Abstract
The neonatal Fc receptor (FcRn) is important for the metabolic fate of IgG antibodies in vivo. Analysis of the interaction between FcRn and IgG in vitro might provide insight into the structural and functional integrity of therapeutic IgG that may affect pharmacokinetics (PK) in vivo. We developed a standardized pH gradient FcRn affinity liquid chromatography method with conditions closely resembling the physiological mechanism of interaction between IgG and FcRn. This method allows the separation of molecular IgG isoforms, degradation products and engineered molecules based on their affinity to FcRn. Human FcRn was immobilized on the column and a linear pH gradient from pH 5.5 to 8.8 was applied. FcRn chromatography was used in comparison to surface plasmon resonance to characterize different monoclonal IgG preparations, e.g., oxidized or aggregated species. Wild-type and engineered IgGs were compared in vitro by FcRn chromatography and in vivo by PK studies in huFcRn transgenic mice. Analytical FcRn chromatography allows differentiation of IgG samples and variants by peak pattern and retention time profile. The method can distinguish: 1) IgGs with different Fabs, 2) oxidized from native IgG, 3) aggregates from monomer and 4) antibodies with mutations in the Fc part from wild-type IgGs. Changes in the FcRn chromatographic behavior of mutant IgGs relative to the wild-type IgG correlate to changes in the PK profile in the FcRn transgenic mice. These results demonstrate that FcRn affinity chromatography is a useful new method for the assessment of IgG integrity.
Keywords: antibody, FcRn, neonatal Fc receptor, methionine oxidation, degradation, pharmacokinetics, PK, affinity chromatography, column, pH gradient
Introduction
Monoclonal antibodies (mAbs) are established as a critical therapeutic modality for a range of diseases.1 The pipeline of antibody-based drug candidates is steadily growing and totals nearly 350 development projects in early 2012.2 Apart from full-length therapeutic antibodies, modified antibodies, such as antibody-drug conjugates (ADCs), bispecific antibodies, Fc- and glyco-engineered antibodies and antibody fragments/ domains, comprise a substantial portion of the antibody-based molecules in clinical development. The preclinical and clinical research and development of therapeutic mAbs requires careful analysis of the pharmacokinetic properties of antibody drug candidates.3 Changes in integrity of the Fc part during storage of a therapeutic antibody may affect the pharmacokinetic (PK) and pharmacodynamic properties of the antibody, e.g., by degradation processes such as aggregation, deamidation or oxidation, disulfide bond scrambling and isomerization.4,5 These changes need to be monitored by appropriate analytical methods.
Therapeutic IgGs are composed of two variable antigen binding regions (Fab) that mediate specificity for the target antigen and the constant (Fc) region which is responsible for unique effector functions and for long half-life. Two main classes of mammalian Fc receptors exists that bind to IgG: the Fcγ receptor (FcγR) family and the neonatal Fc receptor (FcRn). The FcγR family members mediate effector responses, e.g., antibody-dependent cell-mediated cytotoxicity (ADCC), inflammation, cell activation, antibody production.6
FcRn regulates IgG and albumin homeostasis, mediates maternal IgG transport, and it takes an active role in antigen-IgG immune complex phagocytosis and delivery of antigens for presentation in the case of antigen-IgG immune complexes. Human FcRn is a heterodimeric protein consisting of two polypeptides, a 48 to 52 kDa glycosylated class I major histocompatibility complex-like protein (α-FcRn) containing a single N-glycan moiety and a β2-microglobulin (β2m) subunit of approximately 14 kDa.7 FcRn binds with high affinity to the CH2-CH3 portion of the Fc domain of IgG.8-11 The interaction between IgG and FcRn is strictly pH-dependent and has been proposed to occur in a 1:2 stoichiometry, with one IgG binding to two FcRn molecules via its two heavy chains.14,15 The pH-sensitive nature of the interaction facilitates the FcRn-mediated protection of IgGs pinocytosed into cells from intracellular degradation by binding to the receptor within the acidic environment of endosomes.10,12,13 Within capillary endothelial cells, FcRn facilitates the recycling of IgG to the cell surface and subsequent release into the blood stream upon exposure of the FcRn-IgG complex to the neutral pH environment outside the cell. Bone marrow derived cells (e.g., macrophages, dendritic cells, some B cell populations) also express FcRn and are involved in IgG protection.16
Because FcRn plays an important role in IgG catabolism, its in vitro FcRn binding properties should be indicative of non-target related in vivo PK properties. In vitro methods to analyze FcRn interaction would be of great value during antibody development because they may help to eliminate repetition of in vivo studies and thereby reduce animal experiments, time and costs. Such analyses generally have been performed using surface plasmon resonance (SPR) assays,17-21 although calorimetric and asymmetrical flow field flow fractionation methods have also been described for assessing IgG binding affinity to FcRn.15,22 The deficits of SPR assays include their complexity, and the fact that studies of the correlation between in vitro FcRn binding parameters determined by SPR and the serum half-life of antibodies in vivo have given inconsistent results, despite improved binding reaction conditions and appropriate modeling.23-25 Engineering of the Fc of IgG1 to improve affinity to FcRn at pH 6.0 and at neutral pH as measured by SPR technology did not result in improved PK in cynomolgus monkeys;26 however, mutants (e.g., N434A) showing only modest increases in FcRn affinity at pH 6.0 without concomitant significant binding to FcRn at pH 7.4 resulted in improved area under the curve (AUC) and half-life in primates, thereby demonstrating the importance of the FcRn release at pH 7.4, otherwise the antibody will be trapped and cleared from serum.26,27
In studies of five humanized IgG4 antibodies and selected variants thereof, no correlation between FcRn affinity and in vivo PK could be established.18 In contrast, a triple mutation YTE (M252Y/S254T/T256E) in the Fc portion of an antibody against respiratory syncytial virus (MEDI-524) resulted in a 10-fold increase of affinity to FcRn at pH 6.0, as analyzed by SPR, and was associated with a 4-fold increase in serum half-life in cynomolgus monkeys.28 In another study, two engineered IgG1 variants (N434A and T307A/E380A/N434A) of the humanized antibody trastuzumab (Herceptin®) directed against human epidermal growth factor receptor-2 (HER2) with enhanced in vitro binding to FcRn as measured by flow cytometry showed a considerably extended half-life in mice expressing the human FcRn transgene.29 It was recently shown that antibodies with the same wild-type human Fc sequences, but different Fab domains, bind FcRn with considerable differences, which suggested that the Fab domain also may have an effect on FcRn interactions.17 Binding of an antibody to FcRn may also be influenced by chemical degradation, such as oxidation of labile methionines (Met 252 and Met 428) in the constant region. Impaired PK properties of such modified species highlight the need to monitor oxidation in therapeutic antibodies during development.30-32
One of the challenges for the establishment of a conclusive relationship of FcRn binding and PK are the different methodologies, technologies and assay designs used to measure the interaction between IgG and FcRn, which include cell-based approaches, isothermal calorimetry and SPR. Variability in the study results may be due to the fact that an FcRn heterodimer can bind the Fc part on both sides, with the consequence that a high avidity can be expected, which should be taken into account and be reflected in an in vitro setup.19
The work presented here describes a standardized pH gradient huFcRn affinity liquid chromatography method to study interactions between IgG and FcRn under conditions closely resembling the physiological mechanism of interaction. The method uses a column with immobilized FcRn in a liquid chromatography system with a linear pH gradient from pH 5.5 to 8.8. Our study demonstrates that FcRn affinity chromatography method can be used to evaluate FcRn binding of oxidized and aggregated IgG, as well as wild-type and engineered IgG1 variant antibodies with increased and reduced FcRn affinity. We found the FcRn column chromatography to be a valuable tool for biochemical characterization because it allows the detection, separation and the re-analysis of micro-heterogeneous samples, which is not possible by other methods. Results from in vivo PK studies in mice transgenic for human FcRn demonstrate their correlation with in vitro FcRn affinity liquid chromatography data.
