Abstract
Jasmonic acid (JA) and salicylic acid (SA) play important roles in plant defense systems. JA and SA signaling pathways interact antagonistically in dicotyledonous plants, but, the status of crosstalk between JA and SA signaling is unknown in monocots. Our rice microarray analysis showed that more than half of the genes upregulated by the SA analog BTH are also upregulated by JA, suggesting that a major portion of the SA-upregulated genes are regulated by JA-dependent signaling in rice. A common defense system that is activated by both JA and SA is thus proposed which plays an important role in pathogen defense responses in rice.
Keywords: common defense system, induced resistance; jasmonic acid; salicylic acid; rice
Two phytohormones, jasmonic acid (JA) and salicylic acid (SA) play important roles in transducing the activation of plant defense systems against pathogen attacks. SA usually induces resistance mechanisms which are active against biotrophic and hemi-biotrophic pathogens, whereas JA induces resistance against necrotrophic phathogens.1 In most cases, JA and SA defense signaling pathways are mutually antagonistic in dicotyledonous species.2,3 In Arabidopsis, SA downregulates the expression of JA-responsive marker genes such as PDF1.2 and VSP1 as well as genes encoding key enzymes in the JA biosynthesis pathway, such as LOX2, AOS, AOC2 and OPR3.4 On the one hand, this antagonistic crosstalk between JA and SA-dependent defense signaling is unclear in rice, which serves as a model for molecular studies of other monocotyledonous species. A previous study reported that the expression of PR genes and overall resistance to Magnaporthe oryzae were higher in OsAOS2-overexpression rice plants, even though M. oryzae is a hemi-biotrophic pathogen.5 In addition, our recent study demonstrated that exogenous JA application induces resistance to Xanthomonas oryzae pv oryzae (Xoo) in rice, though Xoo is a biotrophic pathogen.6 These results indicate that JA and its signaling pathway make important contributions to both hemi-biotrophic or biotrophic pathogen defense response in rice. These results are supported by a number of previous reports.7,8 Recently, it has been demonstrated a positive contribution of JA and SA signaling in the immunity against both biotrophic and necrotrophic pathogens in Arabidopsis.9 Here, we discuss JA and SA signaling crosstalk and propose that a common defense system is activated by both JA and SA in rice.
It has been reported that 313 BTH-upregulated genes were identified by microarray analysis in rice.10 Because BTH is a functional analog of SA, these 313 genes are very likely the genes responsible for SA defense signaling in rice. Our resent study explored JA-responsive genes using microarray analysis and demonstrated that 1,320 genes were upregulated in response to JA in rice.6 To determine if there is crosstalk between JA and SA-transducing pathways in rice, we measured the expression of BTH-upregulated genes after JA treatment. Although a third of BTH-upregulated genes were downregulated by JA, more than half were upregulated (Fig. 1), suggesting that much of the SA signaling pathway is independent of JA downregulation. In addition, expression of about a fifth of SA-upregulated genes doubled in response to JA, suggesting that JA and SA signaling coordinately interact during induction of a defense response. The expression of rice PR1b gene, a commonly used marker of disease resistance, is induced by treatment of JA or SA,11 suggesting that there is a common defense system, or at least a partly shared signal transduction pathway used for both JA and SA signaling in rice. OsWRKY45 is a BTH-up-regulated gene and is a key protein in BTH-induced resistance to M. oryzae and Xoo.10,12 Microarray analysis showed that expression of OsWRKY45 nearly doubled in response to JA treatment,6 supporting our hypothesis that JA and SA signaling, or at least a critical part of the signaling cascade, interacts coordinately in rice defense response.
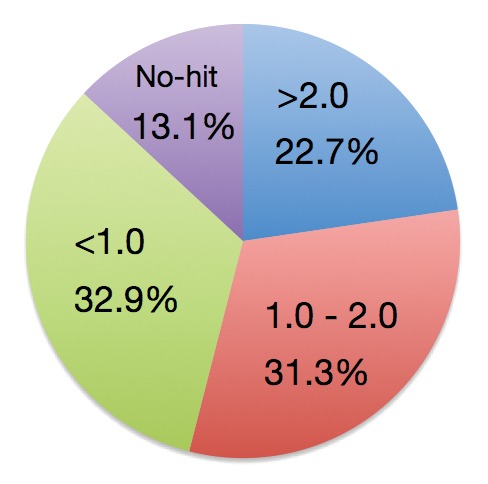
Figure 1. Categories of BTH-upregulated genes after JA treatment in rice. Percentage of BTH-upregulated genes that are also upregulated by JA are shown with the qualifying induction change. “No-hit” indicates BTH-upregulated genes that were not correlated with a corresponding gene in our microarray data.6 BTH-upregulated genes refer Shimono et al.10
Rice has high endogenous SA levels (> 1 µg g−1 fresh weight in leaves).13 To understand the relationship between JA and SA signaling on the rice immune system, it is important to understand the dynamics of endogenous JA and SA relative concentration. However, it is not known whether endogenous SA concentrations are affected by exogenous JA application in rice. We measured the effect of exogenous application of JA on SA contents and found that SA dramatically decreased in response to exogenous JA (Fig. 2). This result suggests that SA signaling is suppressed by JA because of a decrease in tissue SA concentrations.
