Abstract
In developing biosimilar or biobetter products, comparability to the reference product is required to claim similar integrity or intended purpose. In this work, an anti-CD20 monoclonal antibody developed using RNA interference to decrease core fucosylation (RNAi-mediated) was comprehensively characterized by LC-MS and compared with the commercially-available anti-CD20 rituximab (MabThera®). As anticipated, < 30% core fucose was found within the RNAi-produced molecule (compared with > 90% in rituximab), and the reduction in fucose resulting in a significant improvement in FcγRΙΙΙa binding and antibody-dependent cell-mediated cytotoxicity. Two mutations, S258Y (fully mutated) and F174I/L (partially mutated), however, were detected in the production of the RNAi-mediated molecule. An alternative LC-MS approach using dimethyl labeling (i.e., 2CH2 for rituximab and 2CD2 for the RNAi-mediated molecule) was developed to additionally compare the two mAbs and confirm the full sequence with the two mutation sites. Furthermore, disulfide linkages were found to be the same for the two antibodies, with a small portion of unpaired cysteines in both products. Disulfides were correctly linked if the samples were prepared at low pH (i.e., enzymatic digestion by pepsin at pH 2); however, trace amounts of scrambling were found by trypsin digestion at pH 6.8, and this scrambling increased significantly at pH 8. Typical modifications, such as pyro-Glu formation at the N-terminus, K clipping at the C-terminus, oxidation at Met, and deamidation at Asn, were also detected with no significant differences between the two products. Using the LC-MS approaches for the comparability study, product integrity with critical structure information was revealed for confirmation of intended purpose (core fucosylation), identification of critical parameters (e.g., sample pH), and correction as needed (amino acid mutation).
Keywords: biosimilar, LC-MS, sequence mutation, free cysteine, disulfide scrambling, structure characterization
Introduction
Rituximab, a therapeutic monoclonal antibody (mAb) targeting CD20 in B cells, is used to treat B cell non-Hodgkin lymphoma and rheumatoid arthritis.1-3 The product’s brand names are Rituxan® (in the United States) and MabThera® (in Europe). One important function for the antibody is to induce antibody-dependent cell-mediated cytotoxicity (ADCC), in which the Fc domain, including the glycans, binds specifically to Fc receptors in human effector cells, such as macrophages and natural killer cells, to induce ADCC.4-6 Because the glycan structure, particularly the core fucose, is important to mediate ADCC, the reduction of the core fucose (i.e., by RNAi) should enhance the effect.7-11 Thus, RNAi-mediated fucosyltransferase (FUT8) and GDP-man-4,6-dehydratase (GMDS) was used to produce anti-CD20 mAb for this purpose.12 Although the aim was for a bio-better product, the overall structure, except for the level of the core fucose, was intended to be as similar as possible to the reference product to maintain the drug integrity.
In this study, we first used state of the art mass spectrometric methods to characterize the structure of the newly developed RNAi-mediated anti-CD20 mAb and then compared it to the structure of the commercial rituximab molecule. As expected, reduction of core fucosylation was observed for the RNAi-mediated molecule. On the other hand, the primary structure, disulfide linkages, and common modifications such as pyro-Glu formation at the N-terminus, K clipping at the C-terminus, oxidation at Met, and deamidation at Asn were found to be similar between the two products. The liquid chromatography–mass spectrometry (LC-MS) used for full sequence analysis, however, identified two amino acid residues mutated on the RNAi-mediated molecule. An alternative LC-MS method, using dimethyl labeled with 2CH2 for rituximab, and isotopically-dimethyl labeled with 2CD2 for the RNAi-mediated molecule confirmed the amino acid changes on the RNAi-mediated molecule. Moreover, both approaches were in agreement that one variant was fully mutated and the other partially mutated. Small amounts of free cysteines in both molecules were also observed. Disulfide scrambling, which could be caused by the free cysteines, was detected in both mAbs. At pH 6.8 or pH 8, which are typical enzymatic digestion conditions, a small amount of disulfide scrambling was observed (a trace amount at pH 6.8 and significantly more at pH 8), but no scrambling was seen at pH 2. The pH used for sample preparation is shown to be critical to measure correctly the free cysteines and disulfide linkages.
Results
To establish identity, the sequence of the newly developed RNAi-mediated molecule was compared with the amino acid sequence of rituximab found in US Patent 5736137.13 Additionally, disulfide linkages, glycosylation structure, and amino acid modifications in the two mAbs were characterized and compared as described in the following sections.
Peptide mapping
Enzymatic peptide mapping was used for the primary sequence identification. A typical tryptic peptide map of the RNAi-mediated mAb is illustrated in Figure 1, with the identifications of all peptides summarized in the Supplementary Material (Table S1A for the heavy chain and Table S1B for the light chain). As listed in Table S1, several small peptides were identified through miscleavage or by digestion using different enzymes, such as Lys-C or pepsin. Importantly, many peptides with overlapping amino acids were repeatedly identified in the different enzymatic maps. Thus, complete sequence coverage (100%) was successfully achieved by the combined analysis of these enzymatic peptide fragments.

Figure 1. Tryptic map of RNAi-mediated mAb. T1H stands for the first tryptic peptide from the N-terminal heavy chain, T1L for the first tryptic peptide from the N-terminal light chain, pyro-T1H for the pyro-Glu form of T1H, G0F for the glycopeptide with core fucose, T24H* for the deamidated T24H. Similar nomenclature is used for the other peptides.
