Abstract
To take advantage of the large number of well-characterized mouse immunoglobulins (IgGs) for the study of antibody-dependent cell-mediated cytotoxicity (ADCC) in human cells, we armed human cytotoxic lymphocytes with a mouse receptor for the Fc portion of IgG antibodies. The human ΝΚ−92 natural killer cell line was transduced with a mouse receptor gene (mCD16), which was stably expressed on the cell surface (referred to as NK-92mCD16). When tested against a B-lymphoblastoid cell line (BLCL) coated with mouse anti-CD20 IgG1, IgG2a or IgG2b monoclonal antibodies (mAbs), the newly expressed mouse Fc receptor enabled the NK-92mCD16 cells to kill the BLCL by ADCC. Next, using the NK-92mCD16 we compared mouse mAbs directed at B lineage specific CD antigens for their ability to induce ADCC against human Epstein-Barr virus- infected B lymphoblastoid (for anti-CD19, -CD20 and -CD21) or against myeloma (for anti-CD38 and –CD138) target cells. Our results demonstrated that the “NK-92mCD16 assay” allows convenient and sensitive discrimination of mouse mAbs for their ability to mediate ADCC in a human cellular system. In addition, our results provide examples of dissociation between opsonization and target cell killing through ADCC. These “murinized” human effector cells thus represent a convenient cellular tool for the study of ADCC.
Keywords: ADCC, transfection, mouse, CD16, human, lymphocyte, NK, xenogenic
Introduction
Antibody-dependent cell-mediated cytotoxicity (ADCC) is one of the mechanisms by which therapeutic antibodies achieve clinical efficacy. This mechanism combines humoral immunity, which involves specific antigen (Ag) recognition by an antibody (Ab), with cellular immunity, which involves cell-mediated cytolytic destruction of Ab-coated target cells. While the specificity of target cell recognition resides within the Fab portion of the Ab molecule, ADCC occurs upon the interaction between the Fc portion of the target cell-bound Ab and the Fc receptors (FcR) expressed by effector cells, such as FcγRIIIA/CD16A, which recruit and activate effector cells.
In the context of ADCC-mediated tumor cell lysis, Fab-dependent specificity is essential for tumor cell discrimination (and consequently low toxicity), while Fc-dependent effector recruitment is essential for tumor cell killing. An ideal therapeutic Ab would be tumor-specific; however, most of the Ags that are currently targeted in clinical practice are tumor-associated rather than tumor-specific. In addition, because a particular Ag may be adequately tumor-associated, but not expressed by the entire tumor cell population, two or more tumor-associated Ags may be considered targets to improve tumor cell killing. ADCC depends not only on the Ag/Ab and the FcR/Fc affinities, but also on the access of the FcR to the Fc once the Ab is associated with the tumor Ag. Thus, at least two levels of Ab screening could be considered a priori: first, to identify an Ag; and second, to identify the best epitope to be targeted on this particular Ag. Indeed, over 25 y ago, ADCC by effector human lymphocytes was suggested to be “apparently sensitive to spatial orientation and organization of target cell-bound Ab.”1 Accordingly, Fc accessibility for the FcR and its consequences on ADCC efficiency may be different for each Ag, depending on the epitope that is recognized. Thus, to optimize tumor cell destruction through ADCC, the monoclonal antibody (mAb) that allows for the best effector cell activation should be chosen.
While considerable technological efforts have been made to assess ADCC optimization through Fc modifications, no straightforward technique has been identified to associate epitope specificity and ADCC performance against a particular Ag. For this purpose it would be advantageous to be able to test in an effector/target human system the currently available mouse mAbs and those that are newly produced by hybridomas, a technology more available than animals that are humanized for the immunoglobulin locus. To this end, we describe here the production and characterization of human cytotoxic lymphocytes armed with a mouse FcγR and show how these “murinized” human effector cells can become useful cellular tools to analyze the ADCC potential of mouse Abs. Moreover, using this approach, we found that the ADCC-mediated lysis of a given target cell opsonized to the same extent by mAbs directed to different Ag can be dramatically different, demonstrating that opsonization is necessary, but not sufficient, to induce ADCC.