Results
FcRn affinity chromatography of antibodies
For the development of a new FcRn chromatography method, we first optimized FcRn coupling conditions by investigating different buffers, pH conditions and FcRn quantities with regard to IgG binding capacity. In a second step, binding and elution conditions were assessed by applying various equilibration buffers, elution gradients and flow rates. The optimized method using a linear pH gradient from 5.5 to 8.8 as described in materials and methods yields a symmetrical single peak for a homogeneous standard monoclonal IgG1 antibody preparation, eluting reproducibly at a specific retention time (Fig. 1A). The elution by a pH gradient mimics the physiological pH-dependent FcRn-IgG interaction. Under standard conditions, we were able to show a linear relationship between the amount of IgG1 (20−100 µg) applied to the FcRn affinity column and the peak area (Fig. 1B). To assess the resolution ability of this novel analytical method, we applied various IgGs, Fc-engineered mutants, antibody fragments and degradation products.
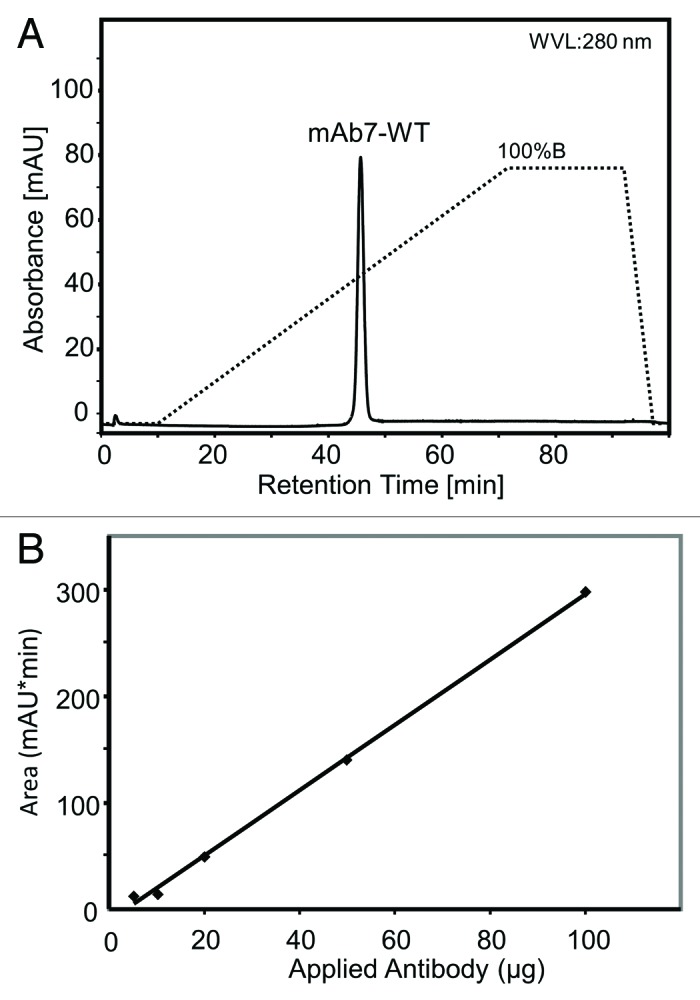
Figure 1. (A) Standard wild-type IgG1 retention profile under standard pH-gradient conditions. (B) Relationship between amount of mAb1 injected into the FcRn column and peak area (retention time vs. absorbance) in the FcRn affinity chromatography.
Role of Fc and F(ab’)2 in FcRn interaction
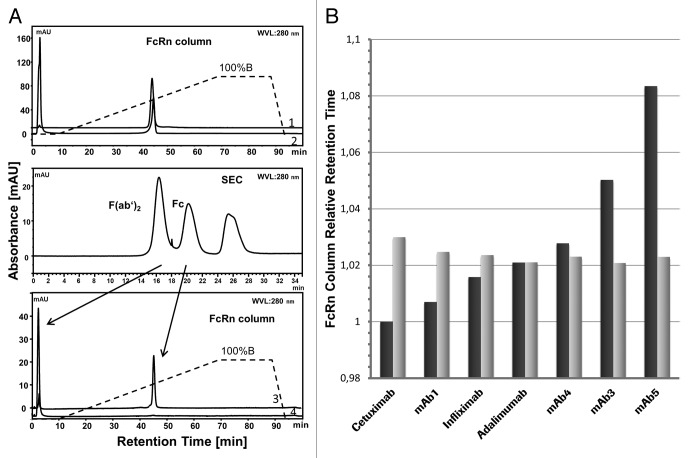
A comparison of full-length mAb2 with its F(ab’)2 and Fc portions obtained by IdeS digestion and separated by size exclusion chromatography (SEC) demonstrated that full-length mAb2 and the Fc portion of mAb2 virtually had the same retention time and eluted at the same pH in the FcRn affinity chromatography (Fig. 2A). The F(ab’)2 of mAb2 did not bind to the column, but was eluted rapidly after injection into the column and contributed only slightly to the retention of full-length mAb2. To further investigate the effect of the Fab part on FcRn interactions, three marketed (cetuximab, infliximab, adalimumab) and four investigational (mAb1, mAb3–5) therapeutic antibodies, with the same human allotype (G1m1.17) were investigated in the FcRn affinity chromatography for differences in the retention time between the full-length antibodies and their Fc portion after cleavage by IdeS protease. Figure 2B illustrates that, relative to the column retention time of full-length cetuximab (Erbitux®), retention times of the other six full-length antibodies were up to 8% longer and varied notably. In contrast, the retention times of the respective Fc portions of all seven antibodies were essentially the same. When plasmin was used for cleavage of the full-length antibodies, the same findings were obtained (data not shown). These results thus show that the Fab parts of seven different antibodies contribute measurably to the FcRn interaction on the column. The specific influence of the Fab part has also been observed for the SPR experiments with a subset of the seven antibodies used (data not shown).
Figure 2.(A) Role of F(ab’)2 in FcRn interaction. Comparison of the FcRn chromatographic profiles of full-length mAb2 and its F(ab’)2 and Fc portions obtained by IdeS digestion and separated by SEC. The upper picture shows retention times vs. absorbance in the FcRn affinity chromatography with linear pH gradient of full length mAb2 before cleavage and of the F(ab’)2 and Fc portions after cleavage in the same graph. The middle picture shows the peaks of F(ab’)2 and Fc of mAb2 in the SEC and assigns them to the corresponding peaks in the FcRn chromatography in the lower picture. Chromatography conditions: buffer A (20 mM MES, 150 mM NaCl, pH 5.5), buffer B (20 mM Tris/HCl, 150 mM NaCl, pH 8.8), flow 0.5 mL/min, gradient from buffer A to buffer B: 60 min (standard). 1, mAb2 before cleavage; 2, mAb2 after cleavage; 3, Fc part; 4, Fab part. (B) Interaction of Fc with FcRn. Comparison of relative retention times (cetuximab = 1) in the FcRn affinity chromatography between full-length antibodies (black bars) and their respective Fc fragments (gray bars) obtained by IdeS protease cleavage. Retention times are relative to that of the full-length cetuximab (set as 1.0).