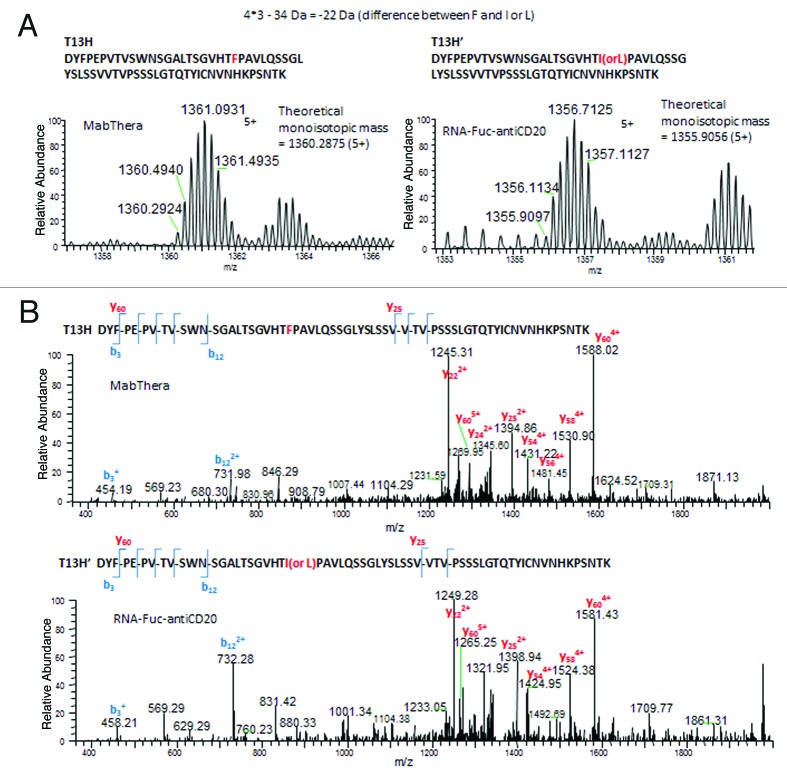
In the comparison of the two molecules, an amino acid mutation of S258Y was found from the tryptic peptide T19H of the RNAi-mediated molecule (Fig. 2). The reason for the mutation was likely due to an error in the PCR procedure (clonal error) as direct DNA sequencing confirmed an X to Y nucleotide substitution at position XXX (data not shown), which changed the serine codon (XXX) to a tyrosine (YYY). Also in the RNAi-mediated molecule, a second mutation F174I or F174L, which is partially mutated (~40%), was found for the T13H tryptic peptide (Fig. 3). This mutation could be attributable to extensive passaging (> 21 passages) of the CHO cell line producing the RNAi-mediated molecule. To confirm the exact site of the mutation, a non-specific cleavage form of the T13H peptide, which has a shorter length, was chosen for collision induced dissociation-tandem mass spectrometry (CID-MS2) to obtain sufficient backbone cleavage for the site assignment (Fig. 3B). The 100% sequence coverage is critical to confirm the sequence or identify a variation, as in this case. Once the position of variation is identified, the site can be pinpointed for correction.
Figure 2.(A) Precursor mass of normal (top left) and S258Y mutated tryptic peptide (top right). Theoretical and observed monoisotopic mass are indicated in the figure. (B) CID-MS2 of the precursor ion from Figure 2A, normal (top) and S258Y mutated tryptic peptide (bottom).
Figure 3.(A) Precursor mass of normal (top left) and F174L/I mutated tryptic peptide (top right). (B) CID-MS2 of the precursor ion from Figure 3A, normal (top) and F174L/I mutated tryptic peptide (bottom).
Because two amino acid mutations were found, additional potential mutations were explored. For this study, we used dimethyl labeling of the digest peptides, with dimethyl (2CH2) for rituximab, and isotopically-labeled dimethyl (2CD2) for the RNAi-mediated molecule to examine more carefully the entire amino acid sequence. With this approach, the difference of the mass for each tryptic peptide between the two products should be constant (2CD2 – 2CH2 = 4 Da per primary amine) if there is no amino acid mutation. For the mutation of S258Y, the delta mass would be 80 Da (Fig. 4A), and with the corresponding MS2 spectrum indicating the mutation site (Fig. 4B). For the mutation of F174I or F174L, the delta mass would be -22 Da (Fig. 5A), and the MS2 spectrum provides site confirmation (Fig. 5B). Importantly, we did not observe any other mutation sites following the complete assignment of the peptides.
Figure 4.(A) Precursor mass of the peptide with CH2 dimethyl labeling (top left) and the precursor mass of the peptide with CD2 dimethyl labeling (top right). The mass difference is not a constant 4 Da but 80 Da, accounting for the difference between S and Y (76 Da), as indicated in the figure. (B) CID-MS2 of the precursor ion from Figure 4A, CH2 dimethyl labeling (top) and CD2 dimethyl labeling (bottom).
Figure 5.(A) Precursor mass of the peptide with CH2 dimethyl labeling (top left) and the precursor mass of the peptide with CD2 dimethyl labeling (top right). The mass difference is not constant 4*3 = 12 Da but 22 Da, accounting for the difference between F and I or L (34 Da), as indicated in the figure. (B) CID-MS2 of the precursor ion from Figure 5A, CH2 dimethyl labeling (top) and CD2 dimethyl labeling (bottom).