Results
Human FcγRIIIA-human FcεRIγ (hCD16A) and mouse FcγRIII-human FcεRIγ chimeric (mCD16) vectors
The chimeric cDNA coding for human FcγRIIIA-V158 linked to human FcεRIγ (hCD16) has been described previously.2 The chimeric cDNA coding for mouse FcγRIII linked to human FcεRIγ (mCD16) was synthesized, and it comprised the extracellular domain of the C57BL/6 mouse FcγRIII haplotype T3 linked to the cDNA coding for the human FcεRIγ (nucleotides 83 to 283). The human FcεRIγ comprised a two amino acid (aa) sequence (Pro4-Gln5) of the extracellular domain and the intact transmembrane and intracytoplasmic domains, as previously described.4 The mCD16 chimeric cDNA was cloned into the HindIII and NotI sites of the pMX retroviral vector (Fig. 1).

Figure 1. Schematic representation of the chimeric mouse FcγRIII-humanFcεRIγ molecule. The mCD16/hγ chimeric cDNA comprised the leader (L) and the extracellular (EC) domain of mouse CD16 (T haplotype), two amino-acids (aa) of the extracellular domain of the human FcεRIγ, as well as the intact transmembrane (TM) and intracellular (IC) domains.
Generation of the hCD16 (NK-92hCD16)- and the mCD16 (NK-92mCD16)-transduced NK-92 cell line
Amphotropic retroviral vector particles were produced by the transfection of Phoenix-Ampho packaging cells and used for the transduction of the human natural killer (NK) cell line NK-92, which does not express human CD16.5 Five days after transduction, 35% of the NK-92hCD16 and 41% of the NK-92mCD16 cells expressed the hCD16 and mCD16 receptors, respectively, as assessed by flow cytometry (Fig. 2). After immuno-selection, the expression of both transgenes remained stable for the 3 mo of follow-up (data not shown).
Figure 2. Surface expression of murine mCD16 and hCD16 on human NK-92 cell line. After retroviral transduction, transduced NK-92 cell lines were selected by immunomagnetic separation with rat-anti-mouse CD16/32 mAb (clone 93) and mouse-anti-human CD16 mAB (clone 3G8) respectively and with magnetic beads. Surface expression of murine CD16 or human CD16 was determined by flow cytometry with a PE- (black areas) and in control with isotype (open areas). Mean fluorescence intensities are indicated.
ADCC by NK-92hCD16 and NK-92mCD16 cells
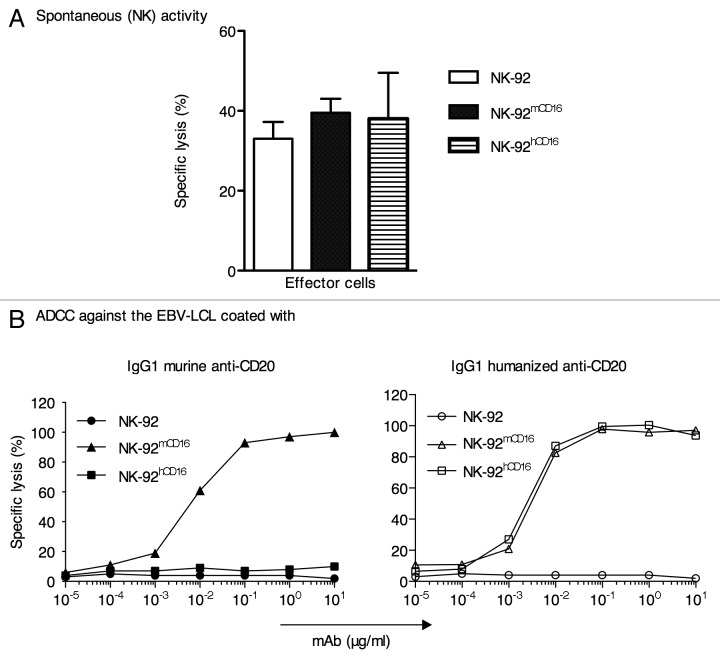
Before assessing the ADCC activity, we first examined whether the expression of the mCD16 or hCD16 receptors had any effect on the natural (CD16-independent) cytotoxic activity of the NK-92 cell line. As shown in Figure 3A, no significant differences were observed between the NK-92, NK-92hCD16 and NK-92mCD16 cells in their ability to kill the NK-sensitive K562 cell line. Next, the ADCC activity of the NK-92, NK-92hCD16 and NK-92mCD16 cells was evaluated using a standard 4 h 51Cr release assay against a human CD20-positive Epstein-Barr virus (EBV)-infected B lymphoblastoid cell line (BLCL) in the presence of increasing concentrations of mouse anti-CD20 IgG1 (clone AT80) (Fig. 3B, left panel) or the chimeric anti-CD20 rituximab (Fig. 3B, right panel).
