI: 97.3–99.6%) of patients were cured [63]. A similar RCT in New Delhi (2005–2006) involved 42 participants; loss to follow-up was high, 52.4%, but all 22 subjects who returned for a test of cure had their N. gonorrhoeae infections cured [64].
In vitro evidence
Over the past decade, in vitro studies have documented the loss of N. gonorrhoeae sensitivity to azithromycin. There are no standard breakpoints of minimum inhibitory concentrations (MICs) used to categorize N. gonorrhoeae resistance to azithromycin, but >1 µg/ml [65] and >2 µg/ml [66] have both been used. In this section, we summarize the key regional observations from 36 in vitro studies (Tables 6 & 7).
Table 6.
Sensitivity testing of Neisseria gonorrhoeae isolates to azithromycin.
| Study (year) | Country | Year(s) | Sample size |
MIC range
(µg/ml) |
Percent strains susceptible | Additional details | Ref. |
|---|---|---|---|---|---|---|---|
| Olsen et al. (2013) | Vietnam | 2011 | 108 | NR | 62% | 11% isolates fully resistant, 29% intermediate susceptibility | [144] |
| Lahra et al. (2012) | Australia | 2011 | 3,293 | ≤4 | 98.1% | Isolates from all states in Australia | [145] |
| Sethi et al. (2013) | India/Pakistan/Bhutan | 2007–2011 | 65 | 0.016–4.0 | 76.9% | 7.7% isolates fully resistant, 15.4% intermediate susceptibility | [72] |
| Lo et al. (2012) | Hong Kong | 2010 | 485 | <0.25 to >256 | 69.7% | [146] | |
| Lefebvre et al. (2011) | Canada | 2010 | 831 | ≤16 | 98.7% | [147] | |
| Hottes et al. (2013) | Canada | 2006–2011 | 1,837 | 0.064–16 | 99% | Elevated MIC showed increasing trend over time | [148] |
| CDC (2011) | USA | 2002–2009 | 87,566 | 99.9% | 39 (0.04%) had MICs ≥8 µg/ml (25 with 8 µg/mL; 14 with 16 µg/mL) | [69] | |
| Yuan et al. (2011) | China | 2008–2009 | 318 | NR | 94.7% | [149] | |
| Takahashi et al. (2013) | Japan | 2007–2009 | 52 | 0.016–1 | 100% | 100% of isolates from men† | [150] |
| Herchline et al. (2010) | USA | 2006–2008 | 286 | 0.032–1.0 | 99.0% | Median MIC 0.125 µg/ml | [151] |
| Cole et al. (2010) | Europe (17 countries) | 2006–2008 | 3,528 | ≤256 | 2.0–7.0% | High resistance (>256 µg/ml) in 4 isolates from Scotland and 1 in Ireland | [68] |
| Olsen et al. (2012) | Guinea-Bissau | 2006–2008 | 31 | NR | 100% | Of resistant strains, two had MIC >64 µg/mL | [152] |
| Tanaka et al. (2011) | Japan | 2001–2009 | 242 | 0.004–1.0 | 99.9% | Modal shift of MIC was 0.25–0.5 µg/mL | [153] |
| Martin et al. (2011) | Canada | 2000–2009 | 40,875 | ≤64 | 99.8% | 100% isolates susceptible also to sefixime, ceftriaxone and spectinomycin. | [66] |
| Bala et al. (2011) | India | 2000–2009 | 274 | NR | 99.7% | One isolate was resistant to azithromycin, quinolones and penicillin | [154] |
| Chisholm et al. (2008) | UK | 2001–2007 | 108 | <0.03 to >256 | 94.0% | 6/108 isolates had MIC >256 µg/mL; shift to high level resistance | [155] |
| Khaki et al. (2007) | India | 2004–2005 | 60 | 0.016–0.25 | 100% | [156] | |
| Enders et al. (2006) | Southern Germany | 2004–2005 | 65 | 0.016–5.0 | 100% | 100% of isolates susceptible to azithromycin | [157] |
| Vorobieva et al. (2007) | Russia | 2004 | 76 | NR | 100% | Although all susceptible, reduced susceptibility seen in 14% | [158] |
| Sutrisna et al. (2006) | Indonesia | 2004 | 163 | NR | 100% | 53% resistant to ciprofloxacin | [159] |
| Martin et al. (2004) | Western Europe | 2004 | 965 | NR | 91.8% | Variation from 31.2% (Austria 30/96) to 0% (France 0/101, Greece 0/79, Portugal 0/17) | [160] |
| Chaudhary et al. (2005) | Nepal | 2003 | 16 | 0.008–0.5 | 100% | No isolates resistant, but 3/16 (19%) had reduced susceptibility | [161] |
| Chen et al. (2009) | Taiwan | 1999–2004 | 65 | NR | 100% | [162] | |
| Hsueh et al. (2005) | Taiwan | 1999–2003 | 55 | 0.03–9.0 | 72.7% | [163] | |
| Aydin et al. (2005) | Turkey | 1998–2002 | 78 | 0.004–0.25 | 100% | 100% of isolates from men† | [164] |
| Moodley et al. (2001) | South Africa | 1995–2000 | 58 | 0.015–1.0 | 100% | 37% (37/100) strains resistant to penicillin; tetracyclines had reduced susceptibility. | [165] |
| Kobayashi et al. (2003) | Japan | 1995–1999 | 699 | 0.015–1 | 100% | 100% of isolates from men† | [166] |
| Llanes et al. (2003) | Cuba | 1995–1999 | 52 | 0.064–0.5 | 76.9% | 23.1% intermediate susceptibility: MIC= 0.125 (10 isolates) MIC= 0.5 (2 isolates) | [167] |
| Sosa et al. (2003) | Cuba | 1995–1999 | 91 | 0.063–4.0 | 90.1% | Isolates with reduced susceptibility also resistant to penicillin and tetracycline | [168] |
| Dillon et al. (2001) | Brazil | 1998 | 81 | 0.032–0.5 | 100% | 28% reduced susceptibility | [169] |
| Zarantonelli et al. (1999) | Uruguay | 1996–1997 | 51 | 0.032–0.5 | 100% | Decreased susceptibility (MIC 0.025 to 0.5) in 72%; isolates from men† | [170] |
| Young et al. (1997) | Scotland | 1996 | 67 | 0.023–0.75 | 100% | Isolates randomly selected from with penicillin MIC≥1 | [171] |
| Mehaffey et al. (1996) | USA | NR | 105 | 0.06–2.0 | 100% | Two tests compared. Data from agar dilution method not Etest. | [172] |
| Dillon et al. (2001) | Guyana & St Vincent | 1992–1996 | 136 | 0.032–8.0 | 85.5 and 97.0% | Two isolates had MIC = 8 µg/l. 49% (67/137) reduced susceptibility (combined) | [173] |
| Van Rijsoort-Vos et al. (1995) | Netherlands | 1991–1993 | 114 | 0.03–1.0 | 100% | One isolate reduced susceptibility | [174] |
| Ison et al. (1993) | South Africa | 1989–1990 | 192 | 0.03–1.0 | 100% | Study in migrant mine workers (men only)‡ | [175] |
Unspecified low-risk populations.
Sexual practices were not reported; isolates are assumed not to be from men who have sex with men;
Dislocated males workers are among high-risk populations.
NR: Not reported.
Table 7.
Sensitivity testing of N. gonorrhoeae isolates to azithromycin.
| Study (year) | Country | Year(s) | Sample size |
MIC range
(µg/ml) |
Percent of strains susceptible | Additional details | Ref. |
|---|---|---|---|---|---|---|---|
| High-risk populations | |||||||
| CDC (2011) | USA | 2009 | 55 | NR | 90.9% | 9.1% resistant (95% CI: 4.0 to 19.6%); of 5 resistant (all from MSM), 3 had MIC = 8 µg/ml and two had MIC = 16ug/ml | [69] |
| Starnino et al. (2009) | Italy | 2007–2008 | 219 | 1.0–256.0 | 90.0% | 72.7% (95% CI: 51.6–86.8%) of resistant isolates from MSM | [176] |
| Donegan et al. (2004) | Bali | 2004 | 147 | 0.013–0.512 | 100% | Study in FSWs; prevalence of NG estimated to be 35-60% | [177] |
| Morris et al. (2009) | USA | 2000–2002 | 79 | 0.03–0.5 | 100% | Increased MIC values seen in MSM subject isolates | [178] |
| Leven et al. (2003) | Indonesia | 1996 | 267 | 0.032–0.5 | 100% | Study in FSWs; prevalence of NG estimated to be 18–44% | [179] |
| CDC (2000) | USA | 1999 | 12 | 1.0–4.0 | NR | Median MIC was 2.0 µg/ml. 6 of 12 samples were from men who had sex with a CSW; 2 of 12 were from HIV positive men | [180] |
| Mixed-risk populations | |||||||
| Bruck et al. (2012) | UK | 2005–2006 | 147 | NR | 99.3% | Mixed male heterosexual, MSM and female heterosexual isolates | [181] |
| Dan et al. (2010) | Israel | 2002–2007 | 406 | 0.023–8.0 | 91.8% | Mixed male heterosexual, MSM and female heterosexual; resistance to azithromycin did not appear to rise over 5 year period | [182] |
| McLean et al. (2004) | USA | 1999–2001 | 1,248 | ≤4 | 97.4% | Mixed high- and low-risk population. Median MIC was 2.0ug.mL | [183] |
| Arreaza et al. (2003) | Spain | 1992–2001 | 63 | 0.03–4.0 | 96.8% | 58.7% of strains had reduced susceptibility (0.25–1.0 µg/ml). 50% of resistant isolates were from FSW | [184] |
BV: Bacterial vaginosis; CSW: Commercial sex worker (gender not specified); FSWs: Female sex workers; GI: Gastrointestinal; MIC: Minimum inhibitory concentration; MSM: Men who have sex with men; NG: Neisseria gonorrhoeae; NR: Not reported; PROM: Preterm premature rupture of the membranes; SD: Standard deviation; TOC: Test of cure.
The Public Health Agency of Canada reported that 0.17% (n = 40,875; 95% CI: 0.001–0.002%) of N. gonorrhoeae samples were resistant to azithromycin between 2000 and 2009, although the modal value of the MIC shifted from 0.25 µg/ml in 2001 to 0.5 µg/ml between 2007 and 2009 [67]. During the same 10-year period, the Centers for Disease Control and Prevention in the USA reported that 0.04% (n = 87.566; 95% CI: 0.03–0.06%) of N. gonorrhoeae isolates tested had MICs ≥8 µg/ml (including 25 with 8 µg/ml and 14 with 16 µg/ml) [68]. This did not include five cases of azithromycin-resistant N. gonorrhoeae found between August and October 2009 among men who have sex with men; three had MICs of 8 µg/ml and two had 16 µg/ml [69]. Resistance may have appeared in Europe slightly before North America. Analysis of isolates from 17 European countries found that 3.2% (n = 836; 95% CI: 0.02–0.05%) of gonococcal isolates were resistant to azithromycin in 2006. By 2007, 6.8% (n = 973; 95% CI: 0.05–0.09) of samples were resistant. The overall proportion of resistant isolates declined in 2008 to 1.8% (n = 940; 95% CI: 0.01–0.03%), although only 5.2% (95% CI: 4.1–6.8%) of strains tested in the same year were fully susceptible to azithromycin and ciprofloxacin. Four isolates from Scotland and one from Ireland exhibited MICs >256 mg/l [68].
Gonococcal isolates examined from South America and Cuba exhibited high but stable levels of resistance between 2000 and 2009 in most settings [70]. Collectively, azithromycin resistance was 13.0% (n = 8,373; 95% CI: 12.3–13.7%) based on data from six countries including Chile, an outlier. Averaged over the decade, 26.7% (n = 3,116; 95% CI 25.2–28.3%) of samples from Chile were resistant, rising to 45.6% (n = 463; 95% CI: 41.1–50.1%) according to the most recent data from 2009. Removing Chile from the regional summary, 4.4% (n = 5,257; 95% CI: 3.9–5.1%) of isolates were resistant over the decade.
All 60 gonococcal isolates from India between 2004 and 2005 were susceptible to azithromycin [71]. Pooled analysis of samples collected from India, Pakistan and Bhutan between 2007 and 2011 found that 76.9% (n = 65; 95% CI: 65.3–85.5%) were susceptible. Results were not stratified by country and, therefore, it is not known whether the sensitivity of isolates from India had changed [72]. Applying the more conservative breakpoint of >1 µg/ml to the in vitro studies identified in this review, 35% (7 of 20) of the in vitro studies reported upper range MICs that included gonococcal isolates resistant to azithromycin. This percentage does not include 16 studies we identified and included in Tables 6 & 7 that did not report MICs.
Chlamydia trachomatis
In vivo evidence
The WHO recommends treating pregnant women with Chlamydia trachomatis infection using 1 g azithromycin as a single oral dose [50]. We found eight RCTs in the literature that reported the treatment efficacy of 1 g azithromycin among pregnant women (Table 8) [73–80]. Using random effect models, we estimate the pooled treatment efficacy to be 92.1% (n = 268; 95% CI: 88.4–95.7%). The estimated efficacy would be higher if we excluded two trials that were conducted in the USA. The first trial (1995–1997) reported a 3-week test of cure rate to be 88.1% (n = 42; 95% CI: 74.9–94.7%) [76], whereas the second trial (1998–2000) was terminated early due to poor efficacy, 63.3% (n = 55; 95% CI: 50.4–75.1%), based on test of cure ≥4 weeks post-treatment [74]. These results need to be interpreted with caution because no distinction was made between treatment failures and new infections, sex partners were not treated by trial staff, but were referred to a treatment center, and only 35% of women were seen within 7 days of the scheduled test of cure.
