Abstract
Abiotic stress factors can interfere with the emission of herbivore-induced plant volatile organic compounds (VOCs) and thus disrupt chemical communication channels between plants and other organisms. We investigated whether copper (Cu) stress alone or in conjunction with insect damage modifies the kinetics of (1) VOCs, (2) the VOC-inducing phytohormone jasmonic acid (JA) and (3) its putative antagonist salicylic acid (SA). Hydroponically grown Zea mays exposed to 10 and 80 µM of Cu showed no increases in JA or VOC levels in the absence of herbivory. However when challenged by herbivores, Cu (80 µM) caused ROS generation in root tissues and primed for increased JA accumulation and VOC emission in leaves. SA synthesis was equally primed but higher concentrations were also apparent before insects started feeding. In contrast, plants grown at 10 µM Cu did not differ from controls. These results show that abiotic and biotic stresses result in concentration-dependent, non-additive defense responses. Further support is given to the notion that JA-SA antagonism is absent in Z. mays.
Keywords: heavy metals, Zea mays, induced indirect defense, herbivory, crosstalk
Plants respond to many biotic and abiotic stress factors by eliciting a network of signaling pathways that involve the phytohormones jasmonic acid (JA)/ethylene (ET) and salicylic acid (SA). These endogenous signals mediate a large range of protective responses including the biosynthesis of volatile organic compounds (VOCs) in vegetative tissues. Plant VOCs are elicited in particular by JA and can serve multiple functions. Physiologically, they have been suggested to alleviate certain environmental stresses through mitigating the effects of oxidative radicals while ecologically, VOCs can protect plants from damaging insects as they attract their natural enemies. Furthermore, they may directly repel herbivores or function as external signaling molecules in inter-plant and within-plant communication.1
Although much is known about volatile defense responses to single stressors, the effects of combined abiotic and biotic factors have not been well understood. Knowledge on such simultaneous stress effects is important as the outcomes are not necessarily additive or opposing and therefore difficult to predict.2 Combined stresses can change the composition and quantity of a VOC blend and in some cases may affect chemical communication between the emitting plant and other organisms because the altered blend may no longer code for meaningful information. Such effects have recently been termed “info-disruption.”3 For example, high CO2 levels can alter herbivore-induced VOCs in Brassica plants and thus interfere with the host-foraging behavior of the herbivore’s parasitoid.4 In several other cases though, chemically-mediated interactions have remained stable despite the impact of external stress factors on VOC emission.5,6
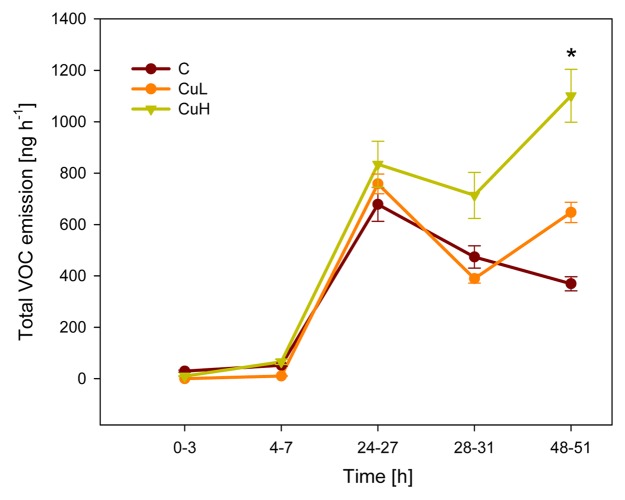