Figure 3. Cytotoxicity activity of NK-92, NK-92 mCD16 and NK-92 hCD16 cell lines. (A) Spontaneous (NK) cytotoxic activity of NK-92, NK-92 mCD16 and NK-92 hCD16 was determined against K562 in 51Cr-release assays at an E/T ratio of 10:1. (B) ADCC activity by NK-92, NK-92 mCD16 and NK-92 hCD16 against an EBV-LCL in the presence the increasing concentrations of murine or humanized (IgG1 anti-CD20 (clone AT80) or rituximab) anti-CD20.
As shown in Figure 3B (left panel), only the NK-92mCD16 cells killed the human BLCL incubated with the mouse anti-CD20 Ab. By contrast, both the NK-92hCD16 and NK-92mCD16 cells killed the human BLCL that was pre-incubated with the chimeric anti-CD20 Ab (Fig. 3B, right panel). Although these data demonstrate that mouse CD16 can perform ADCC in the presence of rituximab, the complete superposition of the two curves is fortuitous because the expression of human and mouse CD16 by transduced NK-92 cells and the affinity of rituximab for these two Fc receptors are likely dissimilar. All the dose responses reached a plateau at the Ab concentration of 10−1 µg/ml.
Mouse isotype and NK-92mCD16-mediated ADCC
We next compared the NK-92mCD16-mediated ADCC in the presence of mouse IgG1, IgG2a and IgG2b anti-human CD20 mAbs (Table 1), which recognize closely related epitopes in the large extracellular loop of CD20.6,7 The three mAbs mediated ADCC against the EBV-LCL in the presence of NK-92mCD16, with the following lysis ranking by isotype: IgG2a ≥ IgG2b > IgG1 (Fig. 4). The minimum concentration of Ab able to induce detectable ADCC in our assay was between 10−4 and 10−3 μg/ml for IgG2a and IgG2b and between 10−3 and 10−2 μg/ml for IgG1. In the presence of the NK-92hCD16 effectors, only IgG2a anti-CD20 induced detectable ADCC against the EBV-LCL. The cytotoxic score in the latter case, however, was almost negligible compared with that observed in the presence of the NK-92mCD16 effectors cells, i.e., those armed with the cognate (mCD16) FcγR.
Table 1. Specificities, clones/isotypes and sources of antibodies used in study.
| Specificity | Clone | Isotype | Source |
|---|---|---|---|
| Human CD20 | AT80 | Mouse IgG1 | AbDSerotec |
| B9H9 (HCR20) | Mouse IgG2a | Beckman coulter/Immunotech | |
| 2H7 | Mouse IgG2b | Beckman coulter/Immunotech | |
| HI47 | Mouse IgG3 | ABBIOTEC | |
| Rituximab | Chimeric IgG1 Hu Fc | Roche | |
| Human CD19 | J3–119 | Mouse IgG1 | Beckman coulter/Immunotech |
| A3-B1 | Mouse IgG2a | Immunostep | |
| Human CD21 | BL13 | Mouse IgG1 | Beckman coulter/Immunotech |
| HI21a | Mouse IgG2a | Immunostep | |
| Human CD38 | T16 | Mouse IgG1 | Beckman coulter/Immunotech |
| Human CD138 | B-A38 | Mouse IgG1 | Exbio Antibodies |
| Human CD16 | 3G8 | Mouse IgG1 | Beckman coulter/Immunotech |
| Mouse CD16/32 | 93 | Rat IgG2b | Beckman coulter/Immunotech |

Figure 4. Comparison of ADCC activity of NK-92 mCD16 and NK-92 hCD16 cell lines toward human EBV-LCL preincubating with increasing concentrations of murine IgG, IgG2a and IgG2b anti-CD20. All Data are presented as the mean of 2 or 3 independent experiments ± SD of the percent of specific lysis at effector-to-target ratio = 10:1.