Table 8.
Treatment efficacy studies of 1 g azithromycin for the treatment of Chlamydia trachomatis in pregnant women.
| Study (year) | Country | Year(s) | Number cured of evaluated | Percent cured | 95% CI | Diagnostic method | Birth outcomes | Additional details | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Kacmar et al. (2001) | USA | 1998–2000 | 18/19 | 94.7% | 84.4–105.1% | Ligase chain reaction; TOC 28–42 days | NR | 52.6% (n = 19; 95% CI: 31.5–72.8%) had side effects; passive or active solicitation not reported; 13.6 weeks (±8.0 SD) mean gestational age at enrolment | [73] |
| Jacobson et al. (2001) | USA | 1998–2000 | 35/55 | 63.6% | 47.7–79.6% | DNA LCx STD assay; TOC 28 days | 13.3% (6/45) preterm | 10.1% (n = 55; 95% CI: 5.2–21.9%) had side effects; passive or active solicitation not reported; 20.6 weeks (±8.8 SD) mean gestational age at enrolment | [74] |
| Wehbeh et al. (1998) | USA | NR | 26/27 | 96.3% | 89.0–103.6% | Culture | NR | 100% (n = 27) of partners treated | [75] |
| Adair et al. (1998) | USA | 1995–1997 | 37/42 | 88.1% | 77.7–98.5% | DNA assay; TOC 28 days | NR | 11.9% (n = 5; 95% CI: 5.3–25.1%) had side effects; passive or active solicitation not reported; 21.6 weeks (±9.5 SD) mean gestational age at enrolment; 54.8% (n = 23) of partners treated | [76] |
| Edwards et al. (1996) | USA | 1993–1994 | 61/65 | 93.8% | 87.8–99.9% | DNA assay; TOC 14 days | 9.2% (6/65) preterm, 3 due to PROM; mean birth age 38.8 weeks ±1.6 | 20.4 weeks mean gestational age at enrolment | [78] |
| Rosenn et al. (1995) | USA | 1994–1995 | 21/23 | 91.3% | 79.3–103.4% | PCR (Amplicor); TOC 21 days | NR | 19.3 weeks (±3.5 SD) mean gestational age at enrolment | [79] |
| Gunter et al. (1996) | USA | NR | 22/22 | 100% | NA | DNA assay; TOC 14 days | NR | 13.6% (n = 3; 95% CI: 5.0–33.6%) had gastrointestinal side effects; passive or active solicitation not reported | [77] |
| Bush & Rosa (1994) | USA | NR | 15/15 | 100% | NA | DNA assay; TOC 14 days | NR | 0% of women experienced side effects; 100% (n = 15) of partners treated | [80] |
| Rahangdale et al. (2006) | USA | 1999–2000 | 137/141 | 97.2% | 94.4–99.9% | DNA assay; TOC days 7–20; 21–34; 35–55; >56 | 7.5% (16/221 'any' preterm azithromycin) | 13.1% (n = 191; 95% CI: 9.0–18.6%) also had BV 14.1 weeks (±6.3 SD) mean gestational age at enrolment |
[185] |
| Miller et al. (1995) | USA | 1993–1994 | 132/138 | 95.6% | 92.2– 99.1% | DNA assay; TOC 10–14 days | 15.2% (19/125) preterm | 5.5% (n = 146; 95% CI: 2.8–10.4%) had side effects; 23.9% (n = 138; 17.6–31.7%) women also had NG; 17.4% (4/17) reported side effects (all GI); mean gestational age at enrolment not reported | [186] |
NR: Not reported; TOC: Test of cure.
Studies investigating sexual activity following treatment offer some perspectives on post-treatment infections and the extent to which they may be failures or de novo infections. A trial in Seattle (1998–2003) found that persistent or recurrent chlamydial or gonorrheal infection occurred in 7.6% (n = 289; 95% CI: 5.1–4.9%) of female patients who reported no sexual intercourse after treatment [81]. Another study reported that 19.0% (n = 79; 95% CI 11.9–29.0%) of women were positive for C. trachomatis 3 months after treatment using 1 g azithromycin. Of these women, 13.3% (n = 15; 95% CI: 4.0–38.3%) reported being sexually inactive during the post-treatment period [82]. These findings may be attributable to false reporting of sexual contact, or treatment failure or may lend credence to the hypothesis that C. trachomatis enters a latent asymptomatic state that is undetectable by culture or, possibly, Nucleic Acid Amplification Tests, but can later reactivate [83].
In vitro evidence
Thresholds for antimicrobial susceptibility and resistance of C. trachomatis are not universally standardized, although MICs >4 μg/ml are often used to characterize therapeutic failure [84–87]. The lowest concentration of antimicrobial compound needed to inhibit chlamydial formation is between 0.03 and 0.125 μg/ml, whereas the minimum bactericidal concentration (MBC; also referred to as the minimum chlamydicidal concentration, or MCC) is between 0.06 and 0.5 μg/ml [88,89]. The published in vitro studies of azithromycin collectively suggest the persistence of high and widespread treatment efficacy (Table 9). One noted exception is the study of six isolates from three patients who experienced treatment failure in Russia (2000–2002); four isolates were resistant to azithromycin, doxycycline and ofloxacin at MICs and MBCs >5.12 μg/ml [90]. Not surprisingly, in vitro resistance appears to be more common in individuals with greater severity of disease or recurrent disease. A study in the USA during the early 1990s described decreased susceptibility and emerging resistance to azithromycin and doxycycline in isolates from women with mucopurulent cervicitis but not in isolates from women with asymptomatic infections [91]. Similar observations were reported in 2010 from India; six of eight isolates with modified susceptibility had been obtained from recurrently infected individuals, whereas the remaining two were from nonrecurrently infected patients. MICs and MBCs for azithromycin were 8 µg/ml for two of the patients from which the modified susceptibility isolates were taken. One individual had chronic cervicitis and the other had pelvic inflammatory disease [92].
Table 9.
Sensitivity testing of Chlamydia trachomatis isolates to azithromycin.
| Study (year) | Country | Year(s) |
Azithromycin
|
Other macrolides
|
Additional details | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | MBC/MCC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | |||||
| No resistance observed | ||||||||
| Ljubin-Sternak et al. (2012) | Croatia | 2010 | 0.064 to 0.125 | 0.064–2.0 | Doxycycline: 0.016–0.064 | 0.032–1.0 | 24 urogenital strains assessed | [187] |
| Donati et al. (2010) | Italy | 2005–2006 | 0.25–0.5 | 0.5–1.0 | Doxycycline: 0.03–0.06 Erythromycin: 0.5–1.0 |
0.06–0.125 1.0–2.0 |
All serovars had comparable susceptibilities. Azithromycin and doxycycline bactericidal with MBC at one to two-times the MIC. (50 strains) | [188] |
| Hong et al. (2009) | Ethiopia | 2006 | 0.25–0.5 | 0.25–1.0 | Doxycycline: 0.015–0.03 | 0.03–0.06 | Azithromycin unchanged between pre- and post-mass biannual treatment of trachoma (10 strains) | [189] |
| Samra et al. (2001) | Israel | 1997–1999 | 0.06–0.125 | 0.06–0.25 | Doxycycline: 0.125–0.25 Tetracyclines: 0.25–0.5 | 0.125–4.0 0.25–4.0 | Smallest MBC and MIC difference in azithromycin versus doxycycline (4 dilutions differences; 50 isolates) | [84] |
| Lefèvre et al. (1993) |
France | NR | 0.06–0.125 | 0.25–0.5 | Clarithromycin: 0.008 Erythromycin : 0.06–0.125 Tetracyclines: 0.125–0.25 |
0.03–0.125 0.25–2.0 1.0–4.0 |
15 clinical isolates tested | [190] |
| Agacfidan et al. (1993) | United States | 1993 | ≤0.06–1 | 0.12–2.0 | Doxycycline: 0.015–0.06 Tetracyclines: 0.03–0.12 | 0.015–0.06 0.06–0.12 | Azithromycin highly active against CT in isolates from urethral and cervical samples (azithromycin 10 strains, doxycycline 22 strains, tetracycline 22 strains) | [191] |
| Scieux et al. (1990) | UK | 1990 | 0.064–0.25 | 2.0–8.0 | Doxycycline: 0.016–0.064 Erythromycin: 0.064–0.128 |
0.5–8.0 0–64.0 |
10 strains used from the USA | [192] |
| Resistance observed | ||||||||
| Bhengraj et al. (2010) | India | 2006–2007 | 0.12–8 | ≤8.0 | Doxycycline: 0.025–8.0 | ≤8.0 | Decreased antimicrobial susceptibility in recurrently infected female patients (21 isolates) | [92] |
| Misyurina et al. (2004) | Russia | 2000–2002 | >5.12 | >5.12 | Erythromycin: >5.12 | >5.12 | Isolates from salpingitis, endocervicitis, and urethritis showed resistance (6 isolates) | [90] |
| Somani et al. (2000) | USA | 1995–1998 | 0.5 to >4.0 | >4.0 | Doxycycline: 0.125–>4.0 | >4.0 | Resistance of strains causing relapsing or persistent infection in 3 patients (3 isolates) | [193] |
| Rice et al. (1995) | USA | 1995 | 0.125–2.0 | 0.5 to >4.0 | Doxycycline: 0.008–0.06 | 0.015–4.0 | Isolates susceptible to azithromycin and doxycycline in asymptotic infection | [91] |
Unspecified low-risk populations.
CT: Chlamydia trachomatis; MBC: Minimum bactericidal concentration; MCC: Minimum chlamydicidal concentrations; MIC: Minimum inhibitory concentrations; NR: Not reported; PID: Pelvic inflammatory disease.
Trichomonas vaginalis
In vivo evidence
Trichomonas vaginalis is a protozoal infection which causes cervicitis and nongonococcal urethritis. The WHO recommends treating pregnant women with T. vaginalis infection after the first trimester using 2 g metronidazole orally as a single dose, or 400–500 mg twice daily for 7 days or 300 mg clindamycin orally twice a day for 7 days [36]. If treatment is imperative during the first trimester of pregnancy, the single-dose regimen of 2 g metronidazole orally is recommended [36]. Azithromycin has not been used directly for prevention or treatment purposes because T. vaginalis is anaerobic. Nevertheless, azithromycin has demonstrated protection against T. vaginalis in studies of mass STI/RTI treatment (Tables 10 & 11).
Table 10.
Trials using azithromycin alone and in combination with other drugs reporting protection curable STIs/RTIs among pregnant women.
| Study (year) | Country | Year(s) | Regimen |
Number of cases post-treatment (%)
|
Additional details | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treponema pallidum † | Neisseria gonorrhoeae | Chlamydia trachomatis | Trichomonas vaginalis | Bacterial vaginosis | ||||||
| Luntamo et al. (2010) | Malawi | 2003–2006 |
Intervention
1 g AZ two-times + SP monthly |
NR | 0.5% (2/391) |
0.3% (1/391) |
11.0% (46/419) |
NR | Significant protection against T. vaginalis p = 0.05 | [94] |
|
Intervention
SP monthly |
NR | 2.1% (8/384) |
0.3% (1/384) |
15.1% (62/411) |
NR | |||||
|
Control
SP two-times |
NR | 0.7% (3/391) |
0.3% (1/391) |
16.7% (69/411) |
NR | |||||
| van den Broek et al. (2009) | Malawi | 2004–2005 |
Intervention
1 g AZ two-times + SP two-times |
NR | NR | NR | NR | NR | No difference in preterm birth (16.8% versus 17.4%); potential explanatory factors include use of sub-optimal syphilis treatment [196,197] | [194] |
|
Control
SP two-times |
NR | NR | NR | NR | NR | |||||
| Gray et al. (2001) | Uganda | 1994–1998 |
Intervention
1 g AZ + 250 mg CIPX + 2 g MTZ |
3.4% (57/1,677) | 0.9% (14/1,503) |
1.1% (16/1,503) |
4.7% (4/1,779) |
36.3% (645/1,779) |
Neonatal death RR: 0.83; 95% CI: 0.71–0.97; Low birth weight RR: 0.68; 95% CI: 0.53–0.86; Preterm delivery RR: 0.77; 95% CI: 0.56 to 1.05. Vertical transmission of HIV was no different between intervention and control groups | [104] |
|
Control
Iron-folate + 100 mg MBZ two-times |
3.3% (46/1,376) | 1.7% (24/1,394) |
2.7% (38/1,394) |
15.9% (248/1,569) |
48.5% (764/1,576) |
|||||
| Wawer et al. (1999) | Uganda | 1994–1998 |
Intervention
1 g AZ + 250 mg CIPX + 2 g MTZ |
6.0% (80/1,323) | 1.0% (8/770) |
1.2% (9/770) |
5.3% (72/1,350) |
39.1% (533/1,364) |
Vertical transmission of HIV was no different between intervention and control groups | [95] |
|
Control
Iron-folate + 100 mg MBZ two-times |
7.1% (75/1,056) | 2.1% (15/714) |
3.5% (25/714) |
17.4% (198/1,137) |
52.8% (609/1,154) |
|||||
Low-risk populations
2.4 mu benzathine Penicillin G was administered to pregnant women in all treatment groups per national guidelines (exception being [194]).
AZ: Azithromycin; CIPX: Ciprofloxacin; NR: Not reported; RR: Risk ratio; SP: Sulfadoxine-pyrimethamine.