Affect of Met252 and Met428 oxidation on FcRn interaction
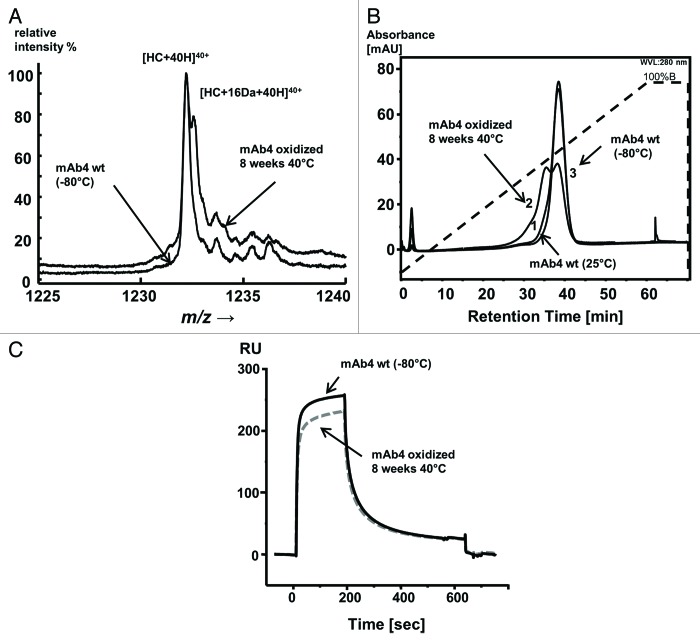
Met252 and Met428 oxidation impairs binding of antibodies to FcRn and negatively affect PK.30-32 Therefore, we analyzed oxidized antibody preparations using our FcRn chromatography method. For this, mAb4 was stored under accelerated conditions at 40°C for two months. ESI-MS data of this sample revealed that approximately 50% of the heavy chains were oxidized at the solvent-exposed Met252 and Met428. A fully functional and biophysical characterization of this sample revealed no further degradations (Fig. 3A). When this stressed mAb4 sample was applied to the FcRn affinity column, two major peaks could be separated, in contrast to the single peak observed for the reference material (Fig. 3B). The later-eluting peak corresponded to the elution time of the unmodified mAb4 reference material, while the earlier-eluting peak likely represented Met252 and Met428 oxidized mAb4. SPR analysis of the same samples merely showed a minor reduction in overall response units (Fig. 3C). The FcRn affinity chromatography resolves two species differing in their FcRn binding ability; our data thus suggest that the oxidized species elute earlier than the native species due to an impaired binding capacities to FcRn. For verification of this hypothesis, isolated fractions need to be characterized with regard to methionine oxidation.
Figure 3.(A) Electrospray ionization mass spectrometry (ESI-MS) analysis of stressed mAb4. Application of a sample of mAb4 stored for 2 mo at 40°C to the ESI-MS shows a 16 Da mass shift of the profile of the stressed mAb4 samples relative to the unstressed mAb4 sample. (B) Effect of Met252 and Met428 oxidation on FcRn interaction. Application of a sample of mAb4 stored for 2 mo at 40°C (curve 2) to the FcRn column leads to an earlier eluting species with a double peak indicative of oxidized mAb4 while application of samples of mAb4 stored for 2 mo at 25°C (curve 1) and -80°C (curve 3) to the FcRn column leads to later eluting, virtually overlapping peaks. Chromatography conditions: buffer A (20 mM MES, 150 mM NaCl, pH 5.5), buffer B (20 mM Hepes, 150 mM NaCl, pH 8.2), flow 0.5 mL/min, gradient from buffer A to buffer B: 60 min (standard). (C) Surface plasmon resonance (SPR) analysis of stressed mAb4. Application of a sample of mAb4 stored for 2 mo at 40°C to the Biacore for SPR analysis shows different sensorgrams for wild-type and for Met252 and Met428 oxidized mAb4 species.
Effect of IgG aggregation on FcRn interaction
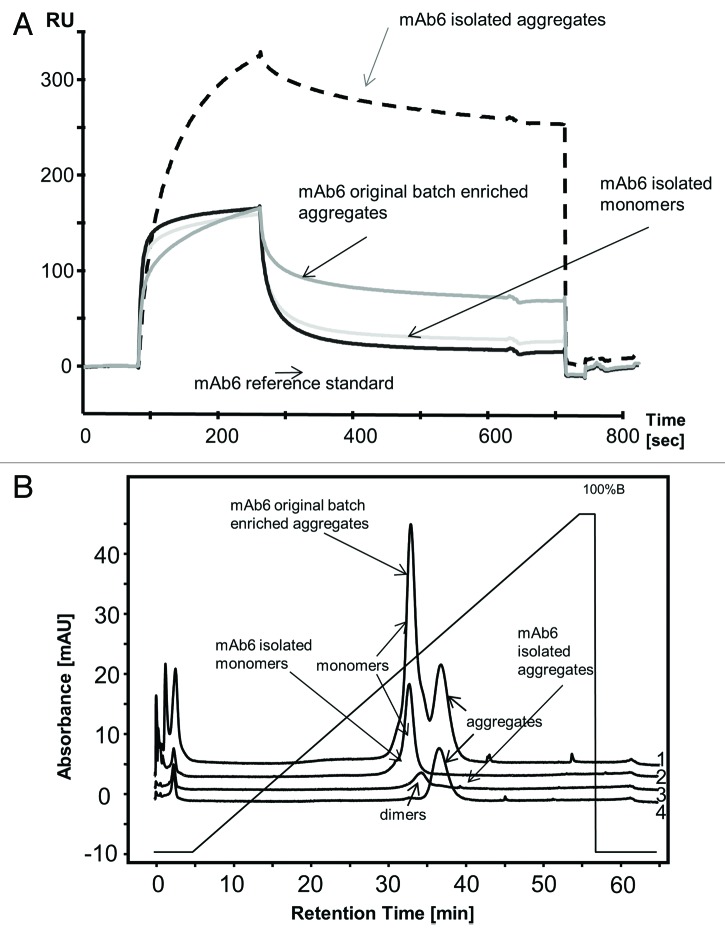
To evaluate the effect of IgG aggregation on FcRn affinity, a mAb6 preparation was used for FcRn interaction studies. This batch (KS64) contained 8.6% Fc-dimer and 0.4% aggregates. SEC was used to isolate two mAb6 fractions from this batch: mAb6 monomers (pool 2) and Fc-dimers and multimeric aggregates (pool 1). The exact compositions of the original batch (KS64) and the fractions are described in Table 1. These two samples were analyzed by SPR and compared with the original KS64 batch and the reference material, which was essentially free of dimers and aggregates. The sensorgram of the monomer-enriched pool 2 could be superimposed with the reference standard material, whereas the original KS64 batch showed differences in the on- and off-rate behavior. More significant differences were observed for the isolated aggregates comprising pool 1, which exhibited a strongly increased response signal and the slowest off-rate (Fig. 4A).