Suitability of NK-92mCD16 to screen the ADCC potential of mouse mAbs
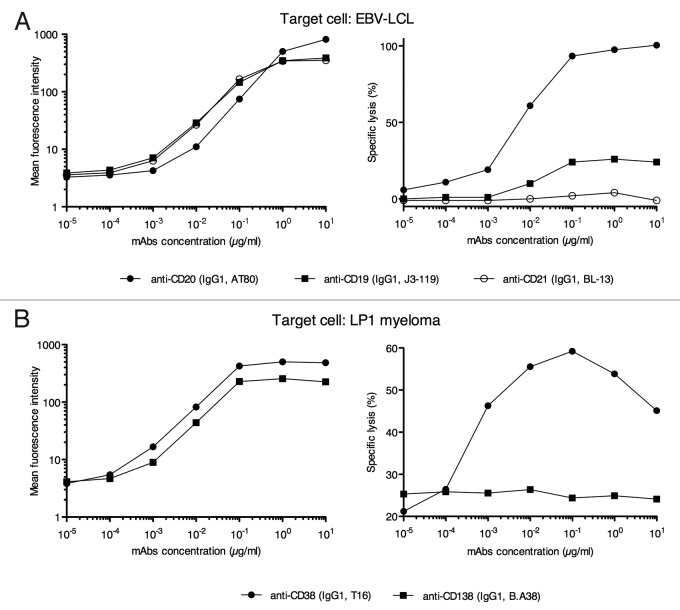
To compare the ADCC potential of mouse mAbs directed at different B cell target Ags, we used an EBV-LCL that expressed CD19, CD20 and CD21. Figure 5A (left panel) shows staining of the EBV-LCL with the mouse IgG1 mAbs J3–119, AT80 and BL13 (Table 1), which are specific for CD19, CD20 and CD21, respectively. After incubation with the first Ab, cells were washed before incubation with the R-phycoerythrin (PE) goat anti-mouse mAb used at saturating concentration. In addition an enzyme-linked immunosorbent assay was performed to verify that the anti-IgG binds similarly to the three Abs (data not shown). In these conditions, the levels of staining reflect the levels of opsonization of each mAb, and at it is shown in Figure 5A (left panel), at 1 μg/ml, levels of opsonization were very close for CD19, CD20 and CD21. Next, using NK-92mCD16 and the above mouse mAbs, we compared the ADCC score mediated by each mAb. As shown on Figure 5A, right panel, no correlation was observed between the level of opsonization and the level of ADCC. Of note, in contrast to reports in the literature (see discussion), the mouse anti-CD20 mAb AT80 initiated ADCC against the EBV-LCL. A second example is presented in Figure 5B. First, CD38 and CD138 were tested with mAbs T6 and BA38 for Ag expression and the ability to drive ADCC upon Ag recognition. The target cell was the LP1 myeloma cell line. As in the previous case, when a specific mAb was used, the assay clearly dissociated opsonization from the induction of ADCC. Indeed, although CD38 and CD138 showed close levels of surface staining (Fig. 5B, left panel), the targeting of CD38, but not CD138, by any of the four mAbs tested (BA38 shown in Figure 5B, right panel as well as MI15, BB4 and DL101; data not shown) triggered ADCC.
Figure 5. Target antigen evaluation for ADCC against an EBV-LCL and a myeloma cell lines using NK-92mCD16. (A) Target cell is a human EBV transformed LCL. Left panel shows the EBV-LCL staining with the murine mAbs against CD19, CD20 and CD21 and the right panel shows the ADCC scores. (B) Target cell is the human LP1 myeloma cell line. Left panel shows the SP1 staining with the murine mAbs against CD38 and against CD138 and the right panel shows the ADCC scores. All murine mAbs are of the IGg1 isotype and ADCC scores represent the mean of triplicates of percentage of specific lysis at a NK92mCD16: EBV-LCL or LP1 ratio = 10:1
Discussion
In the present work, we demonstrate that human lymphocytes can be modified by gene transfer to gain ADCC capacity in the presence of mouse mAbs.