Stable isotope labeling by amino acids in cell culture (SILAC) can be used to detect sequence variations between material produced from different manufacturing processes,14,15 e.g., light SILAC labeling in a reference material in comparison to another manufacturing lot with heavy SILAC labeling. For the sequence variation in biosimilar or biobetter products, however, it is not practical to use the metabolic approach (i.e., SILAC) to reproduce the cell culture conditions used to make the reference product because these conditions may be known by only the company that markets the product. Thus, we purchased the reference product (rituximab) and used the above external chemical approach (i.e., dimethyl labeling). It should be noted that dimethyl labeling is typically used for relative quantitation,16 but, in this case, we adopted the labeling method for mutation analysis. The labeling approach provides an additional measure of assurance to the results of peptide mapping sequence analysis. Moreover, the delta mass shift can provide a reasonable indication of the potential sequence variation, which can be subsequently determined by MS2 analysis.
Disulfide linkage analysis
Enzymatic digestion without reduction followed by LC-MS was used for disulfide analysis. All the expected disulfides were identified by accurate precursor mass measurement and CID or electron transfer dissociation (ETD) fragmentation of the precursor ion. The assignment approach is illustrated in Figure 6, with panel A the MS spectrum for the precursor ion, panel B for the CID-MS2 spectrum, and panel C for the ETD-MS2 spectrum. Using this approach, all the disulfide linkages were successfully identified (Fig. S1–S7 and summarized in Table 1). Both anti-CD20 mAbs were found to contain identical disulfide linkages.
Figure 6. Precursor mass of 1029. 1143 (3+) (A), CID-MS2 of the precursor (B), and ETD-MS2 of the precursor (C) for the disulfide-linked peptide (Cys22-Cys96). For ETD-MS2, a different charge of the precursor ion, 772.59 (4+), was used for fragmentation.
Table 1. Summary of disulfide identification in rituximab and RNAi-mediated mAb.

In the analysis, unpaired cysteines were also identified (Fig. S8–S15). These unpaired cysteines were found as free cysteines at pH 6.8 with trypsin digestion and at pH 2 with pepsin digestion (Table 2), but they were not observed at pH 8. It is likely that the free cysteines became linked disulfides (scrambled) in the alkaline pH (see Table 3). For accurate results, it is thus important to measure the free cysteine levels at low pH, and, especially, the digestion pH needs to be optimized to minimize disulfide scrambling.17
Table 2. Free cysteine determined at different digestion pHs for rituximab and RNA-mediated mAb.
| pH 8.0 | pH 6.8 | pH 2.0 | ||||
|---|---|---|---|---|---|---|
| Rituximab (%) | RNAi-mediated (%) | Rituximab (%) | RNAi-mediated (%) | Rituximab (%) | RNAi-mediated (%) | |
| T12H (C148) | N.D. | N.D. | 5.4 ± 0.9 | 0.4 ± 0.0 | 1.0 | 0.2 |
| T13H (C204) | N.D. | N.D. | 1.3 ± 0.3 | N.D. | 0.2 | 0.1 |
| T20H (C265) | N.D. | N.D. | 3.0 ± 0.3 | 0.2 ± 0.0 | 3.4 | 1.2 |
| T26H (C325) | – | — | — | — | 3.1 | 0.8 |
| T34H (C371) | N.D. | N.D. | 10.7 ± 1.8 | 0.2 ± 0.1 | 1.6 | N.D. |
| T39H (C429) | N.D. | N.D. | 4.7 ± 0.3 | 0.1 ± 0.0 | 1.0 | N.D. |
| T2L (C23) | N.D. | N.D. | 0.4 ± 0.2 | 0.0 ± 0.0 | N.D. | N.D. |
| T5L (C87) | N.D. | N.D. | 0.2 ± 0.0 | 0.0 ± 0.0 | N.D. | N.D. |
| T9L (C133) | N.D. | N.D. | 1.7 ± 0.2 | 0.1 ± 0.0 | N.D. | N.D. |
| T16L (C193) | N.D. | N.D. | 1.1 ± 0.1 | 0.1 ± 0.0 | 1.0 | N.D. |
| T17L (C213) | — | — | — | — | 0.2 | 0.1 |
The percent of free cysteine at pH 8.0 and 6.8 was obtained by the measured amount of the free cysteine form divided by the total cysteines on the specific tryptic peptide, assuming each cysteine is alkylated. The percent of free cysteine at pH 2 was obtained by the ratio of the observed free cysteines divided by the total cysteines after reduction with TCEP. “N.D.” represents not detectable (the values is too low to be observed), and “–” means not measurable due to the tryptic peptide length (too short to retain in the LC chromatogram).
Table 3. Percent scrambled disulfides at different digestion pHs for rituximab and RNA-mediated mAb.