Table 1. Composition of native and enriched pooled fractions of a technical fermentation batch of mAb6.
| Monomers (% of total) | Fc-dimers (% of total) | Aggregates (% of total) | |
|---|---|---|---|
| Native pool KS64 | 91.0 | 8.6 | 0.4 |
| Pool 1 | 27.7 | 26.9 | 45.4 |
| Pool 2 | 98.3 | 1.4 | 0.4 |
Figure 4. (A) SPR analysis of mAb6 aggregates. Sensorgrams of mAb6 as reference standard (curve 1), in the original technical fermentation batch KS64 (3), of isolated mAb6 monomers (curve 2) and isolated mAb6 aggregates (curve 4). (B) Effect of antibody aggregates on FcRn interaction. FcRn chromatographic analysis of mAb6 in the original technical fermentation batch KS64 (lane 1), its isolated monomers (lane 2), isolated Fc-dimers (lane 3) and isolated aggregates (lane 4). Chromatography conditions: buffer A (20 mM MES, 150 mM NaCl, pH 5.5), buffer B (20 mM Tris/HCl, 150 mM NaCl, pH 8.8), flow 0.5 mL/min, gradient from buffer A to buffer B: 50 min (standard).
For the investigation of the effect of aggregation on FcRn chromatography, a differently stressed KS64 batch containing 57% monomers, 13% Fc-dimers and 30% aggregates, was analyzed. Isolated SEC fractions containing the individual species were analyzed in parallel. These data clearly demonstrate that Fc-dimeric and aggregated antibodies exhibit stronger FcRn binding as reflected by a longer retention time (Fig. 4B). The column chromatography even allowed separation between Fc-dimers and higher aggregates.
Although there are differences in the on- and off-rate and overall signal in the SPR analysis, this method does not allow for discrimination between individual aggregation species, i.e., monomer, Fc-dimer and aggregates. In contrast, FcRn chromatography is able to separate the above species based on their binding properties to FcRn-enabling quantification and functional assessment of such species.
Effect of Fc mutations on FcRn affinity
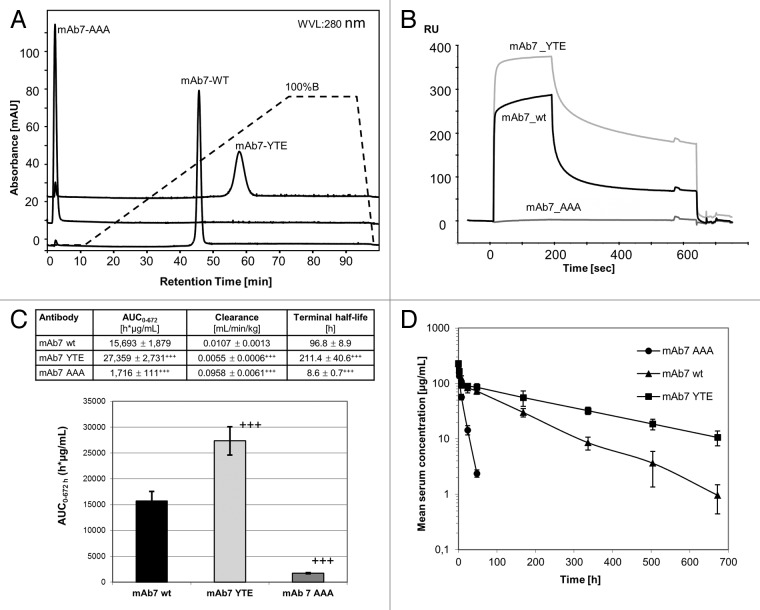
Native mAb7 and its triple mutants YTE and AAA(I252A, H310A, H435A) were analyzed using FcRn column chromatography and SPR. Compared with the retention time of wild-type mAb7 (46 min), the triple AAA mutant did not bind to the FcRn affinity column (flowthrough) while the YTE-mutant was detected after 58 min (Fig. 5A). Consistently, similar behavior was observed in the SPR analysis of these samples, showing enhanced response of the YTE mutant relative to wild-type mAb7 and virtually no response of the AAA mutant (Fig. 5B). These results clearly demonstrate that the effect of antibody Fc engineering can be monitored by FcRn column chromatography as an additional method to SPR.
Figure 5.(A) Effect of Fc mutations of mAb7 on FcRn interaction. FcRn column chromatography of wild-type mAb7 in comparison with its triple mutants YTE and AAA. Chromatography conditions: buffer A (20 mM MES, 150 mM NaCl, pH 5.5), buffer B (20 mM Tris/HCl, 150 mM NaCl, pH 8.8), flow 0.5 mL/min, gradient from buffer A to buffer B: 60 min (standard). (B) SPR analysis of mAb7 mutations. Sensorgrams of wild-type mAb1 (curve 2) compared with triple mutants YTE (curve 3) and AAA (curve 1) of mAb7. (C and D) Effect of Fc mutations of mAb7 on pharmacokinetics in FcRn transgenic mice. Wild-type mAb7 or its triple mutants YTE and AAA were given as a single intravenous bolus injection of 10 mg/kg to 8 animals per group. Results are presented as the mean ± standard deviation (SD), ANOVA analysis of significance in comparison with wild-type mAb7 (+++, p < 0.001). (A) Area under the serum concentration-time curve from time 0 to 672 h (AUC0–672h). 5d. Time concentration profile.
Affect of Fc mutations of mAb7 on pharmacokinetics in mice transgenic for human FcRn
The PK study in C57BL/6J mice deficient for mouse FcRn, but homozygously transgenic for human FcRn (huFcRn (276) -/tg) showed that the YTE mutation enhanced the AUC of mAb7, whereas the AAA mutation reduced it in comparison to the wild-type antibody (Fig. 5C and D). Statistically significant, the YTE mutant of mAb7 had a 1.74-fold higher AUC0–672h, a 1.95-fold slower clearance and a 2.2-fold longer terminal half-life in comparison to wild-type mAb7 (Fig. 5D). In contrast, the triple A mutant exhibited only 10.9% of the AUC0–672h of wild-type mAb7, a 9-fold faster clearance and an 11.3-fold shorter terminal half-life (Fig. 5C).
Discussion
The neonatal Fc receptor (FcRn) plays an important role in the metabolic fate of antibodies. Hence, analysis of the interaction between FcRn and mAbs in vitro is extremely valuable to determine their structural and functional integrity and predict the corresponding FcRn-dependent PK properties. The FcRn column chromatography method described here is an ideal new tool to monitor this interaction. Our results showed that analytical FcRn chromatography was able to differentiate these antibody samples by their retention time profile: (1) IgGs with different Fabs, with separation probably due to variations in the electrical charge distribution or hydrophobic interactions that influence the interaction with the Fc; (2) Fc methionine oxidized samples, which had reduced retention times; (3) aggregates exhibiting stronger interaction probably due to avidity effects of the Fc parts; and (4) samples with mutations in the Fc, which imparted varying effects. We were also able to demonstrate that changes in the FcRn chromatography profile of Fc-engineered antibodies relative to the wild-type antibodies correlated with the PK properties in human FcRn-transgenic mice.