Table 11.
Trials using azithromycin alone and in combination with other antimicrobial therapies not contraindicated in pregnancy and reporting protection curable STIs/RTIs among commercial sex workers.
| Study (year) | Country | Year(s) | Regimen |
Proportion of cases cured (cases pre-treatment/cases post-treatment)
|
Additional details | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treponema pallidum † | Neisseria gonorrhoeae | Chlamydia trachomatis | Trichomonas vaginalis | Bacterial vaginosis | |||||||
| Kaul et al. (2004) | Kenya | 1998–2002 |
Intervention
1 g AZ monthly (multi-yr pd) |
3.9% | 2.6% | 1.1% | 11.3% | 53.0% | Incidence reported per 100 women-years | [93] | |
|
Control
Placebo |
3.8% | 5.7% | 6.5% | 20.4% | 57.4% | ||||||
| Labbe et al. (2012) | Benin Ghana | 2001 |
Intervention
1 g AZ or 500 g CIPX monthly for 9 months alternating AZ & CIPX |
NR | 5.6% (7/126) |
1.6% (2/126) |
NR | NR | Significant protection against N. gonorrhoeae p = 0.05 | [197] | |
|
Control
Placebo |
NR | 12.5% (14/112) |
2.7% (3/112) |
NR | NR | ||||||
| Cowan et al. (2005) | Zimbabwe | NR |
Intervention
1 g AZ+2 g MTZ+500 mg CIPX |
Base: 5.0% (2.8–8.7%); V2, V3, V4: NR in any form |
Base: 1.9% (0.5–3.4%) V2, V3, V4: visible inaccuracies in graphs; cannot estimate |
Base: 1.7% (0.3–3.0%) V2, V3, V4: visible inaccuracies in graphs; cannot estimate |
Base: 19.3% (15.2–23.4%) V2: 4.3% (7.7–2.2%) V3: 12.6% (17.7–8.8%) V4: 11.5% (15.7–7.8%) |
[96] | |||
|
Intervention
1 g AZ+2 g MTZ |
|||||||||||
| Wi et al. (2006) | Philippines | 2001 |
Intervention
1 g AZ one time |
Prior to intervention 1 month post-intervention |
NR | 18.3% (207/1,130) 11.9% (82/687) |
28.6% (323/1,130) 15.1% (104/687) |
NR | NR | [198] | |
| Williams et al. (2003) | South Africa | 1998–2000 |
Intervention
1 g AZ nine-times in 9 months |
Prior to intervention 9-month post-intervention |
9.8% (68/691) 18.7% (166/893) |
6.9% (48/691) 8.6% (77/893) |
7.9% (55/691) 13.8% (123/893) |
NR | NR | HIV prevalence among CSW in the mining community was 68.6% | [199] |
| Steen et al. (2000) | South Africa | 1996–1997 |
Intervention
1 g AZ every month for 9 months |
Prior to intervention 9-month post-intervention |
NR | 17.3% (70/407) 4.7% (5/108) |
14.3% (58/407) 0.9% (1/108) |
NR | NR | Pre-intervention NG and/or CT = 24.9% (101/407); Post-intervention NG and/or CT = 5.7% (6/108) | [200] |
Italicized values are approximate based on enlarged graphs published in Cowan et al. and percentages in parentheses reflect the 95% confidence intervals [96].
2.4 mµ benzathine Penicillin G was administered to commercial sex workers who tested positive for syphilis in all treatment groups per national guidelines.
AZ: Azithromycin; NR: Not reported; CIPX: Ciprofloxacin; CT: C. trachomatis; CSW: Commercial sex worker; MTZ: Metronidazole; NG: Neisseria gonorrhoeae.
In Kenya (1998–2002), 1 g azithromycin or placebo was given once per month to 466 HIV-negative female sex workers [93]. At the end of the trial, HIV incidence was the same across treatment groups, the primary endpoint, but the incidence of T. vaginalis was reduced significantly among those given azithromycin versus placebo (RR: 0.56; 96% CI: 0.40–0.78; p < 0.001). A similar observation was made in a three-arm IPTp trial in Malawi (2003–2006) [94]. Pregnant women received standard IPTp-SP, or monthly IPTp-SP or monthly IPTp-SP plus 1 g azithromycin during two antenatal visits; the prevalence of T. vaginalis at delivery was 16.7% (n = 411; 95% CI: 13.5–20.7%), 15.1% (n = 411; 95% CI: 12.0–18.9%) and 11.0% (n = 419; 95% CI: 8.3–14.3%), respectively. Thus, women who received azithromycin had 35% (RR: 0.65; 95% CI: 0.46–0.93; p = 0.02) fewer T. vaginalis infections at delivery compared with monthly recipients of IPTp-SP.
A cluster randomized trial in Uganda (1994–1998) compared the incidence of HIV infections among nonpregnant adults who received 1 g azithromycin, 250 mg ciprofloxacin and 2 g metronidazole versus multivitamins plus antihelminthics [95]. Although the trial was terminated early for lack of protection against the primary endpoint, the incidence of several curable STIs/RTIs was lower in the control group, most notably T. vaginalis. The cumulative incidence of newly diagnosed T. vaginalis infection was 4.8/100 person-years (116/2,397 person-years) in the intervention group compared with 9.1/100 person-years (182/1,993 person-years) in the control group (RR: 0.52; 95% CI: 0.35–0.79).
The same combination of antimicrobials was provided to female sex workers in rural Zimbabwe as a one-time treatment followed by 3 monthly check-ups [96]. The prevalence of T. vaginalis was just under 20% at baseline, decreased to approximately 5% at visit 2, rose to nearly 13% at visit 3 and lowered again to just over 10%, that is, one-half of the pretreatment levels.
Bacterial vaginosis
The WHO recommends treating bacterial vaginosis in pregnant women, preferably after the first trimester, with 200 or 250 mg metronidazole three-times per day for 7 days, or 5 g metronidazole gel (0.75%) applied intravaginally twice a day for 5 days or 300 mg clindamycin 300 mg orally twice a day for 7 days [36]. As with T. vaginalis, if treatment is imperative during the first trimester of pregnancy, 2 g metronidazole orally is recommended [36]. Bacterial vaginosis has no single causative agent, but is thought to result from destabilization of Lactobacillus species (spp.) with secondary colonization of anaerobic organisms that include Gardnerella vaginalis, Bacteroides spp, Mobiluncus spp. and Mycoplasma hominis alongside an increase in vaginal pH [97,98].
In vivo evidence
Our review identified no trials that have attempted to measure the treatment efficacy of azithromycin alone against bacterial vaginosis. Only one study in the USA (2002–2005) investigated the use of azithromycin as a partner drug with metronidazole for the treatment of symptomatic bacterial vaginosis. Nonpregnant women received one of four treatments: 750 mg metronidazole once per day for 7 days, or metronidazole once per day for 7 days plus 1 g azithromycin on days 1 and 3, or metronidazole for 14 days or metronidazole for 14 days plus azithromycin on days 1 and 3 [99]. No additional benefit of cure was observed among women who received metronidazole plus azithromycin compared with metronidazole alone.
Antibiotic treatment for bacterial vaginosis is challenging, in part, because it is a syndrome that involves multiple microorganisms rather than a single etiological agent. Comparable data from other macrolides suggest potential therapeutic value for azithromycin against bacterial vaginosis (Table 12). Analysis of azithromycin against the anaerobic and carboxyphilic bacteria that replace the normal vaginal flora may provide a better understanding as to the potential role of azithromycin against bacterial vaginosis.
Table 12.
Sensitivity of macrolides and structurally related agents against key causative organisms in bacterial vaginosis
| Study (year) | Country | Year(s) |
Minimum inhibitory concentrations of specific macrolides (µg/ml)
|
Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Azithromycin | Erythromycin | Clarithromycin | Roxithromycin | Clindamycin † | Telithromycin † | ||||
| Gardnerella vaginalis | |||||||||
| Jones et al. (1998) | UK | NR | <0.03–0.125 | <0.03–0.06 | NR | NR | NR | NR | [201] |
| Shanker et al. (1982) | Australia | NR | NR | 0.007–0.06 | NR | NR | 0.007–0.06 | NR | [202] |
| King † et al. (1987) | NR | NR | 0.008–0.016 | NR | 0.016 | NR | NR | [203] | |
| Bacteroides species | |||||||||
| Jones et al. (1998) | UK | NR | 0.06 –16 | <0.03–32 | NR | NR | NR | NR | [201] |
| Dubreuil et al. (1987) | England, France, Germany, Japan | NR | NR | 0.003–>64 | NR | 0.003–>64 | NR | NR | [204] |
| Maskell et al. (1990) | UK | NR | 0.5–>16 | <0.25–16 | NR | NR | NR | NR | [205] |
| Chang et al. (1995) | Taiwan | 1989–1992 | 1–>256 | 0.25–>256 | ≤0.03–>256 | 0.25–>256 | NR | NR | [206] |
| Ednie et al. (1997) | USA | NR | 1–>64 | 0.5–>64 | 0.5–>64 | 2–>64 | ≤0.06–>64 | NR | [207] |
| Mikamo et al. (2003) | Japan | 2000 | 0.125– 32 | 0.125–32 | 0.063 to16 | NR | NR | 0.032–16 | [208] |
| Marina et al. (2009) | Bulgaria | 1983–2007 | NR | 0.5–>64 | NR | NR | 0.125–32 | NR | [209] |
| Chen et al. (1992) | Australia | 1986–1991 | 0.5 to128 | 0.25–128 | NR | NR | NR | NR | [210] |
| Wexler et al. (2001) | USA | NR | NR | NR | NR | NR | 0.25–>64 | [211] | |
| Mycoplasma hominis | |||||||||
| Ridgway et al. (1987) | UK | NR | NR | >32 | NR | 8 to16 | NR | NR | [203] |
| Mobinculus species | |||||||||
| Spiegel et al. (1987) | USA | NR | NR | ≤0.2–>200 | NR | NR | ≤0.015–4 | NR | [212] |
Unspecified low-risk population.
Not a macrolide but has similar mechanism of action and included for comparability.
NR: Not reported.
Discussion
Azithromycin has been used against curable STIs/RTIs for 25 years. It has been an attractive option for preventive and curative treatment because it is efficacious as a single dose and offers reasonable tolerability against T. pallidum, N. gonorrhoeae and C. trachomatis. During the 1990s, and prior to the advent of antiretroviral therapies for HIV, the management of curable STIs/RTIs received renewed importance, particularly as trials showed that treatment of N. gonorrhoeae, C. trachomatis and T. vaginalis reduced the genital viral load of HIV among men and women [100–103]. Groups at high risk for transmitting HIV have since been targeted by treatment campaigns using 1 g azithromycin. Thus, it is not a surprise that changes in azithromycin sensitivity within high-income settings have often been observed first among members of high-risk groups. Pregnant women attending ANC facilities in sub-Saharan Africa do not have the same risk profile. Thus, on this basis alone, it is less likely that the use of ABC therapies in IPTp would be a catalyst for the rapid emergence of azithromycin resistance, although its emergence cannot be ruled out. The potential benefits of ABC therapies may be best viewed through prior experience with mass drug administration among pregnant women. In the context of the AIDS epidemic and before the age of antiretroviral therapies, researchers attempted to prevent maternal-to-child transmission (MTCT) of HIV by providing pregnant women in Uganda 1 g azithromycin, in combination with 250 mg ciprofloxacin and 2 g metronidazole [104]. The data safety monitoring board suspended the trial early for reasons of futility, despite having cut neonatal deaths by 17% (RR: 0.83; 95% CI: 0.71–0.97), decreased the incidence of low birthweight by 32% (RR: 0.68; 95% CI: 0.53–0.86), and reduced the incidence of preterm delivery by 23% (RR: 0.77; 95% CI: 0.56–1.05). These impressive results were achieved at a time when neither IPTp-SP nor insecticide treated bed nets for the control of malaria in pregnancy had been deployed.
If ABC therapies are used in IPTp, then there are several key factors to consider that are pathogen specific. Regarding syphilis, 1 g azithromycin should be used alongside 2.4 mu BPG for three reasons: combination therapy has been shown to achieve higher rates of cure than either therapy alone [105]; use of ABC therapy with BPG would likely reduce selection of the A→G mutation associated with azithromycin and preserve T. pallidum sensitivity; and only BPG can be expected to cure congenital infection if the placenta has been invaded by spirochetes [106]. As for N. gonorrhoeae, 1 g azithromycin may be just above the MIC of fully susceptible strains. Thus, ABC therapies containing >1 g azithromycin may be preferable from the standpoint of reducing selection pressure. However, a single 2 g dose may not be well tolerated as 6 in 10 patients reported self-limiting gastrointestinal discomfort when treated for syphilis infection with such a regimen [42]. The dose could be split over 2 days to improve tolerability. ABC therapies that contain 2 g azithromycin, either a single- or split-dose, would be protective against C. trachomatis. Although the data are limited and the mechanism of action is not understood, ABC therapies that have 1 g azithromycin may protect against T. vaginalis based on reports from Malawi among pregnant women [107] and commercial sex workers in Kenya [93]. It is curious, however, that T. vaginalis infection during pregnancy is associated with adverse birth outcomes, but the first-line treatment of 2 g metronidazole does not always improve birth outcomes. A trial in Uganda reported that pregnant women treated for T. vaginalis infection were 2.5-times more likely to deliver a low birthweight infant than untreated women (RR: 2.49; 95% CI: 1.12–5.50) [108]. The authors suggest that this may be attributable to metronidazole exposure. Another trial in the USA reported an increase in the risk of preterm delivery among pregnant women exposed to metronidazole for the treatment of asymptomatic trichomoniasis compared with those who were not treated (RR: 1.8; 95% CI: 1.2–2.7) [109]. In contrast to these findings from high-income settings, data from a multicenter trial in sub-Saharan Africa indicate that treatment of T. vaginalis infection using metronidazole does not increase the chances of preterm birth [110]. Apart from bacterial vaginosis, which is not transmitted through sexual contact, re-infection will remain a risk for pregnant women and, therefore, providers should continue to offer education and screening as appropriate.