| pH 8.0 | pH 6.8 | pH 2.0 | ||||
|---|---|---|---|---|---|---|
| Rituximab (%) | RNAi-anti-CD20 (%) | Rituximab (%) | RNAi-anti-CD20 (%) | Rituximab (%) | RNAi-anti-CD20 (%) | |
| T2H(C22)-T20H(C265) | 2.2 | 0.4 | 1.6 | 0.0 | N.D. | N.D. |
| T2H(C22)-T34H(C371) | 0.4 | 0.1 | 0.3 | N.D. | N.D. | N.D. |
| T9H(C96)-T26H(C325) | 0.2 | 0.1 | N.D. | N.D. | N.D. | N.D. |
| T12H(C148)-H26H(C325) | 0.3 | 0.6 | 0.0 | 0.1 | N.D. | N.D. |
| T17H(C224)-T20H(C265) | 0.3 | 0.6 | N.D. | N.D. | N.D. | N.D. |
| T18H(C230C233) – intra-linked | 9.4 | 0.4 | 5.8 | 0.9 | N.D. | N.D. |
| T20H(C265)-T20H(C265) | 4.9 | 1.4 | 1.5 | 0.3 | N.D. | N.D. |
| T20H(C265)-T39H(C429) | 1.1 | 0.5 | 1.5 | 0.3 | N.D. | N.D. |
| T20H(C265)-T16L(C193) | 1.0 | 1.7 | N.D. | N.D. | N.D. | N.D. |
| T26H(C325)-T34H(C371) | 1.6 | 1.7 | 0.6 | 0.1 | N.D. | N.D. |
| T26H(C325)-T39H(C429) | 0.7 | 0.3 | 0.5 | N.D. | N.D. | N.D. |
| T26H(C325)-T16L(C193) | 0.2 | 0.8 | N.D. | N.D. | N.D. | N.D. |
The percent scrambled disulfide was obtained by the measured amount of scrambled disulfide divided by the total disulfide-linked forms.
To prove that the scrambled disulfides were caused by the sample preparation and were not in the original samples, a study was conducted to examine the correlation of scrambling as a function of pH (pH 8, 6.8, and 2). For optimum digestion efficiency, trypsin was used at pH 8 and pH 6.8, and pepsin at pH 2. Scrambled disulfides were found in trace amounts for digestion at pH 6.8, and significant amounts at pH 8, but no scrambling was observed at pH 2 (Table 3). Rituximab appears to have a higher amount of scrambling than the RNAi-mediated molecule due to a higher amount of free cysteines in rituximab (Table 2). Nevertheless, scrambling was not observed in either product at pH 2. It can be concluded that if scrambled disulfides are observed when examining a biopharmaceutical, a good test is to explore if scrambling decreases with digestion pH. Any “scrambling” observed at pH 2 likely represents the true structure of the molecule and not an artifact of sample preparation. In this case, a careful study should be conducted to obtain additional data points with different digestion pH to extrapolate the extent of scrambling at the intercept (i.e., extrapolate to pH 0) to reflect the true scrambling.
The Ellman reaction, coupling a chromophore (dithionitrobenzoic acid or DTNB) to a thiol group, has been used to detect free cysteine in a protein.18-21 This method, however, is insensitive and often requires hundreds of milligrams of mAb if the free cysteine is at low levels (i.e., ≤ 1%). Instead, we used the LC-MS approach for free cysteine analysis because it uses minute amounts of mAb (2 µg) and is quite sensitive for detecting free cysteine at low levels. Because the ionization efficiency in the LC-MS analysis could be different for free and disulfide linked cysteines, the relative proportion of the various cysteines would, however, only be an estimate unless synthetic standards are used for correction of potentially different responses. To minimize the difference in responses in the LC-MS experiment, we first converted the free cysteines at pH 8.0 and 6.8 to the alkylated forms, and then divided that value by the value derived from reduction and alkylation of all cysteines in a separate experiment. Because the alkylation experiment is difficult to perform at pH 2, the percentage of free cysteine at this pH was obtained by the ratio of the observed free cysteine divided by the total free cysteines; the latter was derived from the reduction with TCEP at low pH in a separate experiment (see the section of enzymatic digestion in Materials and Methods). These results are presented in Table 2. It should be noted that disulfide scrambling caused by the free cysteine could be a concern for drug quality or safety. LC-MS has been known to be able to detect structural variants with a high degree of sensitivity and precision; however, proper sample handling and precise quantitation of low-level free cysteine residues have not been well-documented. Thus, it is important to study the disulfide linkages carefully with a sensitive technique, as described here.
It should be noted that most therapeutic antibodies contain low levels of free cysteines.22-25 It has also been reported that human IgGs in serum possess low levels of free sulfhydryl as well (most likely associated with human IgG2, with some evidence in IgG1).26-28 Thus, a proper formulation buffer to control the pH is needed to avoid scrambling while still maintaining high solubility. The pH of the formulation buffer is between 5.5 and 6 for both rituximab and the RNAi-mediated molecules.
Fc glycosylation
The common glycoforms at the conserved Fc site (Asn300) were identified at the tryptic peptide T23H, e.g., glycan without galactose (G0F), with one galactose (G1F), with two galactoses (G2F), without galactose and without core fucose (G0), with one galactose and without core fucose (G1), with two galactoses and without core fucose (G2), without galactoses and without core fucose and without one N-acetyl glucosamine (G0-NGlc).
The reduction of core fucosylation, as expected, was found for the RNAi-mediated molecule. The glycan distribution for the two mAbs are shown in Table 4, the a-fucosylated glycans (without core fucose such as G0, G1, and G0-NGlc) were increased to > 70% for the RNAi-mediated molecule, while being < 10% in rituximab. The percent distribution was obtained through the amount of each glycopeptide, divided by the sum of all glycopeptides including the non-glycosylated form. The reduction of the core fucose induced a 17-fold improvement in FcγRIIIa binding, and an increase in specific cell lysis by up to 30%, as determined in an ADCC assay.12
Table 4. Comparison of glycan distribution for rituximab and RNA-mediated mAb.