The FcRn receptor is a non-covalent hetero-dimer with each of the two FcRn molecules interacting with one heavy chain of the Fc part of an IgG molecule.14,20,21 The analytical methods currently used to study antibody-FcRn binding properties include enzyme-linked immunosorbent assays (ELISA), SPR, isothermal titration calorimetry (ITC), SEC and analytical ultracentrifugation.17,19,21,23 These techniques are characterized by specific advantages and disadvantages. Under standard conditions, they have only limited sensitivity for the detection of small differences in affinity when used as a stand-alone technique.21 Furthermore, the currently available methods do not appropriately reflect the physiologic pH dependency of the FcRn binding characteristics requiring acidic pH to mimic endosomal binding, but neutral pH for IgG release.10,12,13 A physiological pH milieu is also important because it has an influence on the self-association properties of the FcRn heterodimer.33 Current methods, however, work at only one pH at a time making it complicate to investigate the highly complex and pH-dependent FcRn-IgG interaction, preventing a sensitive and physiologically-relevant kinetic evaluation. This fact may be responsible for the lack of correlation between in vitro FcRn affinity and PK found in several studies.23-25
SPR analysis of the IgG-FcRn interaction of samples containing various antibody species differing in their affinity to the receptor only yields overall kinetic or binding values, but is unable to resolve and quantify the individual molecules species in such samples. In contrast, the FcRn affinity chromatography allows investigations under appropriate physiologic conditions with a predominant 2:1 stoichiometry or other stoichiometries including 1:2, 1:1 and 2:2, depending on the amount of immobilized receptor. The pH gradient can be adjusted to optimize the separation of the different species found in a heterogeneous sample of interest. The resolved peaks can be easily quantified by their respective area, and the individual fractionated and isolated peaks are amenable to secondary analyses, e.g., functional characterization, re-chromatography, mass spectrometric analysis or PK assessment. Importantly, and in contrast to all other methods, the FcRn affinity chromatography enables separation of molecule species based on functional differences rather than physicochemical properties facilitating alternative characterization strategies.
Stressed antibody samples containing high levels of molecules oxidized at positions Met252 and Met428 are a good example to illustrate the difference between the SPR technique and the FcRn affinity chromatography as described above. While SPR analysis detected a 20% relative decrease in binding of the stressed sample compared with the reference material, it did not provide insight into the heterogeneity of this sample. In contrast, FcRn chromatography of the same sample displayed two distinct peaks, one with a retention time corresponding to that of the single peak in reference material and a second peak that was significantly shifted toward shorter retention times, which indicated a weaker interaction of the antibody with the FcRn column material at lower pH. These results are in accordance with previously reported observations.30,32 Our method would also enable the preparative isolation of various oxidized antibody species based on their FcRn binding properties. These isolates could then be further characterized with regard to their PK properties and the distinct methionine oxidation status. This method can also distinguish aggregation products from monomeric IgG and allows their quantification.
FcRn chromatography analysis of seven different IgG1 antibodies exhibiting different retention times confirmed previous reports of FcRn binding differences of IgGs with the same Fc sequences.17 Using IdeS or Plasmin digests, the results reported here clearly show that the Fab part of an antibody affects the interaction of Fc and FcRn. This could be due to different biophysical characteristics, including charge, hydrophobic patches or conformational differences. Such properties can be indirectly assessed by FcRn affinity chromatography.
The set of in vitro and in vivo experiments conducted with wild-type IgG and engineered Fc-part AAA-34,37 and YTE-mutants28 showed a correlation of FcRn affinity chromatography results to PK in mice transgenic for human FcRn. The AAA-mutation that showed a shorter FcRn column retention time was associated with faster clearance from plasma and a shorter half-life compared with the wild-type antibody. The YTE-mutation lead to a significantly prolonged half-life and slower plasma clearance in accordance with previous experiments.28 The longer in vivo half-life corresponded to a longer retention time in the FcRn chromatography. These results are in agreement with data of a Fc-engineered trastuzumab variant that exhibits an extended half-life and enhanced in vitro binding properties to FcRn as measured by flow cytometry.29 In addition, a variant of the anti-vascular endothelial growth factor IgG1 bevacizumab with 11-fold improved FcRn affinity was shown to have a 5-fold extended half-life in human FcRn transgenic mice and a 3-fold longer half-life in cynomolgus monkeys.35
An intrinsic limitation of all in vitro FcRn interaction analyses with respect to prediction of in vivo PK is the absence of numerous target-related specific and non-specific interactions that can occur in vivo with target, matrix and blood proteins such as competing serum IgG, which are absent in the in vitro system. Thus, it would be difficult in a quantitative manner to exactly correlate retention times of the in vitro FcRn affinity chromatography with PK parameters. The data presented here suggest, however, that in vitro assessment of FcRn interaction with IgG using FcRn affinity chromatography can help to predict the affect of sequence mutations, chemical modifications and differences in post-translational modifications, e.g., Fc glycosylation, on the PK properties of a given IgG molecule.
By definition, FcRn chromatography only evaluates the structural and functional integrity of the antibody parts relevant for FcRn binding and does not address the Fc receptors binding in general. Additional assays are required for a full evaluation of the functional integrity of antibody preparations. In fact, work is ongoing to establish an Fcγ receptor affinity chromatography to further complement the functional analysis of antibodies.
This study presents a novel affinity chromatography method for characterizing IgGs with respect to their interaction with the FcRn receptor. Analyses with the FcRn chromatography method are performed using a pH gradient that resembles physiologic conditions. Application of this method to antibody samples with biophysical differences, sequence mutations, chemical and post-translational modifications showed its value for quantitative and qualitative characterization of the IgG-FcRn interaction. Importantly, results of the FcRn affinity chromatography correlated with PK parameters as shown for antibody variants with faster and slower plasma clearance.
In general, affinity chromatography allows secondary analysis of isolated peak eluates, thus enabling new function-based separation followed by biophysical or further functional and in vivo characterization (e.g., PK assessment). Different aspects of antibody functionality can be addressed using this kind of methodology by varying the immobilized binding partner, e.g., target and other Fc receptors of human or animal species.
Materials and Methods
Cloning, expression and purification of mAb7
Expression plasmids encoding the light and the heavy chain of the wild-type human IgG1 mAb were a gift of Erhard Kopetzki (Roche Diagnostics GmbH).The expression was driven by the cytomegalovirus (CMV) early promoter and the antibody genes were present in a genomic organization. The introduction of mutations into the constant (Fc) part of the antibody was performed by gene synthesis at GeneArt / Life Technologies Inc. Briefly, the codon for Met at position 252 of the IgG1-Fc was changed from ATG to TAC, resulting in the amino acid Tyr. The codon at position 254 was changed from TCC for Ser to ACC for Thr and the codon at position 256 was changed from ACC for Thr to GAG for Glu according to EU numbering as reported by Kabat et al.36 This triple mutation was called YTE-mutant.28 The triple-A mutant was generated in a similar way. Ile (ATC) at position 253 was changed to Ala (GCC), His (CAC) at position 310 to Ala (GCC) and His (CAC) at position 435 to Ala (GCC). The resulting triple mutant was designated AAA-mutant.211,34,37 The verification of the mutations were performed by DNA sequencing of the corresponding plasmid DNA fragments. The mAb7 wild-type and mutant antibodies were transiently expressed in HEK293 cells by transfection of 1.5 × 106 cells/mL with the corresponding light and heavy chain plasmids in equimolar amounts via lipofection in a 1 L scale. Supernatant was collected seven days after transfection and the antibodies were purified via MabSelect SuRe (GE Healthcare Bio-Sciences AB).