None of the studies identified in this review indicate that azithromycin offers preventive or curative effect against bacterial vaginosis, the most prevalent of curable STIs/RTIs. Antibiotic therapy has only been shown to reduce the risk of preterm delivery by one-half (RR: 0.53; 95% CI: 0.34–0.84) among pregnant women with bacterial vaginosis (Nugent scores 7–10) or intermediate flora (Nugent scores 4–6) [111]. A Nugent score of 0–3 is considered normal [112] for which no protection against adverse birth outcomes has been observed.
Conclusions
ABC therapies are among leading candidates to replace SP for use in IPTp and may offer important public health benefits by also reducing the burden of curable STIs/RTIs in pregnancy. Evidence from nonpregnant adults suggests that ABC therapies containing 1 g azithromycin may cure maternal T. pallidum infection. BPG should still be administered with azithromycin because the combination has been shown to be more efficacious in nonpregnant adults than either treatment alone. Moreover, evidence from pregnant women indicates that eradication of congenital syphilis may require BPG treatment. Neisseria gonorrhoeae infection among pregnant women in sub-Saharan Africa, where strains are likely to be fully sensitive to azithromycin, is likely to be cured by ABC therapies containing 1 g azithromycin. However, 2 g may be needed to reduce persistent and/or recurrent infection, and opportunities for the emergence of drug resistance. ABC therapy containing 1 g azithromycin would be curative of C. trachomatis infection, whereas some protection against T. vaginalis infection could be expected with the same dose. It remains unknown whether ABC therapies could offer protection against bacterial vaginosis if administered during the first half of pregnancy. ABC therapies merit investigation for the use in IPTp given their potential to reduce the dual burden of malaria and curable STIs/RTIs in pregnancy and improve maternal, fetal and neonatal health.
Expert commentary
Current strategies for addressing the dual-burden of malaria and curable STIs/RTIs in pregnancy are suboptimal. In West Africa, IPTp-SP continues to provide protection against the effects of malaria infection but, as previously noted, malaria parasites in East Africa have developed resistance so that IPTp-SP no longer protects against the malaria attributable fraction of low birthweight [3]. Some evidence suggests that IPTp-SP may even be harmful in areas where parasites express the 581G dhps mutation [4–6]. ABC therapies are likely to be more efficacious against malaria parasites in these settings. However, there is an urgent need for trials of ABC therapies to be conducted by independent researchers for policymakers to review alongside the results of trials produced and reported by industry.
In the case of curable STIs/RTIs, the focused ANC package recommended by the WHO includes screening for syphilis and the provision of BPG to women who test positive [50]. Screening would need to continue even if ABC therapies were used in IPTp. The WHO currently recommends the use of rapid point of care tests for syphilis in the ANC setting [113]. Using such tests will expedite case finding and treatment with BPG because results are available during the same consultation. As for the four other curable STIs/RTIs of this review, health care providers are limited to the use of a syndrome-based management algorithm to diagnose and to treat suspected infections. However, 80% of gonococcal and 70–75% chlamydial infections in women are asymptomatic [114] and, therefore, rarely recognized using the syndromic approach. As a result, the diagnostic algorithm has a low sensitivity (30–80%) and specificity (40–80%) for N. gonorrhoeae and C. trachomatis among pregnant women [115–117]. The sensitivity for T. vaginalis (54–83%) and bacterial vaginosis (51–69%) is slightly higher, with moderate specificity for T. vaginalis (40–54%) and bacterial vaginosis (40–58%) [118]. Given the evidence of this review, ABC therapies used in IPTp could be expected to mitigate a considerable proportion of this unattended burden of curable STIs/RTIs.
Much is debated about the utility of a syndrome-based approach to diagnosing and treating many STIs/RTIs. Its shortcomings, as described above, illuminate a much-needed area for research. Specifically, more refined definitions of diagnosis need to be used when characterizing adverse birth outcomes. This is particularly so with T. vaginalis for which successful treatment does not necessarily reduce the risk of adverse birth outcomes. Similarly with bacterial vaginosis, treatment of women who have Nugent scores of 1–3 has not reduced the incidence of preterm birth. With both of these infections, is the recommended regimen of metronidazole inadequate for radical cure? Is it administered too late in pregnancy to alter the course of events? Or are asymptomatic infections simply much less virulent and, therefore, treatment has a marginal effect on selected downstream measures of the adverse birth outcome? Studies of descriptive epidemiology are needed to understand better the extent to which symptomatic versus asymptomatic curable STIs/RTIs contribute to adverse birth outcomes. Such descriptive epidemiology, however, would be incomplete if the prevalence of co-infections were not also considered. The apparent failure to reduce the incidence of adverse birth outcomes following treatment for one infection may be masked by the presence of co-infection(s) that will only be mitigated with the use of combination therapies and consideration of downstream outcomes. The trial in Uganda that failed to reduce the incidence of MTCT of HIV is illustrative. HIV transmission was not interrupted for providing combination treatment against curable STIs/RTIs, but significant reductions were observed in the incidence of neonatal deaths by 17% (RR: 0.83; 95% CI: 0.71–0.97) and low birthweight by 32% (RR: 0.68; 95% CI: 0.53–0.86) [104].
Five-year view
Discussion of the future of IPTp needs to be placed in the context of broader malaria elimination efforts. IPTp-SP has long been considered a malaria control intervention that can be expected to protect less against the fraction of low birthweight attributable to malaria infection as malaria transmission decreases. Recent evidence suggests that IPTp-SP continues to protect against low birthweight among multigravidae in areas where the prevalence of malaria parasitemia measured in children is between 7 and 8%, whereas protection is conferred by IPTp-SP among paucigravidae until very low levels of transmission [119]. Unpublished results from a recently completed multicenter trial in West Africa, where there remain malaria parasites sensitive to SP, indicate that an approach of intermittent screening and treatment (IST) of malaria in pregnancy is noninferior to IPTp-SP (Manuscript under review/personal communication with D. Chandramohan). Thus, there is an urgent need for clinical trials in an area of high SP resistance in East Africa, designed to compare ABC therapies versus IST versus IPTp-SP. ABC therapies, given their action against malaria and curable STIs/RTIs, would be superior to IST and IST would be superior to IPTp-SP, potentially paving the way for adoption of an integrated malaria and curable STI/RTI control package that employs the use of combination treatment.
Financial & competing interests disclosure
MJ Newport is Director of the Wellcome Trust-Brighton and Sussex Centre for Global Health Research (grant number 100715). RM Chico receives funding from Medicines for Malaria Venture, a nonprofit foundation based in Geneva, Switzerland. E Ngulube is a Commonwealth Scholar. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Notice of correction
Since publication of this article the Financial & competing interests disclosure has been updated.
Key issues
Use of azithromycin-based combination (ABC) therapies may have a transformative effect on maternal, fetal and newborn heath by mitigating the dual-burden of malaria and curable sexually transmitted infections and reproductive tract infections sexually transmitted infections and reproductive tract infections (STIs/RTIs) in pregnancy.
ABC therapies containing two or more grams of azithromycin may be less likely to select for resistance when exposed to Treponema pallidum, Neisseria gonorrhoeae and potentially, Chlamydia trachomatis.
In the absence of evidence that azithromycin is curative of congenital syphilis, not simply maternal infection, benzanthine penicillin G (BPG) will still need to be administered to pregnant women who have a syphilis infection; however, the combination of azithromycin plus BPG is more efficacious that BPG alone.
ABC therapies may be preventive of Treponema vaginalis infection during pregnancy, although its impact on birth outcomes needs to be investigated.
The most prevalent of all STIs/RTIs, bacterial vaginosis, may or may not be mitigated by the use of ABC therapies.
Studies of descriptive epidemiology are needed to understand better the extent to which symptomatic versus asymptomatic curable STIs/RTIs contribute to adverse birth outcomes. There is an urgent need for clinical trials in an area of high sulfadoxine-pyrimethamine resistance in East Africa, designed to compare ABC therapies versus IPTp-SP versus providing IST for malaria in pregnancy during ANC visits.
References
- 1.WHO WHO Policy Recommendation: Intermittent Preventive Treatment of Malaria in Pregnancy using Sulfadoxine-Pyrimethamine (IPTp-SP) Apr 11, 2013. 2013.
- 2.World Health Organization World malaria report: 2012. World Health Organziation; Geneva: 2012. [Google Scholar]
- 3.Chico RM. Chandramohan D. Intermittent preventive treatment of malaria in pregnancy: at the crossroads of public health policy . Trop. Med. Int. Health. 2011;16(7):774–785. doi: 10.1111/j.1365-3156.2011.02765.x. [DOI] [PubMed] [Google Scholar]
- 4.Harrington WE, Mutabingwa TK, Muehlenbachs A et al. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment . Proc. Natl Acad. Sci. USA. 2009;106(22):9027–9032. doi: 10.1073/pnas.0901415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington WE, Mutabingwa TK, Kabyemela E, Fried M. Duffy PE. Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance . Clin. Infect. Dis. 2011;53(3):224–230. doi: 10.1093/cid/cir376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrington WE, Morrison R, Fried M. Duffy PE. Intermittent preventive treatment in pregnant women is associated with increased risk of severe malaria in their offspring . PLoS ONE. 2013;8(2):e56183. doi: 10.1371/journal.pone.0056183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitman MS. Tunkel AR. Azithromycin and clarithromycin: overview and comparison with erythromycin . Infect. Control Hosp. Epidemiol. 1992;13(6):357–368. doi: 10.1086/646545. [DOI] [PubMed] [Google Scholar]
- 8.Chico RM, Pittrof R, Greenwood B. Chandramohan D. Azithromycin-chloroquine and the intermittent preventive treatment of malaria in pregnancy . Malar. J. 2008;7(1):255. doi: 10.1186/1475-2875-7-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar M, Woodland C, Koren G. Einarson AR. Pregnancy outcome following gestational exposure to azithromycin . BMC Pregnancy Childbirth. 2006;6:18. doi: 10.1186/1471-2393-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuschner R, Heppner D, Andersen S et al. Azithromycin prophylaxis against a chloroquine resistant strain of Plasmodium falciparum . Lancet. 1994;343(8910):1396–1397. doi: 10.1016/s0140-6736(94)92526-7. [DOI] [PubMed] [Google Scholar]
- 11.Anderson S, Berman J, Kuschner R et al. Prophylaxis of Plasmodium falciparum malaria with azithromycin administered to volunteers . Ann. Intern. Med. 1995;123:771–773. doi: 10.7326/0003-4819-123-10-199511150-00005. [DOI] [PubMed] [Google Scholar]
- 12.Ohrt C, Willingmyre G, Lee P, Knirsch C. Milhous W. Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro . Antimicrob. Agents Chemother. 2002;46:2518–2524. doi: 10.1128/AAC.46.8.2518-2524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chico R. Mayaud P, Ariti C, Mabey D, Ronsmans C, Chandramohan D. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-saharan Africa . JAMA. 2012;307(19):2079–2086. doi: 10.1001/jama.2012.3428. [DOI] [PubMed] [Google Scholar]
- 14.Williams JW. A textbook of obstetrics. D Appleton & Co; New York, USA: 1923. [Google Scholar]
- 15.Ratnam AV, Din SN, Hira SK et al. Syphilis in pregnant women in Zambia . Br. J. Vener Dis. 1982;58(6):355–358. doi: 10.1136/sti.58.6.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C. Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage . BMJ. 1994;308(6924):295–298. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis . Am. J. Obstet. Gynecol. 2003;189(1):139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 18.Wilkowska-Trojniel M, Zdrodowska-Stefanow B, Ostaszewska-Puchalska I, Redzko S, Przepiesc J, Zdrodowski M. The influence of Chlamydia trachomatis infection on spontaneous abortions . Adv. Med. Sci. 2009;54(1):86–90. doi: 10.2478/v10039-009-0008-5. [DOI] [PubMed] [Google Scholar]
- 19.McDermott J, Steketee R, Larsen S. Wirima J. Syphilis-associated perinatal and infant mortality in rural Malawi . Bull. World Health Organ. 1993;71(6):773–780. [PMC free article] [PubMed] [Google Scholar]
- 20.Temmerman M, Gichangi P, Fonck K et al. Effect of a syphilis control programme on pregnancy outcome in Nairobi, Kenya . Sex. Transm. Infect. 2000;76(2):117–121. doi: 10.1136/sti.76.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson-Jones D CJ, Balthazar G, Weiss H et al. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J. Infect. Dis. 2002;186:940–947. doi: 10.1086/342952. [DOI] [PubMed] [Google Scholar]
- 22.Association of Chlamydia trachomatis and Mycoplasma hominis with intrauterine growth retardation and preterm delivery. The John Hopkins Study of Cervicitis and Adverse Pregnancy Outcome. Am. J. Epidemiol. 1989;129(6):1247–1257. doi: 10.1093/oxfordjournals.aje.a115244. [DOI] [PubMed] [Google Scholar]
- 23.Watson-Jones D, Changalucha J, Gumodoka B et al. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy . J. Infect. Dis. 2002;186(7):940–947. doi: 10.1086/342952. [DOI] [PubMed] [Google Scholar]
- 24.Gravett MG, Nelson HP, DeRouen T, Critchlow C, Eschenbach DA. Holmes KK. Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome . JAMA. 1986;256(14):1899–1903. [PubMed] [Google Scholar]