After transduction with a mouse CD16/γ receptor gene (mCD16), the human NK cell line NK-92mCD16 displayed stable cell surface expression of the mCD16 receptor. Using target B cells and a set of mAbs directed at several B cell Ags, we demonstrated that the mouse receptor was functional in the human NK cell line. These NK-92mCD16 cells are useful both for analyzing the ADCC potential of mouse mAbs of different isotypes using human (effector and target) cells and consequently for comparing the abilities of different target cell Ags to induce ADCC. Taking advantage of these “murinized” effector cells, we present several examples of the dissociation between the level of opsonization of a target Ag and the ability of effector cells to lyse the Ag-expressing cells by ADCC.
To analyze the ADCC capacity of the NK-92mCD16 cells, we first focused on the CD20 Ag due to its extensive description as an “ADCC target.” When tested against a target BLCL coated with a mouse IgG1 anti-CD20 mAb (clone AT80), the mCD16 receptor enabled the NK-92mCD16 cells to kill the BLCL by ADCC. Expectedly, mouse IgG2a, IgG2b and IgG1 were all capable of inducing ADCC in the presence of NK92mCD16. Of note, the NK-92mCD16 cells mediated ADCC also in the presence of rituximab (a human IgG1), an observation in agreement with the recent analysis by MB Overdijk et al.8
These “murinized” NK-92mCD16 cells present several advantages over human or mouse NK cells for assessing the ADCC activity of mouse mAbs. In addition to the fact that the killing of a human target cell by a murine effector cell may be hampered by the differences between accessory molecules, the major drawback with human NK cells is the FcR-Fc xenogenic interaction because ADCC by human effector cells can be mediated (at least to some extent) by mouse IgG3, IgG2a and IgG2b, but not mouse IgG1.9,10 For example, using 29 mouse mAbs (21 IgG1s, 4 IgG2as and 4 IgG3s) directed at malignant human B cell lines and human mononuclear cells, Würflein et al. showed that only 3 (anti-HLA-DR) of them consistently induced ADCC. This observation could be explained because the anti-HLA-DR antibodies were of the IgG3 isotype, which in contrast to IgG1, interacts with human CD16.11 The xenogenic situation also explains why the mouse anti-CD20 (clone 2B8, an IgG1), from which rituximab is derived, was initially thought incapable of mediating ADCC12 (in the same way as the anti-Tac initially described by Junghans RP et al.13). In these studies, the ADCC potential of mouse IgG1 mAbs was assessed with human PBMC.12,13 These results were also confirmed by our data showing that no ADCC was observed against the EBV-LCL coated with the IgG1 mouse anti-CD20 (Fig. 3B, left panel). Because most of the murine mAb available are of the IgG1 isotype, human PBMC or NK cells are clearly not suitable for assessing ADCC activity by murine mAbs.
The study of ADCC by mouse effector cells is also affected by technical limitations. Mouse NK cells express low levels of FcγRIII14 and show minimal effector functions when incubated in vitro with target tumor cells. In fact, prior to analyses of NK cell responses, most investigators cultured mouse NK cells in the presence of cytokines or treated mice with IFN-γ to elicit detectable effector functions.15-18 Beyond the requirement of animal facilities and the need to treat animals and grow cells prior to experiments, which require time and money, these constraints can also affect the reproducibility of experiments, particularly in the context of a quantitative evaluation.
By contrast, straightforward experiments using NK-92mCD16 permit reproducible comparisons of ADCC potential with different Ab/Ag pairings, as exemplified by the two sets of data in Figure 5. In the present work, performed to validate the NK-92mCD16 cells as a cellular tool, ADCC assays were performed with a limited number of murine mAbs. Obviously, the ranking of different target Ag for their interest in inducing ADCC would require further testing. Despite this, our results clearly discriminated CD20 as a good target for ADCC compared with CD19 (as already shown by Hooijberg, E et al.19) and CD21, thus providing an a posteriori validation of the screening. Such discriminations could not have been drawn, as specified above, by using human PBMC.
Comparison of ADCC against a myeloma cell line induced by opsonization with anti-CD38 and anti-CD138 identified only CD38 as a potential target CD for ADCC. The experiments presented in Figure 5 also demonstrate that the level of CD opsonization obtained with a particular mAb (rank order: CD19 = CD21 > CD20) was clearly dissociated from the ADCC that was triggered by the same mAb-coated CD (rank order: CD20 > > CD19, while ADCC against CD21 was not detectable).