Cloning, expression and purification of huFcRn
The cDNAs encoding the extracellular domain of human FcRn α-chain (sw:fcgn_human, residues 24–297), human β2-microglobulin (em:ab021288, residues 1–119) were generated by gene synthesis (GeneArt / Life Technologies Inc.). Human FcRn α-chain was synthesized without its signal sequence and cloned into pcDNA3.1(+) under the control of the CMV promoter and with the human CD33-signal peptide (MPLLLLLPLLWAGALA). A decahistidine tag and Biotin AviTag (Avidity LLC) were introduced at the C-terminus of the FcRn α coding sequence. Human β2-microglobulin cDNA was cloned into a variant of the expression vector38 without the neomycin resistance cassette and with intron A under the control of the CMV-promoter. Both expression cassettes end with the poly-adenylation signal from bovine growth hormone. The FcRn α expression vector additionally contains the neomycin resistance gene, which, however, was not used during transient expression explained below. All constructs were sequenced to ensure the absence of any undesirable mutations (SequiServe GmbH).
FcRn was transiently expressed by transfection of 293 suspension cells with two plasmids containing the coding sequence of FcRn and of β2-microglobulin. The 293 cells were cultured in chemically-defined medium in shaker flasks at 36.5°C. The cells were diluted every 2−3 d to a density of 3–4 × 105/ mL. For transient expression, a 14 L stainless steel bioreactor was started with a culture volume of 8 L at 36.5°C, pH 7.0 ± 0.2, pO2 35% and a stirrer speed of 100−400 rpm. When the cell density reached 20 × 105/mL, 10 mg plasmid DNA (equimolar amounts of both plasmids) was diluted in 400 mL chemically-defined medium. Transfection reagent was added to this mixture, which was then incubated for 15 min at room temperature and subsequently transferred into the fermenter. From the next day on, the cells were supplied with nutrients in continuous mode: a feed solution based on chemically-defined medium that contained 6.5 g/L glucose, 3 g/L glutamine and 30 g/L soy peptone was given at a rate of 500 mL per day and glucose as needed to keep the level above 2 g/L. The supernatant was harvested seven days after transfection using a swing head centrifuge with 1 L buckets: 4000 rpm for 90 min. The supernatant (13 L) was cleared by a filter (0.45 µm + 0.2 µm) and then handed over for purification of the desired protein.
The clarified supernatants containing hexahis-tagged proteins were loaded on a Ni-NTA affinity chromatography resin (Qiagen) at 4°C. After two wash steps with 20 mM sodium phosphate buffer comprising 500 mM NaCl at pH 7.4, containing 20 mM imidazole in a first step and 100 mM imidazole in a second step, proteins were eluted at a flow rate of 2 ml/min using batch elution with the same buffer containing 300 mM imidazole on an ÄKTA Prime chromatography system (Amersham Pharmacia Biotech). Fractions were pooled and further purified in sodium phosphate buffer containing 500 mM NaCl on SEC (SuperdexTM 200, GE Healthcare). Purified proteins were quantified using a Nanodrop spectrophotometer (Nanodrop Technologies) and analyzed by SDS PAGE on NuPAGE 4–12% Bis–Tris gels in MES buffer under denaturing and reducing conditions.
Affinity column preparation
Between 1.2 mg and 12 mg FcRn/β2-microglobulin in 5 ml 20 mM sodium citrate buffer, pH 5.5 containing 150 mM KCl, 250 µl PBS and 1 tablet complete protease inhibitor (cOmplete ULTRA Tablets, Roche Diagnostics GmbH) were biotinylated using the biotinylation kit from Avidity according to the manufacturer instructions (Bulk BIRA, Avidity LLC). The biotinylation reaction was done at room temperature overnight. The modified protein was dialyzed against 20 mM sodium phosphate buffer comprising 150 mM NaCl, pH 7.5 at 4°C overnight to remove excess of biotin. For coupling to streptavidin sepharose, one gram streptavidin sepharose (GE Healthcare) was added to the biotinylated and dialyzed receptor (between 1.2 and 12 mg FcRn/β2-microglobulin, for standard analytical application 3 mg were chosen) and incubated for two hours with shaking. The receptor derivatized sepharose was filled in a 1 ml XK column (GE Healthcare) and the FcRn column then was equilibrated with 20 mM 2-(N-morpholine)-ethanesulfonic acid (MES) buffer containing 150 mM NaCl, pH 5.5.
FcRn affinity chromatography
ntibody samples containing 50 to 100 µg of protein were adjusted to pH 5.5 and applied to the FcRn column using ÄKTA explorer 10 XT or Dionex Summit (Dionex). The column with 5 cm bed height was then washed with 5–10 column volumes of equilibration buffer 20 mM MES, 150 mM NaCl, pH 5.5. The affinity-bound Fc-containing proteins were eluted with a pH gradient to 20 mM Tris/HCl, 150 mM NaCl, pH 8.8, in 30 column volumes. Thereby, chromatography using FcRn columns mimicked physiological conditions with binding at acidic pH in the acidified endosome (pH 5.5–6.0) and release at pH 7.4 in the blood.39 For complete elution of modified antibodies, the pH is increased in the gradient up to pH 8.8. The experiments were performed at room temperature. The elution profile was obtained by continuous measurement of the absorbance at 280 nm. The time taken for an analyte peak, X, to reach the detector after sample injection was called the retention time.
Antibodies and in vitro sample preparation
In addition to mAb7 used for in vivo and in vitro experiments, commercially available and in-house antibodies were used for in vitro experiments. The three commercially available antibodies were cetuximab (Erbitux®; Merck KGaA), infliximab (Remicade®; Merck and Co., Inc.) and adalimumab (Humira®; AbbVie). Six recombinant human mAbs directed against cell-bound and soluble target antigens were produced in-house and designated mAb1 to mAb6. mAb1 (native IgG1; 22 mg/mL) was used at amounts of 5; 10; 20; 50; and 100 µg to study its relationship with the corresponding peak area when injected into the FcRn column in a volume of 100 µL.
mAb2 (IgG1; 1 mg/mL) was used as native preparation and for preparation of the bivalent antigen-binding fragment F(ab’)2 and the Fc of the full-length antibody. The F(ab’)2 and Fc were prepared by cleavage of the full-length wild-type antibody mAb2 diluted 1:1 with 100 mM Tris, pH 8.0, by adding 1 µg IdeS cysteine protease per 50 µg antibody and incubation for 2 h at 37°C. The resulting cleavage products F(ab’)2 and Fc were separated on a SEC column (Superdex 200, GE Healthcare) using an ÄKTA Explorer chromatography system (GE Healthcare) and the peak fractions were pooled. Molecular weight standards on the same column served to identify the two cleavage products based on their retention times. Whole antibody mAb2 and its cleavage products were studied by FcRn affinity chromatography.