- 25.McGregor JA, French JI, Parker R et al. Prevention of premature birth by screening and treatment or common genital tract infections: results of a prospective controlled evaluation . Am. J. Obstet. Gynecol. 1995;173(1):157–167. doi: 10.1016/0002-9378(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 26.Blas MM, Canchihuaman FA, Alva IE, Hawes SE. Pregnancy outcomes in women infected with Chlamydia trachomatis: A population-based cohort study in Washington State . Sex. Transm. Infect. 2007;83(4):314–318. doi: 10.1136/sti.2006.022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott B, Brunham RC, Laga M et al. Maternal gonococcal infection as a preventable risk factor for low birth weight . J. Infect. Dis. 1990;161(3):531–536. doi: 10.1093/infdis/161.3.531. [DOI] [PubMed] [Google Scholar]
- 28.Cotch MF, Pastorek JG. Nugent RP et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The vaginal infections and prematurity study group . Sex. Transm. Dis. 1997;24(6):353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Svare JA, Schmidt H, Hansen BB, Lose G. Bacterial vaginosis in a cohort of Danish pregnant women: prevalence and relationship with preterm delivery, low birthweight and perinatal infections . BJOG. 2006;113(12):1419–1425. doi: 10.1111/j.1471-0528.2006.01087.x. [DOI] [PubMed] [Google Scholar]
- 30.Odendaal HJ, Schoeman J, Grove D et al. The association between Chlamydia trachomatis genital infection and spontaneous preterm labour. S. Afr. J. Obstet. Gynaecol. 2006;12(3):146–149. [Google Scholar]
- 31.Watson-Jones D, Weiss HA, Changalucha JM et al. Adverse birth outcomes in United Republic of Tanzania--impact and prevention of maternal risk factors . Bull. World Health Organ. 2007;85(1):9–18. doi: 10.2471/BLT.06.033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rours GI, Duijts L, Moll HA et al. Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study . Eur. J. Epidemiol. 2011;26(6):493–502. doi: 10.1007/s10654-011-9586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson HL, Ghanem KG, Zenilman JM, Erbelding EJ. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner city public sexually transmitted diseases clinics . Sex. Transm. Dis. 2011;38(3):167–171. doi: 10.1097/OLQ.0b013e3181f2e85f. [DOI] [PubMed] [Google Scholar]
- 34.Donders GG, Desmyter J, De Wet DH, Van Assche FA. The association of gonorrhoea and syphilis with premature birth and low birthweight . Genitourin. Med. 1993;69(2):98–101. doi: 10.1136/sti.69.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton MY, Sternberg M, Nsuami M, Behets F, Nelson AM, St Louis ME. Trichomoniasis in pregnant human immunodeficiency virus-infected and human immunodeficiency virus-uninfected congolese women: prevalence, risk factors, and association with low birth weight . Am J Obstet Gynecol. 1999;181(3):656–662. doi: 10.1016/s0002-9378(99)70509-0. [DOI] [PubMed] [Google Scholar]
- 36.Organization WH. Guidelines for the Management of Sexually Transmitted Infections. Switzerland: 2003. [Google Scholar]
- 37.Hook EW. Stephens J, Ennis DM. Azithromycin compared with penicillin G benzathine for treatment of incubating syphilis . Ann. Intern. Med. 1999;131(6):434–437. doi: 10.7326/0003-4819-131-6-199909210-00007. 3rd. [DOI] [PubMed] [Google Scholar]
- 38.Klausner JD, Kohn RP, Kent CK. Azithromycin versus penicillin for early syphilis . N. Engl. J. Med. 2006;354(2):203–205. 203–205. author reply. [PubMed] [Google Scholar]
- 39.Hook EW. Martin DH, Stephens J, Smith BS. Smith K. A randomized, comparative pilot study of azithromycin versus benzathine penicillin G for treatment of early syphilis . Sex. Transm. Dis. 2002;29(8):486–490. doi: 10.1097/00007435-200208000-00010. 3rd. [DOI] [PubMed] [Google Scholar]
- 40.Kiddugavu MG, Kiwanuka N, Wawer MJ et al. Effectiveness of syphilis treatment using azithromycin and/or benzathine penicillin in Rakai, Uganda . Sex. Transm. Dis. 2005;32(1):1–6. doi: 10.1097/01.olq.0000148297.48590.d8. [DOI] [PubMed] [Google Scholar]
- 41.Riedner G, Rusizoka M, Todd J et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis . N. Engl. J. Med. 2005;353(12):1236–1244. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 42.Hook EW. Behets F, Van Damme K et al. A phase III equivalence trial of azithromycin versus benzathine penicillin for treatment of early syphilis . J. Infect. Dis. 2010;201(11):1729–1735. doi: 10.1086/652239. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell SJ, Engelman J, Kent CK, Lukehart SA, Godornes C, Klausner JD. Azithromycin-resistant syphilis infection: San Francisco, California, 2000–2004 . Clin. Infect. Dis. 2006;42(3):337–345. doi: 10.1086/498899. [DOI] [PubMed] [Google Scholar]
- 44.Lukehart SA, Godornes C, Molini BJ et al. Macrolide resistance in Treponema pallidum in the United States and Ireland . N. Engl. J. Med. 2004;351(2):154–158. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 45.Marra CM, Colina AP, Godornes C et al. Antibiotic selection may contribute to increases in macrolide-resistant Treponema pallidum . J. Infect. Dis. 2006;194(12):1771–1773. doi: 10.1086/509512. [DOI] [PubMed] [Google Scholar]
- 46.Grimes M, Sahi SK, Godornes BC et al. Two mutations associated with macrolide resistance in Treponema pallidum: increasing prevalence and correlation with molecular strain type in Seattle, Washington . Sex. Transm. Dis. 2012;39(12):954–958. doi: 10.1097/OLQ.0b013e31826ae7a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen XS, Yin YP, Wei WH et al. High prevalence of azithromycin resistance to Treponema pallidum in geographically different areas in China. Clin. Microbiol. Infect. 2012;8(10):1469–0691. doi: 10.1111/1469-0691.12098. [DOI] [PubMed] [Google Scholar]
- 48.Wu H, Chang SY, Lee NY et al. Evaluation of macrolide resistance and enhanced molecular typing of Treponema pallidum in patients with syphilis in Taiwan: a prospective multicenter study . J. Clin. Microbiol. 2012;50(7):2299–2304. doi: 10.1128/JCM.00341-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Damme K, Behets F, Ravelomanana N et al. Evaluation of azithromycin resistance in Treponema pallidum specimens from Madagascar . Sex. Transm. Dis. 2009;36(12):775–776. doi: 10.1097/OLQ.0b013e3181bd11dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization Sexually transmitted and other reproductive tract infections: Guide to essential practice. World Health Organization; Geneva: 2005. Department of Reproductive Health and Research. [Google Scholar]
- 51.Odugbemi T, Oyewole F, Isichei CS, Onwukeme KE. Adeyemi-Doro FA. Single oral dose of azithromycin for therapy of susceptible sexually transmitted diseases: a multicenter open evaluation . West Afr. J. Med. 1993;12(3):136–140. [PubMed] [Google Scholar]
- 52.Waugh MA. Open study of the safety and efficacy of a single oral dose of azithromycin for the treatment of uncomplicated gonorrhoea in men and women . J. Antimicrob. Chemother. 1993;31(Suppl. E):193–198. doi: 10.1093/jac/31.suppl_e.193. [DOI] [PubMed] [Google Scholar]
- 53.Swanston WH, Prabhakar P, Barrow L, Mahabir BS, Furlonge C. Single dose (direct observed) azithromycin therapy for Neisseria gonorrhoeae and Chlamydia trachomatis in STD clinic attenders with genital discharge in Trinidad and Tobago . West Indian Med. J. 2001;50(3):198–202. [PubMed] [Google Scholar]
- 54.Lassus A. Comparative studies of azithromycin in skin and soft-tissue infections and sexually transmitted infections by Neisseria and Chlamydia species . J. Antimicrob. Chemother. 1990;25(Suppl. A):115–121. doi: 10.1093/jac/25.suppl_a.115. [DOI] [PubMed] [Google Scholar]
- 55.Steingrimsson O, Olafsson JH, Thorarinsson H, Ryan RW, Johnson RB. Tilton RC. Azithromycin in the treatment of sexually transmitted disease . J. Antimicrob. Chemother. 1990;25(Suppl. A):109–114. doi: 10.1093/jac/25.suppl_a.109. [DOI] [PubMed] [Google Scholar]
- 56.Steingrimsson O, Olafsson JH, Thorarinsson H, Ryan RW, Johnson RB. Tilton RC. Single dose azithromycin treatment of gonorrhea and infections caused by C. trachomatis and U. urealyticum in men . Sex. Transm. Dis. 1994;21(1):43–46. doi: 10.1097/00007435-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Gruber F, Grubisic-Greblo H, Jonjic A et al. Treatment of gonococcal and chlamydial urethritis with azithromycin or doxycycline. Chron. Derm. (Roma) 1995;5:213–218. [Google Scholar]
- 58.Gruber F, Brajac I, Jonjic A, Grubisic-Greblo H, Lenkovic M, Stasic A. Comparative trial of azithromycin and ciprofloxacin in the treatment of gonorrhea . J. Chemother. 1997;9(4):263–266. doi: 10.1179/joc.1997.9.4.263. [DOI] [PubMed] [Google Scholar]
- 59.Rustomjee R, Kharsany AB, Connolly CA, Karim SS. A randomized controlled trial of azithromycin versus doxycycline/ciprofloxacin for the syndromic management of sexually transmitted infections in a resource-poor setting . J. Antimicrob. Chemother. 2002;49(5):875–878. doi: 10.1093/jac/dkf034. [DOI] [PubMed] [Google Scholar]
- 60.DerSimonian R, Laird N. Meta-analysis in clinical trials . Control Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 61.Bignell C, Garley J. Azithromycin in the treatment of infection with Neisseria gonorrhoeae . Sex. Transm. Infect. 2010;86(6):422–426. doi: 10.1136/sti.2010.044586. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organization, London School of Hygiene and Tropical Medicine and International Trachoma Initiative Trachoma control: A guide for programme managers. World Health Organization; Switzerland: 2006. [Google Scholar]
- 63.Handsfield HH, Dalu ZA, Martin DH, Douglas JM, Jr, McCarty JM, Schlossberg D. Multicenter trial of single-dose azithromycin vs. ceftriaxone in the treatment of uncomplicated gonorrhea. Azithromycin Gonorrhea Study Group . Sex. Transm. Dis. 1994;21(2):107–111. doi: 10.1097/00007435-199403000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Khaki P, Bhalla P, Sharma A, Kumar V. Correlation between In vitro susceptibility and treatment outcome with azithromycin in gonorrhoea: a prospective study . Indian J. Med. Microbiol. 2007;25(4):354–357. doi: 10.4103/0255-0857.37338. [DOI] [PubMed] [Google Scholar]
- 65.Johnson SR, Sandul AL, Parekh M, Wang SA, Knapp JS, Trees DL. Mutations causing in vitro resistance to azithromycin in Neisseria gonorrhoeae . Int. J. Antimicrob. Agents. 2003;21(5):414–419. doi: 10.1016/s0924-8579(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 66.Martin I, Jayaraman G, Wong T, Liu G, Gilmour M. Laboratory N. Trends in antimicrobial resistance in Neisseria gonorrhoeae isolated in Canada: 2000-2009 . Sex. Transm. Dis. 2011;38(10):892–898. doi: 10.1097/OLQ.0b013e31822c664f. [DOI] [PubMed] [Google Scholar]
- 67.Martin I, Jayaraman G, Wong T, Liu G. Gilmour M. Trends in antimicrobial resistance in Neisseria gonorrhoeae isolated in Canada: 2000-2009 . Sex. Transm. Dis. 2011;38(10):892–898. doi: 10.1097/OLQ.0b013e31822c664f. [DOI] [PubMed] [Google Scholar]
- 68.Cole MJ, Chisholm SA, Hoffmann S et al. European surveillance of antimicrobial resistance in Neisseria gonorrhoeae . Sex. Transm. Infect. 2010;86(6):427–432. doi: 10.1136/sti.2010.044164. [DOI] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention Neisseria gonorrhoeae with reduced susceptibility to azithromycin --- San Diego County, California, 2009 . MMWR Morb. Mortal. Wkly Rep. 2011;60(18):579–581. [PubMed] [Google Scholar]
- 70.Starnino S, Galarza P, Carvallo ME et al. Retrospective analysis of antimicrobial susceptibility trends (2000-2009) in Neisseria gonorrhoeae isolates from countries in Latin America and the Caribbean shows evolving resistance to ciprofloxacin, azithromycin and decreased susceptibility to ceftriaxone . Sex. Transm. Dis. 2012;39(10):813–821. doi: 10.1097/OLQ.0b013e3182631c9f. [DOI] [PubMed] [Google Scholar]
- 71.Khaki P, Bhalla P, Sharma P, Chawla R, Bhalla K. Epidemilogical analysis of Neisseria gonorrhoeae isolates by antimicrobial susceptibility testing, auxotyping and serotyping . Indian J. Med. Microbiol. 2007;25(3):225–229. doi: 10.4103/0255-0857.34763. [DOI] [PubMed] [Google Scholar]
- 72.Sethi S, Golparian D, Bala M et al. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from India, Pakistan and Bhutan in 2007-2011 . BMC Infect. Dis. 2013;13:35. doi: 10.1186/1471-2334-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kacmar J, Cheh E, Montagno A. Peipert JF. A randomized trial of azithromycin versus amoxicillin for the treatment of Chlamydia trachomatis in pregnancy . Infect. Dis. Obstet. Gynecol. 2001;9(4):197–202. doi: 10.1155/S1064744901000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobson GF, Autry AM, Kirby RS, Liverman EM, Motley RU. A randomized controlled trial comparing amoxicillin and azithromycin for the treatment of Chlamydia trachomatis in pregnancy . Am. J. Obstet. Gynecol. 2001;184(7):1352–1354. 1354–1356. doi: 10.1067/mob.2001.115050. [DOI] [PubMed] [Google Scholar]
- 75.Wehbeh HA, Ruggeirio RM, Shahem S, Lopez G, Ali Y. Single-dose azithromycin for Chlamydia in pregnant women . J. Reprod. Med. 1998;43(6):509–514. [PubMed] [Google Scholar]
- 76.Adair CD, Gunter M, Stovall TG, McElroy G, Veille JC, Ernest JM. Chlamydia in pregnancy: a randomized trial of azithromycin and erythromycin . Obstet. Gynecol. 1998;91(2):165–168. doi: 10.1016/s0029-7844(97)00586-3. [DOI] [PubMed] [Google Scholar]
- 77.Gunter ME. Ernest JM, McElroy G. Azithromycin powder versus erythromycin in the treatment of chlamydial cervicitis in pregnancy. Infect. Dis. Obstet. Gynecol. 1996;4(53) doi: 10.1155/S1064744996000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edwards MS, Newman RB, Carter SG, Leboeuf FW, Menard MK, Rainwater KP. Randomized clinical trial of azithromycin vs. erythromycin for the treatment of chlamydia cervicitis in pregnancy . Infect. Dis. Obstet. Gynecol. 1996;4(6):333–337. doi: 10.1155/S1064744996000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosenn MF, Macones GA. Silverman NS. Randomized trial of erythromycin and azithromycin for treatment of chlamydial infection in pregnancy . Infect Dis Obstet Gynecol. 1995;3(6):241–244. doi: 10.1155/S1064744995000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bush MR. Rosa C. Azithromycin and erythromycin in the treatment of cervical chlamydial infection during pregnancy . Obstet. Gynecol. 1994;84(1):61–63. [PubMed] [Google Scholar]
- 81.Golden MR, Whittington WL, Handsfield HH et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection . N. Engl. J. Med. 2005;352(7):676–685. doi: 10.1056/NEJMoa041681. [DOI] [PubMed] [Google Scholar]
- 82.Katz BP FD, Orr DP. Stephens RS, Byrne GI, Christiansen G Factors affecting chlamydial persistence or recurrence one and three months after treatment. Chlamydial infections. Proceedings of the ninth international symposium on human chlamydial infection. 1998. pp. 35–38. Eds.