| Glycans | Rituximab ± SD (%) | RNAi-mediated ± SD (%) |
|---|---|---|
| G0F | 34.2 ± 1.3 | 14.6 ± 0.8 |
| G0F-NGlc | 7.6 ± 0.6 | 2.3 ± 0.6 |
| G1F | 35.6 ± 2.7 | 4.2 ± 0.6 |
| G1F-NGlc | 4.7 ± 0.6 | 0.4 ± 0.1 |
| G2F | 9.6 ± 0.3 | 0.7 ± 0.1 |
| G0 | 2.8 ± 0.1 | 46.8 ± 1.6 |
| G0-NGlc | 1.2 ± 0.1 | 14.6 ± 2.1 |
| G1 | 0.9 ± 0.1 | 9.1 ± 0.6 |
| G1-NGlc | 0.1 ± 0.0 | 1.1 ± 0.1 |
| Man5 | 3.1 ± 0.1 | 5.0 ± 0.4 |
| Man4 | 0.1 ± 0.0 | 0.2 ± 0.1 |
| Man3 | 0.1 ± 0.0 | 0.9 ± 0.0 |
The percent glycosylation was obtained by the measured amount of the specific glycopeptide divided by the total of all forms of the glycopeptides including the non-glycosylated form. The SD was determined from 6 measurements.
Other modifications
MAbs with “Q” or “E” N-terminal amino acids could easily convert to pyro-Glu. The N-terminus of the heavy and light chains of rituximab contains the Q amino acid residue. Thus, loss of NH3 (minus 17 Da) was examined in the N-terminal peptides, and both the non-modified and pyro-Glu forms were found (Figs. S16–S17). The C-terminus of the heavy chain could also be cleaved by carboxypeptidase present during mAb production in the CHO cell. Thus, the K clipping at the C-terminus of the heavy chain was examined (Fig. S18). Both mAbs have the same pyro-Glu formation at the N-terminus, and near complete K clipping at the C-terminus of the heavy chain. Other typical modifications, including Asn deamidation and Met oxidation, were also found. No significant differences were observed for these modifications, except Met286 and pyro-Glu at the light chain (see t-test results in Table S2). The minor difference at Met286 could be due to this amino acid residue being very close to the mutation site (S258Y), and the difference of pyro-Glu on the light chain could be due to the difference in storage time and conditions for the two products.
Discussion
The full structure of the two anti-CD20 mAbs was compared using LC-MS analysis of the enzymatic peptide mixtures. As shown, the full amino acid sequence, including N-terminal, C-terminal, disulfide linkages, and glycosylation site and structure, were successfully identified. The methodology can detect amino acid modifications, mutations, free cysteines, and scrambled disulfides. In such a comparative study, the identification of the entire amino acid sequence is critical. A multi-enzyme digestion strategy is necessary, along with complete LC-MS analysis of all the enzymatically-produced peptides. As shown in this work, dimethyl labeling of H and D isotopically labeled derivatizing agents adds a valuable dimension for verification.
We believe that sensitive LC-MS analysis should detect the structural variants as described. However, it is important not only to detect variants, but also to have a strategy to rule out other possibilities to provide a high degree of assurance that the results are correct. The two orthogonal techniques used in this work independently confirm the findings, but also can determine the degree of similarity of the two products. We believe that such an extensive comparability study by mass spectrometric techniques should be performed early in the research and development process to obtain an accurate sequence and the correct up-stream and down-stream process conditions to generate a highly similar antibody. As suggested in the US Food and Drug Administrations’ biosimilar draft guideline of February 2012, a highly similar antibody could reduce the extent of clinical studies needed for approval, and thus decrease the cost of biosimilar drugs, which could have a significant effect on the cost of healthcare.
For a comparability analysis, it should be emphasized that even 80 to 90% sequence coverage would not be satisfactory for comparison of two antibodies (e.g., reference vs. biosimilar). Although it may be time-consuming and labor-intensive, all peptides must be identified and, where appropriate, quantitated. In the future, top down mass spectrometry29-31 may be able to achieve 100% sequence coverage on the intact protein, without enzymatic digestion. With improving sensitivity and enhanced resolution of mass spectrometric instrumentation, the top down approach could ultimately have a great impact in the characterization of biopharmaceuticals.32 However, detecting many modifications at once is presently too challenging, as is the identification of all the modifications with associated sites using current software. The combination of both peptide mapping and top down MS may alleviate constraints in both approaches. The middle down, also known as the extended range proteomic analysis, approach,33-35 which takes advantage of large peptide fragments for high sequence coverage and with less sophisticated modifications than the entire protein, is at present a useful alternative.
Materials and Methods
Samples
RNAi-mediated mAb was manufactured at Alnylam Pharmaceuticals and provided as 1 mg/mL x 0.8 mL as previously described.12 Rituximab was purchased by Alnylam from Imperial College, London and provided as 10 mg/mL x 0.5 mL. The samples were aliquoted as 10 µL per vial for rituximab (100 µg) and 100 µL per vial for RNAi-mediated mAb (100 µg) and stored at -80°C before analysis.