The three commercial antibodies and the investigational antibodies mAb1, mAb3, mAb4 and mAb5 were analyzed as full-length antibodies and as their Fc part only by FcRn affinity chromatography to compare the retention times between the seven whole antibodies and the respective Fc fragments. The influence of mAb oxidation on the retention time in the FcRn affinity chromatography was studied for mAb4. Oxidation of mAb4 (IgG1; 1 mg/mL) was induced by storing mAb4 at 40°C for 2 mo. The unmodified and the oxidized mAb4 samples were analyzed by FcRn affinity chromatography and by FcRn SPR technology. Oxidation of mAb4 was characterized by peptide mapping and electrospray ionization mass spectrometry (ESI-MS).
The influence of antibody aggregate formation on FcRn interaction in the affinity chromatography was evaluated for mAb6 preparations obtained from a fermentation batch KS64 enriched with aggregates. The antibody monomer, Fc-dimer and aggregate fractions were isolated by SEC and pooling of the respective peaks. The FcRn interaction of the individual fractions was analyzed by affinity chromatography and by SPR technology. The FcRn interaction of wild-type mAb7 and its two triple mutants YTE and AAA were studied in vivo and in vitro by affinity chromatography and by SPR technology.
Electrospray ionization mass spectrometry
Protein aliquots (50 μg) were deglycosylated by adding 0.5 μL N-Glycanase plus (Roche) and sodium phosphate buffer (0.1 M, pH 7.1) to obtain a final sample volume of 115 μL. The mixture was incubated at 37°C for 18 h. Afterwards for reduction and denaturing 60 μL 0.5 M TCEP (Pierce) in 4 M Gua · HCl (Pierce) and 50 μL 8 M Gua HCl were added. The mixture was incubated at 37°C for 30 min. Samples were desalted by SEC (Sepharose G-25, isocratic, 40% acetonitrile with 2% formic acid). ESI mass spectra (+ve) were recorded on a Q-TOF instrument (maXis, Bruker) equipped with a nano ESI source (TriVersa NanoMate, Advion). MS parameter settings were as follows: Transfer: Funnel RF, 400 Vpp; ISCID Energy, 0 eV; Multipole RF, 400 Vpp; Quadrupole: Ion Energy, 4.0 eV; Low Mass, 600 m/z; Source: Dry Gas, 8 L/min; Dry Gas Temperature, 160°C; Collision Cell: Collision Energy, 10 eV; Collision RF: 2000 Vpp; Ion Cooler: Ion Cooler RF, 300 Vpp; Transfer Time: 120 µs; Pre Puls Storage, 10 µs; scan range m/z 600 to 2000. Software (MassAnalyzer) developed in-house was used for data evaluation.
FcRn surface plasmon resonance analysis
The binding properties of mAb7-WT, -AAA, -YTE, mAb4 and mAb6 to FcRn were analyzed by SPR technology using a BiaCore T100 instrument (BiaCore AB). In the current assay, the FcRn receptor was immobilized onto a Biacore CM5-biosensor chip (GE Healthcare Bioscience) via amine coupling to a level of 400 response units (RU). The assay was performed at room temperature with PBS, 0.05% Tween20 pH 6.0 (GE Healthcare Bioscience) as running and dilution buffer. A solution containing 200 nM of native or oxidized mAb samples were injected at a flow rate of 50 µL/min at room temperature. Association time was 180 sec, dissociation phase took 360 sec. Regeneration of the chip surface was reached by a short injection of HBS-P, pH 8.0. Evaluation of SPR-data was performed by comparison of the response signal height at 180 sec after injection and at 300 sec after injection. The corresponding parameters are the RU max level (180 sec after injection) and late stability (300 sec after end of injection).
Pharmacokinetic study in human FcRn mice
All procedures were performed in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (www.aaalac.org). The study was authorized by the Regional Council of Oberbayern, Germany. Male and female C57BL/6J mice (background), which are mouse FcRn-deficient but hemizygous transgenic for human FcRn (huFcRn (276) -/tg,40,41 were used throughout the PK study. At the time of administration, the animals weighed between 17 and 25 g. The respective antibody was given as a single intravenous bolus injection via the tail vein. Due to limited blood volume of mice, three groups of four male and four female animals each were required to cover nine sampling time points, i.e., three sampling time points per animal. Blood samples were taken in group 1 at 5 min, 24 and 336 h; in group 2 at 2, 168 and 504 h; and in group 3 at 8, 48 and 672 h after administration. Blood samples of about 100 µL were obtained by retrobulbar puncture and stored at room temperature for 60 min to allow clotting. Serum samples of at least 40 µL were obtained by centrifugation at 9300 x g at 4°C for 3 min and immediately frozen and stored at -20°C until assayed.
Serum concentrations of the human therapeutic antibodies in murine serum were determined by an antigen-captured ELISA specific for the antigen binding region (Fab) of the mAb7 and its variants (Ab-1). All reagents or samples were incubated at room temperature on a shaker at 400 rpm. Each washing step included three cycles. Briefly, streptavidin-coated microtiter plates were coated with biotinylated Ab-1 antigen diluted in assay buffer. After washing with phosphate-buffered saline-polysorbate 20 (Tween20), serum samples in various dilutions were added and incubated for 1 h. After washing, bound human therapeutic antibodies were detected by subsequent incubation with human Fcγ-specific monoclonal Fab fragments conjugated with digoxigenin that do not cross react with mouse IgG. After washing, an anti-digoxigenin antibody conjugated with horseradish peroxidase (HRP) was added and incubated for 1 h. After washing, ABTS (2,2’azino-di[3-ethylbenzthiazoline sulfonate]; Roche Diagnostics GmbH) was added as the HRP substrate to form a colored reaction product. Absorbance of the resulting reaction product was read at 405 nm with a reference wavelength at 490 nm. All serum samples and positive or negative control samples were analyzed in replicates and calibrated against reference standard.
The PK parameters were calculated by non-compartmental analysis, using the PK evaluation program WinNonlinTM (Pharsight), version 5.2.1. Briefly, the area under the concentration/time curve AUC (0–672) was calculated by linear trapezoidal rule (with linear interpolation) from time 0 to infinity. The apparent terminal half-life (T 1/2) was derived from the equation: T1/2 = ln2 / λz. Total body clearance (CL) was calculated as Dose/AUC. Statistically significant differences in the PK parameters between the wild-type antibody and its variants were determined by ANOVA analysis.
Acknowledgments
We thank Roberto Falkenstein (Roche Penzberg, Bioprocess Development) for providing the KS64 batch and Harald Duerr (Roche Penzberg, Department of Protein Sciences) for the purification of wild-type and engineered mAb7 samples.