- 83.Horner P. The case for further treatment studies of uncomplicated genital Chlamydia trachomatis infection . Sex. Transm. Infect. 2006;82(4):340–343. doi: 10.1136/sti.2005.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Samra Z, Rosenberg S, Soffer Y, Dan M. In vitro susceptibility of recent clinical isolates of Chlamydia trachomatis to macrolides and tetracyclines. Diag. Microbiol. Infect. Dis. 2001;39(3):177–179. doi: 10.1016/s0732-8893(01)00221-8. [DOI] [PubMed] [Google Scholar]
- 85.Somani J, Bhullar VB, Workowski KA, Farshy CE, Black CM. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure . J. Infect. Dis. 2000;181(4):1421–1427. doi: 10.1086/315372. [DOI] [PubMed] [Google Scholar]
- 86.Lefevre JC. Lepargneur JP. Comparative in vitro susceptibility of a tetracycline-resistant Chlamydia trachomatis strain isolated in Toulouse (France) . Sex. Transm. Dis. 1998;25(7):350–352. doi: 10.1097/00007435-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 87.Jones RB, Van der Pol B, Martin DH, Shepard MK. Partial characterization of Chlamydia trachomatis isolates resistant to multiple antibiotics . J. Infect. Dis. 1990;162(6):1309–1315. doi: 10.1093/infdis/162.6.1309. [DOI] [PubMed] [Google Scholar]
- 88.Dreses-Werringloer U, Padubrin I, Zeidler H, Kohler L. Effects of azithromycin and rifampin on Chlamydia trachomatis infection in vitro . Antimicrob. Agents Chemother. 2001;45(11):3001–3008. doi: 10.1128/AAC.45.11.3001-3008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Donati M. Fermepin M, Olmo A, D'Apote L, Cevenini R. Comparative in-vitro activity of moxifloxacin, minocycline and azithromycin against Chlamydia spp . J. Antimicrob. Chemother. 1999;43(6):825–827. doi: 10.1093/jac/43.6.825. [DOI] [PubMed] [Google Scholar]
- 90.Misyurina OY, Chipitsyna EV, Finashutina YP et al. Mutations in a 23S rRNA gene of Chlamydia trachomatis associated with resistance to macrolides . Antimicrob. Agents Chemother. 2004;48(4):1347–1349. doi: 10.1128/AAC.48.4.1347-1349.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rice RJ, Bhullar V, Mitchell SH, Bullard J, Knapp JS. Susceptibilities of Chlamydia trachomatis isolates causing uncomplicated female genital tract infections and pelvic inflammatory disease . Antimicrob. Agents Chemother. 1995;39(3):760–762. doi: 10.1128/AAC.39.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhengraj AR, Vardhan H, Srivastava P, Salhan S, Mittal A. Decreased susceptibility to azithromycin and doxycycline in clinical isolates of Chlamydia trachomatis obtained from recurrently infected female patients in India . Chemotherapy. 2010;56(5):371–377. doi: 10.1159/000314998. [DOI] [PubMed] [Google Scholar]
- 93.Kaul R, Kimani J, Nagelkerke NJ et al. Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: a randomized controlled trial . JAMA. 2004;291(21):2555–2562. doi: 10.1001/jama.291.21.2555. [DOI] [PubMed] [Google Scholar]
- 94.Luntamo M, Kulmala T, Mbewe B, Cheung YB, Maleta K, Ashorn P. Effect of repeated treatment of pregnant women with sulfadoxine-pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial . Am. J. Trop. Med. Hyg. 2010;83(6):1212–1220. doi: 10.4269/ajtmh.2010.10-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wawer MJ, Sewankambo NK, Serwadda D et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group . Lancet. 1999;353(9152):525–535. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 96.Cowan FM, Hargrove JW, Langhaug LF et al. The appropriateness of core group interventions using presumptive periodic treatment among rural Zimbabwean women who exchange sex for gifts or money . J. Acquir. Immune Defic. Syndr. 2005;38(2):202–207. doi: 10.1097/00126334-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 97.Hay PE. Therapy of bacterial vaginosis . J. Antimicrob. Chemother. 1998;41(1):6–9. doi: 10.1093/jac/41.1.6. [DOI] [PubMed] [Google Scholar]
- 98.Eschenbach DA. Bacterial vaginosis: resistance, recurrence, and/or reinfection? . Clin. Infect. Dis. 2007;44(2):220–221. doi: 10.1086/509584. [DOI] [PubMed] [Google Scholar]
- 99.Schwebke JR. Desmond RA. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis . Clin. Infect. Dis. 2007;44(2):213–219. doi: 10.1086/509577. [DOI] [PubMed] [Google Scholar]
- 100.Cohen MS, Hoffman IF, Royce RA et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group . Lancet. 1997;349(9069):1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 101.McClelland RS, Wang CC, Mandaliya K et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS (Lond., Engl.) 2001;15(1):105–110. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 102.Wang CC, McClelland RS, Reilly M et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1 . J. Infect. Dis. 2001;183(7):1017–1022. doi: 10.1086/319287. [DOI] [PubMed] [Google Scholar]
- 103.Price MA, Zimba D, Hoffman IF et al. Addition of treatment for trichomoniasis to syndromic management of urethritis in Malawi: a randomized clinical trial . Sex. Transm. Dis. 2003;30(6):516–522. doi: 10.1097/00007435-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 104.Gray RH, Wabwire-Mangen F, Kigozi G et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda . Am. J. Obstet. Gynecol. 2001;185(5):1209–1217. doi: 10.1067/mob.2001.118158. [DOI] [PubMed] [Google Scholar]
- 105.Aires FT, Soares RP. Bernardo WM. Efficacy of azithromycin on the treatment of syphilis . Rev. Assoc. Med. Bras. 2010;56(5):496. doi: 10.1590/s0104-42302010000500002. [DOI] [PubMed] [Google Scholar]
- 106.Zhou P, Qian Y, Xu J, Gu Z. Liao K. Occurrence of congenital syphilis after maternal treatment with azithromycin during pregnancy . Sex. Transm. Dis. 2007;34(7):472–474. doi: 10.1097/01.olq.0000246314.35047.91. [DOI] [PubMed] [Google Scholar]
- 107.Luntamo M, Kulmala T, Mbewe B, Cheung Y B, Maleta K, Ashorn P. Effect of Repeated Treatment of Pregnant Women with Sulfadoxine-Pyrimethamine and Azithromycin on Preterm Delivery in Malawi: A Randomized Controlled Trial . Am. J. Trop. Med. Hyg. 2010;83:1212–1220. doi: 10.4269/ajtmh.2010.10-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kigozi GG, Brahmbhatt H, Wabwire-Mangen F et al. Treatment of Trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda . Am. J. Obstet. Gynecol. 2003;189(5):1398–1400. doi: 10.1067/s0002-9378(03)00777-4. [DOI] [PubMed] [Google Scholar]
- 109.Klebanoff MA, Carey JC, Hauth JC et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection . N. Engl. J. Med. 2001;345(7):487–493. doi: 10.1056/NEJMoa003329. [DOI] [PubMed] [Google Scholar]
- 110.Stringer E, Read JS, Hoffman I, Valentine M, Aboud S, Goldenberg RL. Treatment of trichomoniasis in pregnancy in sub-Saharan Africa does not appear to be associated with low birth weight or preterm birth. Samj South Afr. Med. J. 2010;100(1):58–64. [PMC free article] [PubMed] [Google Scholar]
- 111.Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst. Rev. 2013;1:CD000262. doi: 10.1002/14651858.CD000262.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation . J. Clin. Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.The use of rapid syphilis tests. Geneva: 2006. World Health Organization and Special Programme for Research and Training in Tropical Diseases. WHO. [Google Scholar]
- 114.World Health Organization Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections: Overview and Estimates. World Health Organization; Geneva: 2001. [Google Scholar]
- 115.Mayaud P, ka-Gina G, Cornelissen J et al. Validation of a WHO algorithm with risk assessment for the clinical management of vaginal discharge in Mwanza, Tanzania . Sex. Transm. Infect. 1998;74(Suppl. 74):S77–S84. [PubMed] [Google Scholar]
- 116.Vuylsteke B, Laga M, Alary M et al. Clinical algorithms for the screening of women for gonococcal and chlamydial infection: evaluation of pregnant women and prostitutes in Zaire . Clin. Infect. Dis. 1993;17(1):82–88. doi: 10.1093/clinids/17.1.82. [DOI] [PubMed] [Google Scholar]
- 117.Costello Daly C, Wangel AM, Hoffman IF et al. Validation of the WHO diagnostic algorithm and development of an alternative scoring system for the management of women presenting with vaginal discharge in Malawi. Sex. Transm. Infect. 1998;74(Suppl. 74):S50–S58. [PubMed] [Google Scholar]
- 118.Tann CJ, Mpairwe H, Morison L et al. Lack of effectiveness of syndromic management in targeting vaginal infections in pregnancy in Entebbe, Uganda . Sex. Transm. Infect. 2006;82(4):285–289. doi: 10.1136/sti.2005.014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chico RM, Ariti C, Cano J, Chandramohan D, Greenwood B. Malaria transmission intensity and the protective effect of intermittent preventive therapy using sulphadoxine-pyrimethamine. Evidence Review Group of the World Health Organization. 2013.