This dissociation, initially described by JE Christiaansen et al.1 in a xenogenic FcγR-Fc model (with human effector cells and mouse IGg2a), reveals other levels of complexity regarding the nature of the cell-Ab-cell interactions that drives ADCC. In conclusion, the “murinized” NK-92mCD16 cells described in the present work represent a sensitive and easy to use cellular tool for the study of ADCC and the screening of mouse mAbs according to their ADCC potential.
Materials and Methods
Cell lines
NK-92, the human NK cell line, (ATCC) was grown in RPMI 1640 culture medium (Gibco, Cergy) supplemented with 10% FBS (PAA Laboratories), 100 UI/ml IL-2 (Proleukin) (Chiron Corporation), 2 mM L-glutamine (Gibco), penicillin (100 UI/ml) and streptomycin (0.1 µg/ml) (Gibco). EBV-BLCLs were derived from donor peripheral-blood mononuclear cells (PBMCs) by in vitro infection with EBV-containing culture supernatants from the Marmoset B95–8 cell line (ATCC) in the presence of 1 µg/ml cyclosporin-A. The BLCL and K562 cell lines were cultured in complete medium consisting of RPMI 1640 (Sigma Aldrich), 10% heat-inactivated fetal calf serum, 2 mM glutamine (Sigma Aldrich), 100 U/ml penicillin and 10 µg/ml streptomycin (Sigma Aldrich).
Antibodies and staining
The specificity, isotype and source of mAbs used in the study are indicated in Table 1. One hundred thousand (0.1 × 106) cells (NK-92, NK-92mCD16, NK-92hCD16, BLCL or LP1) were incubated for 15 min at room temperature at the indicated mAb concentrations diluted with PBS supplemented with 0.1% human albumin (HA) in a final volume of 30μl. After staining, plates were centrifuged, the supernatant was discarded by flicking, and wells were washed twice with 200 μl PBS. Negative controls were set up in the presence of a control isotype in case of direct staining or in the absence of first Abs for indirect staining. For indirect staining, cells were washed after the first incubation and the second Ab was used at saturating concentration.
Construction of the human FcγRIII-human FcεRIγ and mouse FcγRIII-human FcεRIγ chimeric cDNAs
The chimeric cDNA coding for human FcγRIIIA-V158 linked to human FcεRIγ was described previously.2 The chimeric cDNA coding for mouse FcγRIII linked to human FcεRIγ was synthesized (GeneCust). This cDNA was composed of the extracellular domain of mouse FcγRIII (nucleotides 1–215, NCBI Reference Sequence: NP-034318.2 from C57BL/6 strain, haplotype T) linked to the cDNA coding for human FcεRIγ (nucleotides 83–283, GenBank Accession number BC033872). The human FcεRIγ included a two aa sequence (Pro4-Gln5) of the extracellular domain and the intact transmembrane and intracytoplasmic domains, as previously described.4 The chimeric cDNA of mouse FcγRIII-human FcεRIγ (referred to as mCD16) and the chimeric cDNA of human FcγRIII-human FcεRIγ (referred to as hCD16) were cloned into HindIII-NotI and BamHI-NotI sites, respectively, within the Moloney mouse leukemia virus vector pMX under the transcriptional control of the viral long-terminal repeat.20
Retroviral vector production
Transient retroviral supernatants were produced by CaCl2 precipitation with 15 µg of plasmid. Two million Phoenix-Ampho cells21 were seeded into 10-cm-diameter dishes 24 h prior to transfection. The transfection was performed with 15 µg pMX/mCD16 or pMX/hCD16 plasmid DNA using CaCl2 precipitation (Invitrogen). The medium (10 ml) was replaced 6 h after transfection. The conditioned medium was collected 48 h post-transfection, filtered through 0.45-µm pore-size filters and kept at -80°C until use. The viral titer was determined by the transduction of Jurkat T cells (1 × 106 cells per well in 6-well plates) with serial dilutions of virus and analyzed for mouse or human CD16 expression 4 d post-infection. The retroviral supernatant titers were typically 1−5 × 105 IU (Infectious Units)/ml.