Reagents
Trypsin (sequencing grade, V5111) was purchased from Promega, endoproteinase Lys-C (MS grade, 125–05061) from Wako Chemicals USA, pepsin (Porcine Stomach Mucosa, 0219536701) from MP Biomedicals, and endoproteinase Glu-C (sequencing grade, 11420399001) from Roche Diagnostics. Formaldehyde (CH2O) (37% v/v, 252549), labeled formaldehyde (CD2O) (20% v/v, 98% D, 492620), sodium cyanoborohydride (296813), triethylammonium bicarbonate (TEAB, T7408), ammonium hydroxide solution (17837), guanidine hydrochloride (Gn-HCl, G3272), dithiothreitol (DTT, D5545), iodoacetamide (IAM, I6125), ammonium bicarbonate (09830) and 1.0 N hydrochloric acid (HCl, 318949) solution were obtained from Sigma-Aldrich. Tris buffer (17–1321–01) was from GE Healthcare, and tris(2-carboxyethyl)phosphine hydrochloride (TCEP-HCl, 20490) and formic acid (FA, optima LC/MS, A11750) from Fisher Scientific. LC-MS grade water (JT9831–3) and acetonitrile (ACN, EM-AX0145–1) were purchased from VWR. Amicon centrifugal filters (10 kDa molecular weight cutoff, UFC501096) were obtained from EMD Millipore.
Enzymatic digestion
An aliquot of 10 µL of rituximab and 100 µL of RNAi-mediated mAb solution (100 µg) was denatured with 6 M guanidine hydrochloride containing 100 mM ammonium bicarbonate (pH 8), reduced with 5 mM DTT for 30 min at 37°C, and then alkylated with 20 mM IAM in the dark for 45 min at room temperature. The reduced and alkylated protein was buffer exchanged with 100 mM ammonium bicarbonate (pH 8) or 50 mM Tris (pH 6.8) using a 10 kDa molecular weight cutoff filter to a concentration of 1 mg/mL (100 µL). For tryptic digestion, trypsin (1:50, w/w) was added to the protein solution at room temperature. After 8 h, the enzyme was added a second time (1:50, w/w) and the digestion continued at room temperature for 12 h. For Lys-C digestion, the endoproteinase Lys-C (1:50, w/w) was added to the protein solution for 4 h at 37°C. For Lys-C plus trypsin digestion, the protein solution was added with endoproteinase Lys-C (1:50 w/w) for 4 h at 37°C, and then trypsin (1:50 w/w) for 20 h at room temperature. For pepsin digestion, the protein solution was dissolved in 10 mM HCl (pH 2). Pepsin (1:10, w/w) was added to the protein solution and incubated at 37°C for 30 min. The reaction was quenched by adjusting the pH to 5 with 100 mM ammonium bicarbonate. For digestion without reduction (for disulfide assignment), the same digestion protocol as above was applied but without the reduction and alkylation steps. For quantitation of free cysteines at pH 6.8, 100 µg sample was alkylated with 20 mM IAM (in 6M Gn-HCl) in pH 6.8 for 2 h, and then half of the protein solution was digested by trypsin without reduction, and the other half reduced, alkylated and digested by trypisn. For quantitation of free cysteines at pH 2, the protein solution was digested in pepsin, and then half was reduced by 10 mM TCEP at pH 5.0 for an hour at room temperature. In all cases (except pepsin digestion), digestion was terminated by addition of 1% formic acid. An aliquot of 2 µg of the enzyme digest was analyzed per LC-MS run.
Dimethyl labeling
After digestion, the digests (20 µL, nearly completely dried) were reconstituted in 20 µL 0.1 mM TEAB, and 1 µL of 37% (v/v) CH2O was added for the rituximab sample, while 1.9 µL of 20% (v/v) CD2O was added for the RNAi-Fuc-anti-CD20 mAb sample. Each sample was then added 5.8 µL 1M NaBH3CN, and the solution was incubated in a fume hood for 1 h at room temperature. Finally, the reaction was quenched by addition of 4 µL 10% ammonia solution, and then 8 µL of formic acid on an ice top to prevent frothing or heating of the sample.16 The samples of rituximab and RNAi-Fuc-anti-CD20 mAb were mixed equally for subsequent LC-MS analysis.
LC-MS
An Ultimate 3000 nano-LC pump (Dionex) and a self-packed C18 column (Magic C18, 200 Å pore and 5 μm particle size, 75 μm i.d. × 15 cm) (Michrom Bioresources) was coupled online to an LTQ-Orbitrap-ETD XL mass spectrometer (Thermo Fisher Scientific) through a nanospray ion source (New Objective). Mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in acetonitrile) were used for the gradient consisting of (1) 20 min at 2% B for sample loading and 5 min for desalting at 0.3 µL / min (20 min desalting for pepsin or dimethylated digests); (2) linear from 2 to 5% B for 2 min; (3) linear from 5 to 35% B for 60 min; (4) linear from 35 to 90% B for 3 min; and finally (5) isocratic at 90% B for 5 min. The flow rate of the column was maintained at 0.2 µL/min, and the mass spectrometer started to record data after 5 min of the gradient. The LTQ-Orbitrap-ETD XL mass spectrometer was operated initially in the data-dependent mode as follows: survey full-scan MS spectra (m/z 300–2000) were acquired in the Orbitrap with a mass resolution of 30,000 at m/z 400 (with an ion target value of 5 × 105 ions), followed by nine sequential MS2 scans using the LTQ . For disulfide mapping, the MS was switched automatically between MS (scan 1 in the Orbitrap), CID-MS2 (scan 2 in the LTQ), and ETD-MS2 (scan 3 in the LTQ). Briefly, after a survey MS spectrum from m/z 300 to 2000, subsequent CID-MS2 and ETD-MS2 steps were performed on the same precursor ion with a ± 2.5 m/z isolation width. Any incomplete assignment in the CID-MS2 and ETD-MS2 spectra was repeated by targeting the desired ions, e.g., the same precursor but with a different charge state, to gain additional linkage information. This targeted approach was repeated (e.g., targeting multiple charges of a precursor ion or the same disulfide-linked peptide but with different enzymatic cleavages or miscleavages) until the linkage information was complete. In addition, a targeted CID-MS3 after ETD for ions of interest was performed as necessary.