To explore the effects of combined stresses on plant signaling, we investigated how two factors, copper (Cu) and insect damage, influence the kinetics of JA, SA and VOCs. Plants can be exposed to high levels of Cu in their above- and belowground parts due to common use as a fungicide and the fact that heavy metals accumulate in soil over time.7 In our study, experiments were performed with intact Zea mays seedlings as it is a well-established model plant for VOC-mediated interactions. Young maize plants were grown in hydroponic solution at three different Cu levels (0 µM = control, 10 µM = CuL, 80 µM = CuH) for three days and subsequently challenged with 15 second-instar caterpillars of Spodoptera frugiperda. Exposure to Cu led to stunted growth and lower photosynthetic capacities in the plants. Measurements of phytohormones and volatiles in the aboveground plant parts were performed as described previously.8 We found concentration-dependent responses to the combination of both stress factors which were remarkably different to single stress effects. As expected, herbivory alone led to continuous increases in JA levels over a 24 h time course while SA levels remained unchanged (Fig. 1). This finding correlated with increased herbivore-induced emission of a characteristic blend of VOC compounds (Fig. 2). Plants treated with low doses of Cu (CuL) behaved similarly to control plants with no effects on phytohormone and VOC levels in the absence of insects. Significant differences, however, were found in plants grown under CuH conditions: root tissues were characterized by enhanced ROS production while leaves showed elevated levels of JA in the first hours of herbivore feeding compared with control seedlings that were exposed to herbivory without Cu stress.8 This priming event, i.e., the stronger and/or faster response to a stimulus after previous exposure to a biotic or abiotic stimulus, was transient and JA levels at 24 h of insect feeding were in line with those found in control and CuL plants. Comparable to JA kinetics, control and CuL-treated plants responded in the same manner with respect to SA levels: neither CuL nor herbivory enhanced SA concentrations above control levels. In contrast, plants grown in CuH solution showed elevated amounts of endogenous SA in the leaf tissue in the absence of insect damage. During the first 24 h of herbivore-feeding, SA concentrations increased strongly and remained significantly above control levels. In this case, herbivory acted as a synergist that enhanced CuH-induced SA levels rather than Cu priming a response triggered by herbivory.
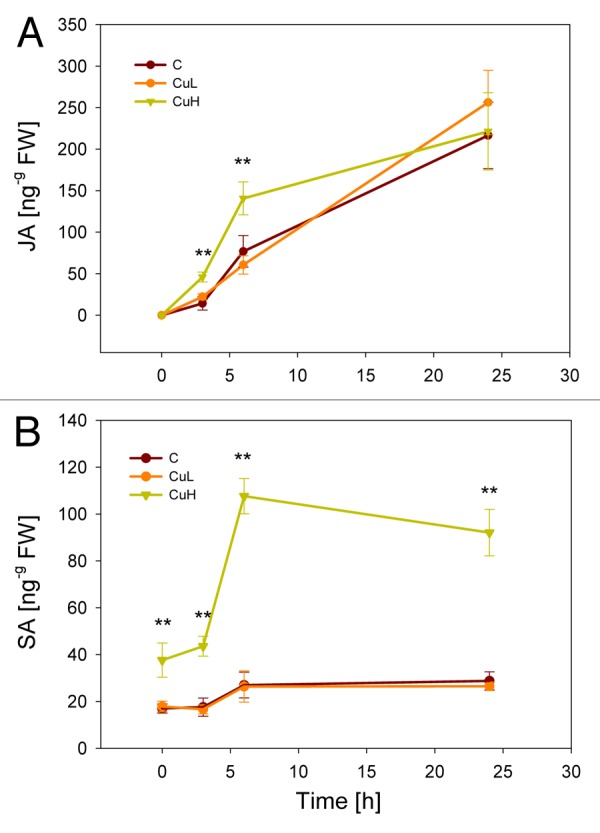
Figure 1. Kinetics of (A) jasmonic acid (JA) and (B) salicylic acid (SA) accumulation in response to herbivory. Phytohormone levels were measured in Zea mays leaves after 0 h, 3 h, 6 h and 24 h of caterpillar feeding. C, control plants grown without added copper; CuL, plants exposed to 10 µM Cu; CuH, exposed to 80 µM Cu. **p < 0.01, Kruskal-Wallis ANOVA followed by median test. Symbols depict mean values (± SE), n = 7.