NK-92 cell line transduction using retroviral supernatant
The NK-92 cell line was resuspended in RPMI 1640 culture medium supplemented with 10% FBS and 100 UI/ml of recombinant IL-2, seeded at 1 × 106 cells in 1 ml per well into 6-well plates and exposed to 2 × 2 ml of retroviral supernatant by spinoculation (2400 g, 1.5 h, 32°C) in the presence of 4 µg/ml polybrene (Sigma). The culture medium was changed 24 h post-infection. Mock (non-transduced) controls were performed in parallel, by which the supernatant of untransfected packaging cells was added to the NK-92 cell line. The transduction efficiencies were assessed 5 d later by staining the mCD16- and hCD16-transduced NK-92 cell lines (referred to as NK-92mCD16 and NK-92hCD16) with a PE-conjugated rat anti-mouse CD16/32 mAb (clone 93) (Beckman Coulter) or a PE-conjugated mouse anti-human CD16 mAb (clone 3G8) (Beckman Coulter), respectively.
Immuno-selection of transduced NK-92 cell line
After transduction, NK-92mCD16 and NK-92hCD16 cells were stained with rat anti-mouse CD16/32 mAb (clone 93) and mouse anti-human CD16 mAb (clone 3G8), respectively and immuno-selected using the cognate sheep anti-rat and anti-mouse-IgG coated beads (Dynabeads M-450, Dynal AS), according to the supplier’s instructions. Based on CD16 expression, the purity of the immuno-selection was > 95%.
Cytotoxicity and ADCC assay
Cytotoxic activity was assessed using a standard 51Cr release assay. The target cells were labeled with 100 μCi 51Cr for 1 h at 37°C, washed four times with culture medium and plated at the indicated effector-to-target cell ratios in 96-well flat-bottom plates. The indicated mAb was incubated with the target cells for 20 min at room temperature before the addition of effector cells. After a 4 h incubation at 37°C, 25 µl of supernatant were removed from each well and mixed with 100 µl scintillation fluid, and 51Cr activity was counted in a scintillation counter (MicroBeta, Perkin Elmer). Each test was performed in triplicate. The results are expressed as the percentage of lysis, which is calculated according to the following equation: (experimental release - spontaneous release) / (maximal release-spontaneous release) X 100, where the experimental release represents the mean counts per minute (cpm) of the target cells in the presence of effector cells; spontaneous release represents the mean cpm of the target cells incubated without effector cells; and maximal release represents the mean cpm of the target cells incubated with 1% Triton X 100 (Sigma).
Enzyme-linked immunosorbent assay
A 96-well plate (Maxisorp Nunc) was coated for 24 h at 4°C with 5 µg/ml of the mouse antibodies anti-CD19, anti-CD20, anti-CD21 in Bicarbonate / carbonate coating buffer, 100 mM, pH 9.6. The plate was washed three times with PBST (PBS buffer containing 0.05% Tween 20) and then blocked with PBST-1% BSA at room temperature for 1 h, followed by three washes with PBST. The plate was incubated for 1 h at room temperature with the secondary antibody (Goat F(ab')2 Fragment Anti-Mouse IgG (H+L)-Peroxidase, Beckman Coulter), diluted 1/5000, 1/10.000, 1/25.000, 1/50.000, 1/100.000 in PBST-1% BSA. The wells were washed three times with PBST and one time with PBS. Bound secondary antibody was detected by an incubation of 15 min with the substrate SIGMAFAST™ OPD (o-Phenylenediamine dihydrochloride). The reaction was stopped by adding 0.5 M H2SO4 and the absorbance at 492 and 620 nm was measured with an iEMS reader MF spectrometer (Labsystems).