Peptide assignment
The spectra generated in the CID-MS2 step were searched against spectra of theoretical fragmentations (b and y ions) of rituximab sequence with a mass tolerance of ≤ 5 ppm for the precursor ions and with enzyme specificity, using a Sequence probability score (> 95% confidence) as the filter. For peptides with miscleavages or a mass tolerance > 5 ppm (but less than 20 ppm) of the precursor ion, confirmation required manual inspection to match all highly abundant product ions. The sequence coverage generated by each enzymatic map was combined as a total coverage.
Disulfide assignment
The anticipated disulfide-linked tryptic or multi-enzyme digested peptide masses with different charges were first calculated and then matched to the observed masses in the LC-MS chromatogram. The matched masses (with < 5 ppm mass accuracy) were further confirmed by the corresponding CID-MS2 and ETD-MS2 fragmentation, as well as by CID-MS3 fragmentation, as needed.
Glycan structure identification
Theoretical masses of glycan structures such as G0F, G1F and G2F were added to the tryptic peptide backbone (EEQYNSTYR). The anticipated glycopeptide masses with different charges were thus obtained to match the observed masses in the LC-MS chromatogram. The matched masses (with ≤ 5 ppm mass accuracy) were further confirmed by the corresponding CID-MS2 fragmentation.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grant GM 15847. The authors thank Alnylam Pharmaceuticals for providing the RNAi-mediated anti-CD20 antibody and rituximab for this study. Contribution Number 1036 from the Barnett Institute.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/24814
References
- 1.Hainsworth JD, Burris HA, 3rd, Morrissey LH, Litchy S, Scullin DC, Jr., Bearden JD, 3rd, et al. Rituximab monoclonal antibody as initial systemic therapy for patients with low-grade non-Hodgkin lymphoma. Blood. 2000;95:3052–6. [PubMed] [Google Scholar]
- 2.Okamoto H, Kamatani N. Rituximab for rheumatoid arthritis. N Engl J Med. 2004;351:1909–, author reply 1909. doi: 10.1056/NEJM200410283511820. [DOI] [PubMed] [Google Scholar]
- 3.Taylor PC. Rituximab in the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2007;3:17–26. doi: 10.1586/1744666X.3.1.17. [DOI] [PubMed] [Google Scholar]
- 4.Flieger D, Renoth S, Beier I, Sauerbruch T, Schmidt-Wolf I. Mechanism of cytotoxicity induced by chimeric mouse human monoclonal antibody IDEC-C2B8 in CD20-expressing lymphoma cell lines. Cell Immunol. 2000;204:55–63. doi: 10.1006/cimm.2000.1693. [DOI] [PubMed] [Google Scholar]
- 5.Harjunpää A, Junnikkala S, Meri S. Rituximab (anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms. Scand J Immunol. 2000;51:634–41. doi: 10.1046/j.1365-3083.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez J, Gutierrez A. Pharmacokinetic properties of rituximab. Rev Recent Clin Trials. 2008;3:22–30. doi: 10.2174/157488708783330495. [DOI] [PubMed] [Google Scholar]
- 7.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–40. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 8.Niwa R, Shoji-Hosaka E, Sakurada M, Shinkawa T, Uchida K, Nakamura K, et al. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004;64:2127–33. doi: 10.1158/0008-5472.CAN-03-2068. [DOI] [PubMed] [Google Scholar]
- 9.Niwa R, Hatanaka S, Shoji-Hosaka E, Sakurada M, Kobayashi Y, Uehara A, et al. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 Is independent of FcgammaRIIIa functional polymorphism. Clin Cancer Res. 2004;10:6248–55. doi: 10.1158/1078-0432.CCR-04-0850. [DOI] [PubMed] [Google Scholar]
- 10.Niwa R, Sakurada M, Kobayashi Y, Uehara A, Matsushima K, Ueda R, et al. Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin Cancer Res. 2005;11:2327–36. doi: 10.1158/1078-0432.CCR-04-2263. [DOI] [PubMed] [Google Scholar]
- 11.Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, et al. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods. 2005;306:151–60. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Tummala S, Titus M, Wilson L, Wang C, Ciatto C, Foster D, et al. Evaluation of exogenous siRNA addition as a metabolic engineering tool for modifying biopharmaceuticals. Biotechnol Prog. 2012 doi: 10.1002/btpr.1667. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson DR, Hanna N, Leonard GE, Newman RA, Reff ME, Rastetter WH. Therapeutic application of chimeric and radiolabeled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma 1998. US patent 5736137.