- 120.Temmerman M, Njagi E, Nagelkerke N, Ndinya-Achola J, Plummer FA, Meheus A. Mass antimicrobial treatment in pregnancy. A randomized, placebo-controlled trial in a population with high rates of sexually transmitted diseases . J. Reprod. Med. 1995;40(3):176–180. [PubMed] [Google Scholar]
- 121.Elliott B, Brunham RC, Laga M et al. Maternal gonococcal infection as a preventable risk factor for low birth weight . J. Infect. Dis. 1990;161(3):531–536. doi: 10.1093/infdis/161.3.531. [DOI] [PubMed] [Google Scholar]
- 122.Johnson HL, Ghanem KG, Zenilman JM, Erbelding EJ. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner city public sexually transmitted diseases clinics . Sex. Transm. Dis. 2011;38(3):167–171. doi: 10.1097/OLQ.0b013e3181f2e85f. [DOI] [PubMed] [Google Scholar]
- 123.Silveira MF, Ghanem KG, Erbelding EJ et al. Chlamydia trachomatis infection during pregnancy and the risk of preterm birth: A case-control study . Int. J. STD AIDS. 2009;20(7):465–469. doi: 10.1258/ijsa.2008.008388. [DOI] [PubMed] [Google Scholar]
- 124.Blas MM, Canchihuaman FA, Alva IE, Hawes SE. Pregnancy outcomes in women infected with Chlamydia trachomatis: A population-based cohort study in Washington State . Sex. Transm. Infect. 2007;83(4):314–318. doi: 10.1136/sti.2006.022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kovacs L, Nagy E, Berbik I, Meszaros G, Deak J, Nyari T. The frequency and the role of Chlamydia trachomatis infection in premature labor . Int. J. Gynaecol. Obstet. 1998;62(1):47–54. doi: 10.1016/s0020-7292(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 126.Investigators of the Johns Hopkins Study of Cervicitis and Adverse Pregnancy Outcome. Association of Chlamydia trachomatis and Mycoplasma hominis with intrauterine growth retardation and preterm delivery. Am. J. Epidemiol. 1989;129:1247–1257. doi: 10.1093/oxfordjournals.aje.a115244. [DOI] [PubMed] [Google Scholar]
- 127.Meis PJ, Goldenberg RL, Mercer B et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal M edicine Units Network . Am. J. Obstet. Gynecol. 1995;173(4):1231–1235. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- 128.Minkoff H, Grunebaum AN, Schwarz RH et al. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy . Am. J. Obstet. Gynecol. 1984;150(8):965–972. doi: 10.1016/0002-9378(84)90392-2. [DOI] [PubMed] [Google Scholar]
- 129.Hillier SL, Nugent RP, Eschenbach DA et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group . N. Engl. J. Med. 1995;333(26):1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 130.Kiddugavu MG, Kiwanuka N, Wawer MJ et al. Effectiveness of syphilis treatment using azithromycin and/or benzathine penicillin in Rakai, Uganda . Sex. Transm. Dis. 2005;32(1):1–6. doi: 10.1097/01.olq.0000148297.48590.d8. [DOI] [PubMed] [Google Scholar]
- 131.Hook EW. Stephens J, Ennis DM. Azithromycin compared with penicillin G benzathine for treatment of incubating syphilis. Ann. Int. Med. 1999;131(6):434–437. doi: 10.7326/0003-4819-131-6-199909210-00007. 3rd. [DOI] [PubMed] [Google Scholar]
- 132.Riedner G, Rusizoka M, Todd J et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis . N. Engl. J. Med. 2005;353(12):1236–1244. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 133.Chen CY, Chi KH, Pillay A, Nachamkin E, Su JR, Ballard RC. Detection of the A2058G and A2059G 23S rRNA gene point mutations associated with azithromycin resistance in Treponema pallidum by use of a TaqMan real-time multiplex PCR assay . J. Clin. Microbiol. 2013;51(3):908–913. doi: 10.1128/JCM.02770-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Muller EE, Paz-Bailey G. Lewis DA. Macrolide resistance testing and molecular subtyping of Treponema pallidum strains from southern Africa . Sex. Transm. Infect. 2012;88(6):470–474. doi: 10.1136/sextrans-2011-050322. [DOI] [PubMed] [Google Scholar]
- 135.The A2058G Prevalence Workgroup Prevalence of the 23S rRNA A2058G point mutation and molecular subtypes in Treponema pallidum in the United States, 2007 to 2009. Sex. Transm. Dis. 2012;39(10):794–798. doi: 10.1097/OLQ.0b013e31826f36de. [DOI] [PubMed] [Google Scholar]
- 136.Matejkova P, Flasarova M, Zakoucka H et al. Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum . J. Med. Microbiol. 2009;58(Pt 6):832–836. doi: 10.1099/jmm.0.007542-0. [DOI] [PubMed] [Google Scholar]
- 137.Martin IE, Gu W, Yang Y, Tsang RS. Macrolide resistance and molecular types of Treponema pallidum causing primary syphilis in Shanghai, China . Clin. Infect. Dis. 2009;49(4):515–521. doi: 10.1086/600878. [DOI] [PubMed] [Google Scholar]
- 138.Tipple C, McClure MO, Taylor GP. High prevalence of macrolide resistant Treponema pallidum strains in a London centre . Sex. Transm. Infect. 2011;87(6):486–488. doi: 10.1136/sextrans-2011-050082. [DOI] [PubMed] [Google Scholar]
- 139.Rekart ML, Patrick DM, Chakraborty B et al. Targeted mass treatment for syphilis with oral azithromycin . Lancet. 2003;361(9354):313–314. doi: 10.1016/s0140-6736(03)12335-5. [DOI] [PubMed] [Google Scholar]
- 140.Muldoon EG, Walsh A, Crowley B, Mulcahy F. Treponema pallidum azithromycin resistance in Dublin, Ireland . Sex. Transm. Dis. 2012;39(10):784–786. doi: 10.1097/OLQ.0b013e318269995f. [DOI] [PubMed] [Google Scholar]
- 141.Martin IE, Tsang RS, Sutherland K et al. Molecular typing of Treponema pallidum strains in western Canada: predominance of 14d subtypes . Sex. Transm. Dis. 2010;37(9):544–548. doi: 10.1097/OLQ.0b013e3181d73ce1. [DOI] [PubMed] [Google Scholar]
- 142.Morshed MG. Jones HD. Treponema pallidum macrolide resistance in BC . CMAJ. 2006;174(3):349. doi: 10.1503/cmaj.1050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Khaki P, Bhalla P, Sharma A, Kumar V. Correlation between In vitro susceptibility and treatment outcome with azithromycin in gonorrhoea: a prospective study . Indian J. Med. Microbiol. 2007;25(4):354–357. doi: 10.4103/0255-0857.37338. [DOI] [PubMed] [Google Scholar]
- 144.Olsen B, Pham TL, Golparian D, Johansson E, Tran HK, Unemo M. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011 . BMC Infect. Dis. 2013;13:40. doi: 10.1186/1471-2334-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lahra MM. Annual report of the Australian Gonococcal Surveillance Programme, 2011 . Commun. Dis. Intell. Q Rep. 2012;36(2):E166–E173. doi: 10.33321/cdi.2012.36.11. [DOI] [PubMed] [Google Scholar]
- 146.Lo JY, Ho KM, Lo AC. Surveillance of gonococcal antimicrobial susceptibility resulting in early detection of emerging resistance . J. Antimicrob. Chemother. 2012;67(6):1422–1426. doi: 10.1093/jac/dks036. [DOI] [PubMed] [Google Scholar]
- 147.Lefebvre B, Bourgault AM. P1-S1.44 Antimicrobial susceptibility profile of Neisseria gonorrhoeae isolates in the Province of Quebec - 2010. Sex. Transm. Infect. 2011;87(Suppl. 87):A117. [Google Scholar]
- 148.Hottes TS, Lester RT, Hoang LM et al. Cephalosporin and azithromycin susceptibility in Neisseria gonorrhoeae isolates by site of infection, British Columbia, 2006 to 2011 . Sex. Transm. Dis. 2013;40(1):46–51. doi: 10.1097/OLQ.0b013e31827bd64c. [DOI] [PubMed] [Google Scholar]
- 149.Yuan LF, Yin YP, Dai XQ et al. Resistance to azithromycin of Neisseria gonorrhoeae isolates from 2 cities in China . Sex. Transm. Dis. 2011;38(8):764–768. doi: 10.1097/OLQ.0b013e318219cdb5. [DOI] [PubMed] [Google Scholar]
- 150.Takahashi S, Kurimura Y, Hashimoto J et al. Antimicrobial susceptibility and penicillin-binding protein 1 and 2 mutations in Neisseria gonorrhoeae isolated from male urethritis in Sapporo, Japan . J. Infect. Chemother. 2013;19(1):50–56. doi: 10.1007/s10156-012-0450-3. [DOI] [PubMed] [Google Scholar]
- 151.Herchline TE, Inkrott BP. Resistance trends in Neisseria gonorrhoeae in southwestern Ohio . Sex. Transm. Dis. 2010;37(2):121–122. doi: 10.1097/OLQ.0b013e3181be356e. [DOI] [PubMed] [Google Scholar]
- 152.Olsen B, Mansson F, Camara C et al. Phenotypic and genetic characterisation of bacterial sexually transmitted infections in Bissau, Guinea-Bissau, West Africa: a prospective cohort study . BMJ Open. 2012;2(2):e000636. doi: 10.1136/bmjopen-2011-000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tanaka M, Koga Y, Nakayama H et al. Antibiotic-resistant phenotypes and genotypes of Neisseria gonorrhoeae isolates in Japan: identification of strain clusters with multidrug-resistant phenotypes . Sex. Transm. Dis. 2011;38(9):871–875. doi: 10.1097/OLQ.0b013e31821d0f98. [DOI] [PubMed] [Google Scholar]
- 154.Bala M. Characterization of profile of multidrug-resistant Neisseria gonorrhoeae using old and new definitions in India over a decade: 2000–2009 . Sex. Transm. Dis. 2011;38(11):1056–1058. doi: 10.1097/OLQ.0b013e31822e6361. [DOI] [PubMed] [Google Scholar]
- 155.Chisholm SA, Neal TJ, Alawattegama AB, Birley HD, Howe RA, Ison CA. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales . J. Antimicrob. Chemother. 2009;64(2):353–358. doi: 10.1093/jac/dkp188. [DOI] [PubMed] [Google Scholar]
- 156.Khaki P, Bhalla P, Sharma P, Chawla R, Bhalla K. Epidemilogical analysis of Neisseria gonorrhoeae isolates by antimicrobial susceptibility testing, auxotyping and serotyping. Indian J. Med. Microbiol. 2007. pp. 225–229. [DOI] [PubMed]
- 157.Enders M, Turnwald-Maschler A, Regnath T. Antimicrobial resistance of Neisseria gonorrhoeae isolates from the Stuttgart and Heidelberg areas of southern Germany . Eur. J. Clin. Microbiol. Infect. Dis. 2006;25(5):318–322. doi: 10.1007/s10096-006-0134-y. [DOI] [PubMed] [Google Scholar]
- 158.Vorobieva V, Firsova N, Ababkova T et al. Antibiotic susceptibility of Neisseria gonorrhoeae in Arkhangelsk, Russia . Sex. Transm. Infect. 2007;83(2):133–135. doi: 10.1136/sti.2006.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sutrisna A, Soebjakto O, Wignall FS et al. Increasing resistance to ciprofloxacin and other antibiotics in Neisseria gonorrhoeae from East Java and Papua, Indonesia, in 2004 - implications for treatment. J. Clin. Pathol. 2007;60(1):90–91. doi: 10.1258/095646206779307595. [DOI] [PubMed] [Google Scholar]
- 160.Martin IM, Hoffmann S, Ison CA. European Surveillance of Sexually Transmitted Infections (ESSTI): the first combined antimicrobial susceptibility data for Neisseria gonorrhoeae in Western Europe . J. Antimicrob. Chemother. 2006;58(3):587–593. doi: 10.1093/jac/dkl265. [DOI] [PubMed] [Google Scholar]
- 161.Chaudhary C, Hasan Chaudhary FA, Pandy AR et al. A pilot study on antimicrobial susceptibility of Neisseria gonorrhoeae isolates from Nepal . Sex. Transm. Dis. 2005;32(10):641–643. doi: 10.1097/01.olq.0000179906.57604.e3. [DOI] [PubMed] [Google Scholar]
- 162.Chen PL, Hsieh YH, Lee HC et al. Suboptimal therapy and clinical management of gonorrhoea in an area with high-level antimicrobial resistance . Int. J. STD AIDS. 2009;20(4):225–228. doi: 10.1258/ijsa.2008.008286. [DOI] [PubMed] [Google Scholar]
- 163.Hsueh PR, Tseng SP, Teng LJ, Ho SW. High prevalence of ciprofloxacin-resistant Neisseria gonorrhoeae in Northern Taiwan . Clin. Infect. Dis. 2005;40(1):188–192. doi: 10.1086/426142. [DOI] [PubMed] [Google Scholar]
- 164.Aydin D, Kucukbasmaci O, Gonullu N, Aktas Z. Susceptibilities of Neisseria gonorrhoeae and Ureaplasma urealyticum isolates from male patients with urethritis to several antibiotics including telithromycin. Clin. Infect. Dis. 2005;40(11):1608–1616. doi: 10.1159/000085616. [DOI] [PubMed] [Google Scholar]
- 165.Moodley P, Pillay C, Goga R, Kharsany AB, Sturm AW. Evolution in the trends of antimicrobial resistance in Neisseria gonorrhoeae isolated in Durban over a 5 year period: impact of the introduction of syndromic management . J. Antimicrob. Chemother. 2001;48(6):853–859. doi: 10.1093/jac/48.6.853. [DOI] [PubMed] [Google Scholar]
- 166.Kobayashi I, Kanayama A, Saika T et al. Tendency toward increase in the frequency of isolation of beta-lactamase-nonproducing Neisseria gonorrhoeae exhibiting penicillin resistance, and recent emergence of multidrug-resistant isolates in Japan. Pediatrics. 2003;112(1 Pt 1):87–95. doi: 10.1007/s10156-002-0229-z. [DOI] [PubMed] [Google Scholar]