Acknowledgments
This work was supported by institutional grant from INSERM, by the CANCEROPOLE Grand Ouest and the Ligue Régionale contre le Cancer. B.C. designed research, performed research, analyzed data and wrote the manuscript. R.V. performed research, C.P. provided myeloma cell lines and analyzed data, G.T. performed research, analyzed data and edit the manuscript, H.V. designed research, analyzed data and wrote the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/25077
References
- 1.Christiaansen JE, Burnside SS, Sears DW. Apparent sensitivity of human K lymphocytes to the spatial orientation and organization of target cell-bound antibodies as measured by the efficiency of antibody-dependent cellular cytotoxicity (ADCC) J Immunol. 1987;138:2236–43. [PubMed] [Google Scholar]
- 2.Clémenceau B, Congy-Jolivet N, Gallot G, Vivien R, Gaschet J, Thibault G, et al. Antibody-dependent cellular cytotoxicity (ADCC) is mediated by genetically modified antigen-specific human T lymphocytes. Blood. 2006;107:4669–77. doi: 10.1182/blood-2005-09-3775. [DOI] [PubMed] [Google Scholar]
- 3.Andrén M, Johanneson B, Alarcón-Riquelme ME, Kleinau S. IgG Fc receptor polymorphisms and association with autoimmune disease. Eur J Immunol. 2005;35:3020–9. doi: 10.1002/eji.200526291. [DOI] [PubMed] [Google Scholar]
- 4.Vivier E, Rochet N, Ackerly M, Petrini J, Levine H, Daley J, et al. Signaling function of reconstituted CD16: zeta: gamma receptor complex isoforms. Int Immunol. 1992;4:1313–23. doi: 10.1093/intimm/4.11.1313. [DOI] [PubMed] [Google Scholar]
- 5.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–8. [PubMed] [Google Scholar]
- 6.Polyak MJ, Deans JP. Alanine-170 and proline-172 are critical determinants for extracellular CD20 epitopes; heterogeneity in the fine specificity of CD20 monoclonal antibodies is defined by additional requirements imposed by both amino acid sequence and quaternary structure. Blood. 2002;99:3256–62. doi: 10.1182/blood.V99.9.3256. [DOI] [PubMed] [Google Scholar]
- 7.Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–71. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 8.Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JH, Bleeker WK, et al. Crosstalk between human IgG isotypes and murine effector cells. J Immunol. 2012;189:3430–8. doi: 10.4049/jimmunol.1200356. [DOI] [PubMed] [Google Scholar]
- 9.Anasetti C, Martin PJ, Morishita Y, Badger CC, Bernstein ID, Hansen JA. Human large granular lymphocytes express high affinity receptors for murine monoclonal antibodies of the IgG3 subclass. J Immunol. 1987;138:2979–81. [PubMed] [Google Scholar]
- 10.Kipps TJ, Parham P, Punt J, Herzenberg LA. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J Exp Med. 1985;161:1–17. doi: 10.1084/jem.161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Würflein D, Dechant M, Stockmeyer B, Tutt AL, Hu P, Repp R, et al. Evaluating antibodies for their capacity to induce cell-mediated lysis of malignant B cells. Cancer Res. 1998;58:3051–8. [PubMed] [Google Scholar]
- 12.Junghans RP, Waldmann TA, Landolfi NF, Avdalovic NM, Schneider WP, Queen C. Anti-Tac-H, a humanized antibody to the interleukin 2 receptor with new features for immunotherapy in malignant and immune disorders. Cancer Res. 1990;50:1495–502. [PubMed] [Google Scholar]
- 13.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 14.Biburger M, Nimmerjahn F. Low level of FcγRIII expression on murine natural killer cells. Immunol Lett. 2012;143:53–9. doi: 10.1016/j.imlet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Djeu JY, Heinbaugh JA, Holden HT, Herberman RB. Role of macrophages in the augementation of mouse natural killer cell activity by poly I:C and interferon. J Immunol. 1979;122:182–8. [PubMed] [Google Scholar]
- 16.Gidlund M, Orn A, Wigzell H, Senik A, Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978;273:759–61. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- 17.Brunda MJ, Rosenbaum D. Modulation of murine natural killer cell activity in vitro and in vivo by recombinant human interferons. Cancer Res. 1984;44:597–601. [PubMed] [Google Scholar]
- 18.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–17. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooijberg E, van den Berk PC, Sein JJ, Wijdenes J, Hart AA, de Boer RW, et al. Enhanced antitumor effects of CD20 over CD19 monoclonal antibodies in a nude mouse xenograft model. Cancer Res. 1995;55:840–6. [PubMed] [Google Scholar]
- 20.Onishi M, Kinoshita S, Morikawa Y, Shibuya A, Phillips J, Lanier LL, et al. Applications of retrovirus-mediated expression cloning. Exp Hematol. 1996;24:324–9. [PubMed] [Google Scholar]
- 21.Swift S, Lorens J, Achacoso P, Nolan GP. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell-based systems. Curr Protoc Immunol 2001; Chapter 10:Unit 10 7C. [DOI] [PubMed] [Google Scholar]