- 14.Manuilov AV, Radziejewski CH, Lee DH. Comparability analysis of protein therapeutics by bottom-up LC-MS with stable isotope-tagged reference standards. MAbs. 2011;3:387–95. doi: 10.4161/mabs.3.4.16237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sousa E, Olland S, Shih HH, Marquette K, Martone R, Lu Z, et al. Primary sequence determination of a monoclonal antibody against α-synuclein using a novel mass spectrometry-based approach. Int J Mass Spectrom. 2012;312:61–9. doi: 10.1016/j.ijms.2011.05.005. [DOI] [Google Scholar]
- 16.Boersema PJ, Raijmakers R, Lemeer S, Mohammed S, Heck AJ. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat Protoc. 2009;4:484–94. doi: 10.1038/nprot.2009.21. [DOI] [PubMed] [Google Scholar]
- 17.Ni W, Lin M, Salinas P, Savickas P, Wu SL, Karger BL. Complete mapping of a cystine knot and nested disulfides of recombinant human arylsulfatase A by multi-enzyme digestion and LC-MS analysis using CID and ETD. J Am Soc Mass Spectrom. 2013;24:125–33. doi: 10.1007/s13361-012-0510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brych SR, Gokarn YR, Hultgen H, Stevenson RJ, Rajan R, Matsumura M. Characterization of antibody aggregation: role of buried, unpaired cysteines in particle formation. J Pharm Sci. 2010;99:764–81. doi: 10.1002/jps.21868. [DOI] [PubMed] [Google Scholar]
- 19.Symes AL, Sourkes TL. Essential sulfhydryl groups of rat liver monoamine oxidase. Can J Biochem. 1975;53:910–3. doi: 10.1139/o75-125. [DOI] [PubMed] [Google Scholar]
- 20.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 21.Riener CK, Kada G, Gruber HJ. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4,4′-dithiodipyridine. Anal Bioanal Chem. 2002;373:266–76. doi: 10.1007/s00216-002-1347-2. [DOI] [PubMed] [Google Scholar]
- 22.Chumsae C, Gaza-Bulseco G, Liu H. Identification and localization of unpaired cysteine residues in monoclonal antibodies by fluorescence labeling and mass spectrometry. Anal Chem. 2009;81:6449–57. doi: 10.1021/ac900815z. [DOI] [PubMed] [Google Scholar]
- 23.Xiang T, Chumsae C, Liu H. Localization and quantitation of free sulfhydryl in recombinant monoclonal antibodies by differential labeling with 12C and 13C iodoacetic acid and LC-MS analysis. Anal Chem. 2009;81:8101–8. doi: 10.1021/ac901311y. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Czupryn MJ. Free sulfhydryl in recombinant monoclonal antibodies. Biotechnol Prog. 2002;18:509–13. doi: 10.1021/bp025511z. [DOI] [PubMed] [Google Scholar]
- 25.Harris RJ. Heterogeneity of recombinant antibodies: linking structure to function. Dev Biol (Basel) 2005;122:117–27. [PubMed] [Google Scholar]
- 26.Schauenstein E, Sorger S, Reiter M, Dachs F. Free thiol groups and labile disulfide bonds in the IgG fraction of human serum. J Immunol Methods. 1982;50:51–6. doi: 10.1016/0022-1759(82)90303-9. [DOI] [PubMed] [Google Scholar]
- 27.Yoo EM, Wims LA, Chan LA, Morrison SL. Human IgG2 can form covalent dimers. J Immunol. 2003;170:3134–8. doi: 10.4049/jimmunol.170.6.3134. [DOI] [PubMed] [Google Scholar]
- 28.Gevondyan NM, Volynskaia AM, Gevondyan VS. Four free cysteine residues found in human IgG1 of healthy donors. Biochemistry (Mosc) 2006;71:279–84. doi: 10.1134/S0006297906030072. [DOI] [PubMed] [Google Scholar]
- 29.Kelleher NL, Lin HY, Valaskovic GA, Aaserud DJ, Fridriksson EK, McLafferty FW. Top down versus bottom up protein characterization by tandem high-resolution mass spectrometry. J Am Chem Soc. 1999;121:806–12. doi: 10.1021/ja973655h. [DOI] [Google Scholar]
- 30.Chait BT. Chemistry. Mass spectrometry: bottom-up or top-down? Science. 2006;314:65–6. doi: 10.1126/science.1133987. [DOI] [PubMed] [Google Scholar]
- 31.Zamdborg L, LeDuc RD, Glowacz KJ, Kim YB, Viswanathan V, Spaulding IT, et al. ProSight PTM 2.0: improved protein identification and characterization for top down mass spectrometry. Nucleic Acids Res. 2007;35(Web Server issue):W701-6. doi: 10.1093/nar/gkm371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fornelli L, Damoc E, Thomas PM, Kelleher NL, Aizikov K, Denisov E, et al. Analysis of intact monoclonal antibody IgG1 by electron transfer dissociation Orbitrap FTMS. Mol Cell Proteomics. 2012;11:1758–67. doi: 10.1074/mcp.M112.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu SL, Kim J, Hancock WS, Karger B. Extended Range Proteomic Analysis (ERPA): a new and sensitive LC-MS platform for high sequence coverage of complex proteins with extensive post-translational modifications-comprehensive analysis of beta-casein and epidermal growth factor receptor (EGFR) J Proteome Res. 2005;4:1155–70. doi: 10.1021/pr050113n. [DOI] [PubMed] [Google Scholar]
- 34.Wu SL, Kim J, Bandle RW, Liotta L, Petricoin E, Karger BL. Dynamic profiling of the post-translational modifications and interaction partners of epidermal growth factor receptor signaling after stimulation by epidermal growth factor using Extended Range Proteomic Analysis (ERPA) Mol Cell Proteomics. 2006;5:1610–27. doi: 10.1074/mcp.M600105-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Pipes GD, Campbell P, Bondarenko PV, Kerwin BA, Treuheit MJ, Gadgil HS. Middle-down fragmentation for the identification and quantitation of site-specific methionine oxidation in an IgG1 molecule. J Pharm Sci. 2010;99:4469–76. doi: 10.1002/jps.22158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.