Figure 2. Kinetics of total emission of volatile organic compounds in response to herbivory. C, control plants grown without added copper; CuL, plants exposed to 10 µM Cu; CuH, exposed to 80 µM Cu. *p < 0.05, Repeated measures ANOVA after log-transformation. Symbols depict mean values (± SE), n = 6.
In addition to JA, priming was also found to play a role in VOC emission. Continuous herbivory over a 51 h time course led to increasingly higher release rates of green leaf volatiles, esters, aromatics and terpenoids while Cu treatments alone had no inducing effects on VOCs. When maize seedlings experienced combined stresses from Cu exposure (CuH) to roots and aboveground insect damage, significantly enhanced emission rates of herbivore-induced VOCs were measured.
In summary, multiple stresses altered the kinetics of internal and external plant signals in a non-additive way through priming and synergism. Our findings also confirm the lack of antagonistic crosstalk between JA and SA pathways in Z. mays which seems to be prevalent in many other plant species.9 Moreover, enhanced levels of SA in Cu stressed plants could be directly involved in priming JA and JA-induced VOCs as demonstrated in a recent study using external SA application.10 The potential for Cu to cause info-disruption through altered VOC signaling awaits further exploration. VOC blends from primed maize have been shown to be more attractive to parasitoids11 but this could prove fatal as caterpillars on stressed plants are suboptimal hosts.5
Acknowledgments
We thank past undergraduate researcher Katharina Kaiser for contributions to this work. Financial support was provided by the Deutsche Forschungsgemeinschaft (SFB 567 “Mechanismen der interspezifischen Interaktionen”).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24264
References
- 1.Loreto F, Schnitzler JP. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010;15:154–66. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Holopainen JK, Gershenzon J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010;15:176–84. doi: 10.1016/j.tplants.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Boyd RS. Heavy metal pollutants and chemical ecology: exploring new frontiers. J Chem Ecol. 2010;36:46–58. doi: 10.1007/s10886-009-9730-5. [DOI] [PubMed] [Google Scholar]
- 4.Vuorinen T, Nerg AM, Ibrahim MA, Reddy GVP, Holopainen JK. Emission of Plutella xylostella-induced compounds from cabbages grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiol. 2004;135:1984–92. doi: 10.1104/pp.104.047084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winter TR, Rostás M. Nitrogen deficiency affects bottom-up cascade without disrupting indirect plant defense. J Chem Ecol. 2010;36:642–51. doi: 10.1007/s10886-010-9797-z. [DOI] [PubMed] [Google Scholar]
- 6.Vuorinen T, Nerg AM, Holopainen JK. Ozone exposure triggers the emission of herbivore-induced plant volatiles, but does not disturb tritrophic signalling. Environ Pollut. 2004;131:305–11. doi: 10.1016/j.envpol.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 7.Zhou XX, He ZL, Liang ZB, Stoffella PJ, Fan JH, Yang YG, et al. Long-term use of copper-containing fungicide affects microbial properties of citrus grove soils. Soil Sci Soc Am J. 2011;75:898–906. doi: 10.2136/sssaj2010.0321. [DOI] [Google Scholar]
- 8.Winter TR, Borkowski L, Zeier J, Rostás M. Heavy metal stress can prime for herbivore-induced plant volatile emission. Plant Cell Environ. 2012;35:1287–98. doi: 10.1111/j.1365-3040.2012.02489.x. [DOI] [PubMed] [Google Scholar]
- 9.Thaler JS, Humphrey PT, Whiteman NK. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012;17:260–70. doi: 10.1016/j.tplants.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Engelberth J, Viswanathan S, Engelberth MJ. Low concentrations of salicylic acid stimulate insect elicitor responses in Zea mays seedlings. J Chem Ecol. 2011;37:263–6. doi: 10.1007/s10886-011-9926-3. [DOI] [PubMed] [Google Scholar]
- 11.Ton J, D’Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, et al. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]