- 167.Llanes R, Sosa J, Guzman D et al. Antimicrobial susceptibility of Neisseria gonorrhoeae in Cuba (1995-1999): implications for treatment of gonorrhea. Sex. Transm. Dis. 2003;30(1):25–29. doi: 10.1097/00007435-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 168.Sosa J, Ramirez-Arcos S, Ruben M et al. High percentages of resistance to tetracycline and penicillin and reduced susceptibility to azithromycin characterize the majority of strain types of Neisseria gonorrhoeae isolates in Cuba, 1995-1998 . Sex. Transm. Dis. 2003;30(5):443–448. doi: 10.1097/00007435-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 169.Dillon JA, Rubabaza JP, Benzaken AS et al. Reduced susceptibility to azithromycin and high percentages of penicillin and tetracycline resistance in Neisseria gonorrhoeae isolates from Manaus, Brazil, 1998 . Sex. Transm. Dis. 2001;28(9):521–526. doi: 10.1097/00007435-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 170.Zarantonelli L, Borthagaray G, Lee EH, Shafer WM. Decreased azithromycin susceptibility of Neisseria gonorrhoeae due to mtrR mutations. J. Antimicrob. Chemother. 1999;44(3):411–414. doi: 10.1128/aac.43.10.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Young H, Moyes A, McMillan A. Azithromycin and erythromycin resistant Neisseria gonorrhoeae following treatment with azithromycin. J. Antimicrob. Chemother. 1997;39(5):623–630. doi: 10.1258/0956462971920127. [DOI] [PubMed] [Google Scholar]
- 172.Mehaffey PC, Putnam SD, Barrett MS, Jones RN. Evaluation of in vitro spectra of activity of azithromycin, clarithromycin, and erythromycin tested against strains of Neisseria gonorrhoeae by reference agar dilution, disk diffusion, and Etest methods. Clin. Infect. Dis. 1996;22(2):233–239. doi: 10.1128/jcm.34.2.479-481.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dillon JA, Li H, Sealy J, Ruben M, Prabhakar P. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates from three Caribbean countries: Trinidad, Guyana, and St. Vincent . Sex. Transm. Dis. 2001;28(9):508–514. doi: 10.1097/00007435-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 174.van Rijsoort-Vos JH, Stolz E, Verbrugh HA, Kluytmans JA. In-vitro activity of a new quinolone (CP-99,219) compared with ciprofloxacin, pefloxacin, azithromycin and penicillin against Neisseria gonorrhoeae . J. Antimicrob. Chemother. 1995;36(1):215–218. doi: 10.1093/jac/36.1.215. [DOI] [PubMed] [Google Scholar]
- 175.Ison CA, Roope NS, Dangor Y, Radebe F, Ballard R. Antimicrobial susceptibilities and serotyping of Neisseria gonorrhoeae in southern Africa: influence of geographical source of infection . Epidemiol. Infect. 1993;110(2):297–305. doi: 10.1017/s0950268800068230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Starnino S, Stefanelli P. Azithromycin-resistant Neisseria gonorrhoeae strains recently isolated in Italy . J. Antimicrob. Chemother. 2009;63(6):1200–1204. doi: 10.1093/jac/dkp118. [DOI] [PubMed] [Google Scholar]
- 177.Donegan EA, Wirawan DN, Muliawan P et al. Fluoroquinolone-resistant Neisseria gonorrhoeae in Bali, Indonesia: 2004 . Sex. Transm. Dis. 2006;33(10):625–629. doi: 10.1097/01.olq.0000216012.83990.bd. [DOI] [PubMed] [Google Scholar]
- 178.Morris SR, Moore DF, Hannah PB et al. Strain typing and antimicrobial resistance of fluoroquinolone-resistant Neisseria gonorrhoeae causing a California infection outbreak . J. Clin. Microbiol. 2009;47(9):2944–2949. doi: 10.1128/JCM.01001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ieven M, Van Looveren M, Sudigdoadi S et al. Antimicrobial susceptibilities of Neisseria gonorrhoeae strains isolated in Java, Indonesia . Sex. Transm. Dis. 2003;30(1):25–29. doi: 10.1097/00007435-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 180.Centers for Disease C and Prevention Fluoroquinolone-resistance in Neisseria gonorrhoeae, Hawaii, 1999, and decreased susceptibility to azithromycin in N. gonorrhoeae, Missouri, 1999. MMWR Morb. Mortal. Wkly Rep. 2000;49(37):833–837. [PubMed] [Google Scholar]
- 181.Bruck PE, Robertson C, Allan PS. Management of Neisseria gonorrhoeae infection over 12 months in a genitourinary medicine setting against British Association for Sexual Health and HIV auditable outcome measures . Int. J. STD AIDS. 2012;23(3):e30–e32. doi: 10.1258/ijsa.2009.009185. [DOI] [PubMed] [Google Scholar]
- 182.Dan M, Mor Z, Gottliev S, Sheinberg B, Shohat T. Trends in antimicrobial susceptibility of Neisseria gonorrhoeae in Israel, 2002 to 2007, with special reference to fluoroquinolone resistance . Sex. Transm. Dis. 2010;37(7):451–453. doi: 10.1097/OLQ.0b013e3181cfca06. [DOI] [PubMed] [Google Scholar]
- 183.McLean CA, Wang SA, Hoff GL et al. The emergence of Neisseria gonorrhoeae with decreased susceptibility to Azithromycin in Kansas City, Missouri, 1999 to 2000 . Sex. Transm. Dis. 2004;31(2):73–78. doi: 10.1097/01.OLQ.0000109514.91508.FC. [DOI] [PubMed] [Google Scholar]
- 184.Arreaza L, Vazquez F, Alcala B, Otero L, Salcedo C, Vazquez JA. Emergence of gonococcal strains with resistance to azithromycin in Spain . J. Antimicrob. Chemother. 2003;51(1):190–191. doi: 10.1093/jac/dkg027. [DOI] [PubMed] [Google Scholar]
- 185.Rahangdale L, Guerry S, Bauer HM et al. An observational cohort study of Chlamydia trachomatis treatment in pregnancy . Sex. Transm. Dis. 2006;33(2):106–110. doi: 10.1097/01.olq.0000187226.32145.ea. [DOI] [PubMed] [Google Scholar]
- 186.Miller JM. Efficacy and tolerance of single-dose azithromycin for treatment of chlamydial cervicitis during pregnancy . Infect. Dis. Obstet. Gynecol. 1995;3(5):189–192. doi: 10.1155/S1064744995000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ljubin-Sternak S, Mestrovic T, Vilibic-Cavlek T et al. In vitro susceptibility of urogenital Chlamydia trachomatis strains in a country with high azithromycin consumption rate. Folia Microbiol. 2012;29:29. doi: 10.1007/s12223-012-0218-2. [DOI] [PubMed] [Google Scholar]
- 188.Donati M, Di Francesco A, D'Antuono A et al. In vitro activities of several antimicrobial agents against recently isolated and genotyped Chlamydia trachomatis urogenital serovars D through K . Antimicrob. Agents Chemother. 2010;54(12):5379–5380. doi: 10.1128/AAC.00553-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hong KC, Schachter J, Moncada J, Zhou Z, House J, Lietman TM. Lack of macrolide resistance in Chlamydia trachomatis after mass azithromycin distributions for trachoma . Emerg. Infect. Dis. 2009;15(7):1088–1090. doi: 10.3201/eid1507.081563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lefevre JC, Escaffre MC, Courdil M, Lareng MB. In vitro evaluation of activities of azithromycin, clarithromycin and sparfloxacin against Chlamydia trachomatis . Pathol. Biol. 1993;41(4):313–315. [PubMed] [Google Scholar]
- 191.Agacfidan A, Moncada J, Schachter J. In vitro activity of azithromycin (CP-62,993) against Chlamydia trachomatis and Chlamydia pneumoniae . Antimicrob. Agents Chemother. 1993;37(9):1746–1748. doi: 10.1128/aac.37.9.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Scieux C, Bianchi A, Chappey B, Vassias I, Perol Y. In-vitro activity of azithromycin against Chlamydia trachomatis. J. Antimicrob. Chemother. 1990. pp. 7–10. [DOI] [PubMed]
- 193.Somani J, Bhullar VB, Workowski KA, Farshy CE, Black CM. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure . J. Infect. Dis. 2000;181(4):1421–1427. doi: 10.1086/315372. [DOI] [PubMed] [Google Scholar]
- 194.van den Broek NR, White SA, Goodall M et al. The APPLe Study: A Randomized, Community-Based, Placebo-Controlled T rial of Azithromycin for the Prevention of Preterm Birth, with Meta-Analysis . PLoS Med. 2009;6(12):e1000191. doi: 10.1371/journal.pmed.1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Luntamo M, Kulmala T, Cheung YB, Maleta K, Ashorn P. The effect of antenatal monthly sulphadoxine-pyrimethamine, alone or with azithromycin, on foetal and neonatal growth faltering in Malawi: a randomised controlled trial . Trop. Med. Int. Health. 2013;18(4):386–397. doi: 10.1111/tmi.12074. [DOI] [PubMed] [Google Scholar]
- 196.Chico RM, Chandramohan D. Azithromycin plus chloroquine: combination therapy for protection against malaria and sexually transmitted infections in pregnancy . Expert Opin. Drug Metab. Toxicol. 2011;7(9):1153–1167. doi: 10.1517/17425255.2011.598506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Labbe AC, Pepin J, Khonde N et al. Periodical antibiotic treatment for the control of gonococcal and chlamydial infections among sex workers in Benin and Ghana: a cluster-randomized placebo-controlled trial . Sex. Transm. Dis. 2012;39(4):253–259. doi: 10.1097/OLQ.0b013e318244aaa0. [DOI] [PubMed] [Google Scholar]
- 198.Wi T, Ramos ER, Steen R et al. STI declines among sex workers and clients following outreach, one time presumptive treatment, and regular screening of sex workers in the Philippines . Sex. Transm. Infect. 2006;82(5):386–391. doi: 10.1136/sti.2005.018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Williams BG, Taljaard D, Campbell CM et al. Changing patterns of knowledge, reported behaviour and sexually transmitted infections in a South African gold mining community . AIDS. 2003;17(14):2099–2107. doi: 10.1097/00002030-200309260-00011. [DOI] [PubMed] [Google Scholar]
- 200.Steen R, Vuylsteke B, DeCoito T et al. Evidence of declining STD prevalence in a South African mining community following a core-group intervention . Sex. Transm. Dis. 2000;27(1):1–8. doi: 10.1097/00007435-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 201.Jones BM, Kinghorn GR, Duerden BI. In vitro activity of azithromycin and erythromycin against organisms associated with bacterial vaginosis and chancroid . Eur. J. Clin. Microbiol. Infect. Dis. 1988;7(4):551–553. doi: 10.1007/BF01962614. [DOI] [PubMed] [Google Scholar]
- 202.Shanker S, Toohey M, Munro R. In vitro activity of seventeen antimicrobial agents against Gardnerella vaginalis . Eur. J. Clin. Microbiol. 1982;1(5):298–300. doi: 10.1007/BF02019975. [DOI] [PubMed] [Google Scholar]
- 203.Ridgway GL. A review of the in-vitro activity of roxithromycin against genital pathogens . J. Antimicrob. Chemother. 1987;20(Suppl. B):7–11. doi: 10.1093/jac/20.suppl_b.7. [DOI] [PubMed] [Google Scholar]
- 204.Dubreuil L. In-vitro comparison of roxithromycin and erythromycin against 900 anaerobic bacterial strains . J. Antimicrob. Chemother. 1987;20(Suppl. B):13–19. doi: 10.1093/jac/20.suppl_b.13. [DOI] [PubMed] [Google Scholar]
- 205.Maskell JP, Sefton AM, Williams JD. Comparative in-vitro activity of azithromycin and erythromycin against Gram-positive cocci, Haemophilus influenzae and anaerobes . J. Antimicrob. Chemother. 1990;25(Suppl. A):19–24. doi: 10.1093/jac/25.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- 206.Chang SC, Chen YC, Luh KT, Hsieh WC. Macrolides resistance of common bacteria isolated from Taiwan. Diag. Microbiol. Infect. Dis. 1995;23(4):147–154. doi: 10.1016/0732-8893(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 207.Ednie LM, Spangler SK, Jacobs MR, Appelbaum PC. Antianaerobic activity of the ketolide RU 64004 compared to activities of four macrolides, five beta-lactams, clindamycin, and metronidazole . Antimicrob. Agents Chemother. 1997;41(5):1037–1041. doi: 10.1128/aac.41.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mikamo H, Yin XH, Ninomiya M, Tamaya T. In vitro and in vivo antibacterial activities of telithromycin . Chemotherapy. 2003;49(1)(2):62–65. doi: 10.1159/000069789. [DOI] [PubMed] [Google Scholar]
- 209.Marina M, Ivanova M, Kantardjiev T. Antimicrobial susceptibility of anaerobic bacteria in Bulgaria . Anaerobe. 2009;15(4):127–132. doi: 10.1016/j.anaerobe.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 210.Chen SC, Gottlieb T, Palmer JM, Morris G, Gilbert GL. Antimicrobial susceptibility of anaerobic bacteria in Australia . J. Antimicrob. Chemother. 1992;30(6):811–820. doi: 10.1093/jac/30.6.811. [DOI] [PubMed] [Google Scholar]
- 211.Wexler HM, Molitoris E, Molitoris D, Finegold SM. In vitro activity of telithromycin (HMR 3647) against 502 strains of anaerobic bacteria . J. Antimicrob. Chemother. 2001;47(4):467–469. doi: 10.1093/jac/47.4.467. [DOI] [PubMed] [Google Scholar]
- 212.Spiegel CA. Susceptibility of Mobiluncus species to 23 antimicrobial agents and 15 other compounds . Antimicrob. Agents Chemother. 1987;31(2):249–252. doi: 10.1128/aac.31.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 301.ReMeD (Network for Medicine and Development) Essential Medicines: Madagascar. www.who.int/selection_medicines/country_lists/mdg/en/ World Health Organization.