Glossary
Abbreviations:
- AUC
area under the concentration-time curve
- Cl
clearance
- ESI-MS
electrospray ionization mass spectrometry
- Fab
antigen-binding fragment
- Fc
constant fragment
- FcRn
neonatal Fc receptor
- HRP
horseradish peroxidase
- IgG
immunoglobulin G
- mAb
monoclonal antibody
- PK
pharmacokinetic
- SEC
size exclusion chromatography
- SPR
surface plasmon resonance
Disclosure of Potential Conflicts of Interests
All authors are current or former employees of Roche Diagnostics GmbH
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/24981
References
- 1.Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9:767–74. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- 2.Reichert JM. Which are the antibodies to watch in 2012? MAbs. 2012;4:1–3. doi: 10.4161/mabs.4.1.18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmood I, Green MD. Pharmacokinetic and pharmacodynamic considerations in the development of therapeutic proteins. Clin Pharmacokinet. 2005;44:331–47. doi: 10.2165/00003088-200544040-00001. [DOI] [PubMed] [Google Scholar]
- 4.Vázquez-Rey M, Lang DA. Aggregates in monoclonal antibody manufacturing processes. Biotechnol Bioeng. 2011;108:1494–508. doi: 10.1002/bit.23155. [DOI] [PubMed] [Google Scholar]
- 5.Filpula D. Antibody engineering and modification technologies. Biomol Eng. 2007;24:201–15. doi: 10.1016/j.bioeng.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 7.Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, Lencer WI, et al. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol. 2010;30:777–89. doi: 10.1007/s10875-010-9468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 9.Martin WL, West AP, Jr., Gan L, Bjorkman PJ. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding. Mol Cell. 2001;7:867–77. doi: 10.1016/S1097-2765(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 10.Goebl NA, Babbey CM, Datta-Mannan A, Witcher DR, Wroblewski VJ, Dunn KW. Neonatal Fc receptor mediates internalization of Fc in transfected human endothelial cells. Mol Biol Cell. 2008;19:5490–505. doi: 10.1091/mbc.E07-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JK, Tsen MF, Ghetie V, Ward ES. Identifying amino acid residues that influence plasma clearance of murine IgG1 fragments by site-directed mutagenesis. Eur J Immunol. 1994;24:542–8. doi: 10.1002/eji.1830240308. [DOI] [PubMed] [Google Scholar]
- 12.Ober RJ, Martinez C, Lai X, Zhou J, Ward ES. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proc Natl Acad Sci U S A. 2004;101:11076–81. doi: 10.1073/pnas.0402970101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol. 2004;172:2021–9. doi: 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez LM, Penny DM, Bjorkman PJ. Stoichiometry of the interaction between the major histocompatibility complex-related Fc receptor and its Fc ligand. Biochemistry. 1999;38:9471–6. doi: 10.1021/bi9907330. [DOI] [PubMed] [Google Scholar]
- 15.Huber AH, Kelley RF, Gastinel LN, Bjorkman PJ. Crystallization and stoichiometry of binding of a complex between a rat intestinal Fc receptor and Fc. J Mol Biol. 1993;230:1077–83. doi: 10.1006/jmbi.1993.1220. [DOI] [PubMed] [Google Scholar]
- 16.Ward ES, Martinez C, Vaccaro C, Zhou J, Tang Q, Ober RJ. From sorting endosomes to exocytosis: association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol Biol Cell. 2005;16:2028–38. doi: 10.1091/mbc.E04-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, Lu P, Fang Y, Hamuro L, Pittman T, Carr B, et al. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab Dispos. 2011;39:1469–77. doi: 10.1124/dmd.111.039453. [DOI] [PubMed] [Google Scholar]
- 18.Datta-Mannan A, Chow CK, Dickinson C, Driver D, Lu J, Witcher DR, et al. FcRn affinity-pharmacokinetic relationship of five human IgG4 antibodies engineered for improved in vitro FcRn binding properties in cynomolgus monkeys. Drug Metab Dispos. 2012;40:1545–55. doi: 10.1124/dmd.112.045864. [DOI] [PubMed] [Google Scholar]
- 19.Vaughn DE, Bjorkman PJ. High-affinity binding of the neonatal Fc receptor to its IgG ligand requires receptor immobilization. Biochemistry. 1997;36:9374–80. doi: 10.1021/bi970841r. [DOI] [PubMed] [Google Scholar]
- 20.Raghavan M, Wang Y, Bjorkman PJ. Effects of receptor dimerization on the interaction between the class I major histocompatibility complex-related Fc receptor and IgG. Proc Natl Acad Sci U S A. 1995;92:11200–4. doi: 10.1073/pnas.92.24.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin WL, Bjorkman PJ. Characterization of the 2:1 complex between the class I MHC-related Fc receptor and its Fc ligand in solution. Biochemistry. 1999;38:12639–47. doi: 10.1021/bi9913505. [DOI] [PubMed] [Google Scholar]
- 22.Pollastrini J, Dillon TM, Bondarenko P, Chou RY. Field flow fractionation for assessing neonatal Fc receptor and Fcγ receptor binding to monoclonal antibodies in solution. Anal Biochem. 2011;414:88–98. doi: 10.1016/j.ab.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Gurbaxani B, Dela Cruz LL, Chintalacharuvu K, Morrison SL. Analysis of a family of antibodies with different half-lives in mice fails to find a correlation between affinity for FcRn and serum half-life. Mol Immunol. 2006;43:1462–73. doi: 10.1016/j.molimm.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 24.Gurbaxani BM, Morrison SL. Development of new models for the analysis of Fc-FcRn interactions. Mol Immunol. 2006;43:1379–89. doi: 10.1016/j.molimm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Gurbaxani B. Mathematical modeling as accounting: predicting the fate of serum proteins and therapeutic monoclonal antibodies. Clin Immunol. 2007;122:121–4. doi: 10.1016/j.clim.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol. 2009;182:7663–71. doi: 10.4049/jimmunol.0804182. [DOI] [PubMed] [Google Scholar]
- 27.Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23:1283–8. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- 28.Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281:23514–24. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 29.Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18:1759–69. doi: 10.1093/intimm/dxl110. [DOI] [PubMed] [Google Scholar]
- 30.Pan H, Chen K, Chu L, Kinderman F, Apostol I, Huang G. Methionine oxidation in human IgG2 Fc decreases binding affinities to protein A and FcRn. Protein Sci. 2009;18:424–33. doi: 10.1002/pro.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertolotti-Ciarlet A, Wang W, Lownes R, Pristatsky P, Fang Y, McKelvey T, et al. Impact of methionine oxidation on the binding of human IgG1 to Fc Rn and Fc gamma receptors. Mol Immunol. 2009;46:1878–82. doi: 10.1016/j.molimm.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Vlasak J, Li Y, Pristatsky P, Fang Y, Pittman T, et al. Impact of methionine oxidation in human IgG1 Fc on serum half-life of monoclonal antibodies. Mol Immunol. 2011;48:860–6. doi: 10.1016/j.molimm.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Vaughn DE, Bjorkman PJ. Structural basis of pH-dependent antibody binding by the neonatal Fc receptor. Structure. 1998;6:63–73. doi: 10.1016/S0969-2126(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 34.Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, et al. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J Exp Med. 2002;196:303–10. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IWL, Sproule TJ, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28:157–9. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of proteins of immunological interest. 5th edition, U.S. Public Health Services, National Institute of Health (NIH) 1991; publication no. 91-3242, Bethesda, MD. [Google Scholar]
- 37.Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, et al. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci U S A. 2008;105:9337–42. doi: 10.1073/pnas.0801717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji Z, Lee JY, Pan Z, Jiang B, Tian B. Progressive lengthening of 3′ untranslated regions of mRNAs by alternative polyadenylation during mouse embryonic development. Proc Natl Acad Sci U S A. 2009;106:7028–33. doi: 10.1073/pnas.0900028106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 40.Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–33. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 41.Roopenian DC, Christianson GJ, Sproule TJ. Human FcRn transgenic mice for pharmacokinetic evaluation of therapeutic antibodies. Methods Mol Biol. 2010;602:93–104. doi: 10.1007/978-1-60761-058-8_6. [DOI] [PubMed] [Google Scholar]