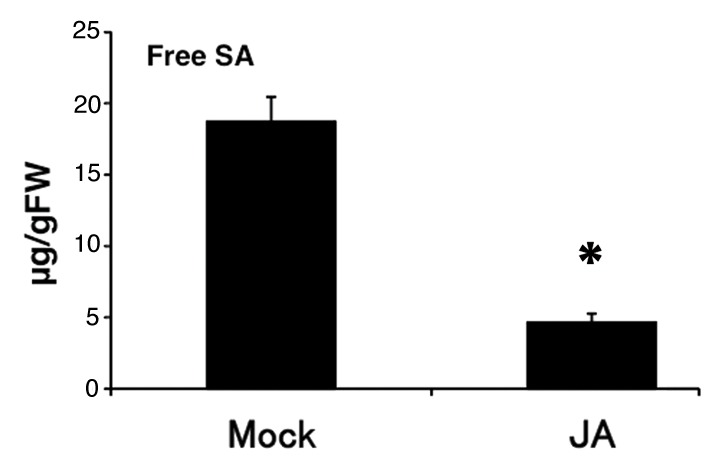
Figure 2. SA content after JA treatment in rice. Free SA content after100 µM JA treatment for 24 h in the fourth leaf blades of rice. Values are means ± SE (n = 3 for Mock, 4 for JA). An asterisk represents a statistically significant difference from the mock-treated control at p < 0.05 (Student’s t-test).
No distinct antagonistic interaction between JA and SA signaling could be verified in the defense response in rice, although JA and SA synthesis may be regulated antagonistically. Rather, our results suggest that some common defense signaling system plays a crucial role in the rice defense response (Fig. 3). Because high endogenous SA concentrations are maintained under normal conditions, SA signaling contributes mainly to the basal defense in rice. Once the JA signal is activated, endogenous SA levels would dramatically decrease and SA signaling would be suppressed. JA would then activate the common defense system instead of SA in rice (Fig. 3). In fact, JA-Ile, a bioactive form of JA, is accumulated following inoculation with M. oryzae.14 Because the JA and SA-activated common defense system is critical to the pathogen defense response, JA signaling must be able to induce resistance against the biotrophic pathogen, Xoo, in rice.
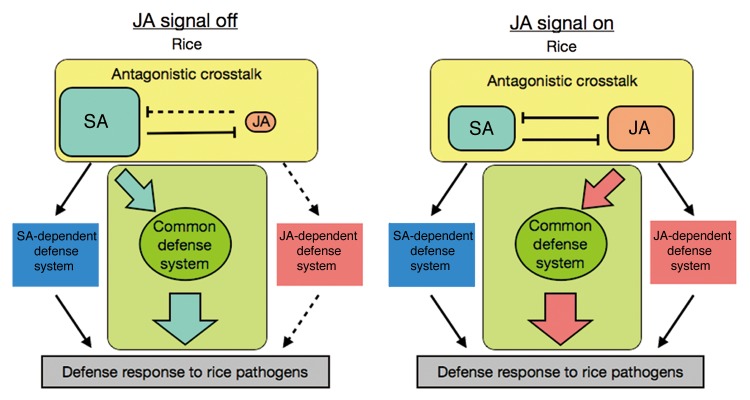
Figure 3. Schematic overview of the common defense system activated by JA and SA in rice. Parallel models of the activation scheme of a common defense system either when endogenous JA is low (JA signal off) or high (JA signal on). Solid lines indicate putative active signaling pathways. Dashed lines indicate putative inactive signaling pathways under the JA signaling condition of the model.
Materials and Methods
Rice growth condition and JA treatment
To examine the effects of JA on SA content in rice leaf blades, rice plants were grown to the four-leaf stage in a growth chamber according to Yamada et al.6 Treatment with 100 µM JA was performed according to Yamada et al.6
Quantitation of SA
Quantitation of free SA was performed essentially as described.15 SA was analyzed by HPLC equipped with a Symmetry C18 (4.6 mm by 25 cm) column maintained at 40°C and a fluorescence detector (Model RF-550A, Shimadzu). Isocratic separation was done with 23% (v/v) methanol in 20 mM sodium acetate, pH 5.0, at a flow rate of 1 ml/min. All data were corrected for losses.
Acknowledgments
We thank M. Satoh (National Agricultural Research Center for Kyushu Okinawa Region, NARO) and Dr H. Kanno (NARO) for laying the foundation of a part of this study. This work was supported in part by a Funding Program for Next Generation World-Leading Researchers from Japan Society for Promotion of Science (No. GS022).
Glossary
Abbreviations:
- BTH
benzothiadiazole
- PR
pathogenesis-related
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24260
References
- 1.Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant Mol Biol. 2009;69:473–88. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- 2.Niki T, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y. Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiol. 1998;39:500–7. doi: 10.1093/oxfordjournals.pcp.a029397. [DOI] [Google Scholar]
- 3.Koornneef A, Pieterse CM. Cross talk in defense signaling. Plant Physiol. 2008;146:839–44. doi: 10.1104/pp.107.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leon-Reyes A, Van der Does D, De Lange ES, Delker C, Wasternack C, Van Wees SC, et al. Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta. 2010;232:1423–32. doi: 10.1007/s00425-010-1265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei C, Qi M, Sheng G, Yang Y. Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact. 2006;19:1127–37. doi: 10.1094/MPMI-19-1127. [DOI] [PubMed] [Google Scholar]
- 6.Yamada S, Kano A, Tamaoki D, Miyamoto A, Shishido H, Miyoshi S, et al. Involvement of OsJAZ8 in jasmonate-induced resistance to bacterial blight in rice. Plant Cell Physiol. 2012;53:2060–72. doi: 10.1093/pcp/pcs145. [DOI] [PubMed] [Google Scholar]
- 7.Kanno H, Hasegawa M, Kodama O. Accumulation of salicylic acid, jasmonic acid and phytoalexins in rice, Oryza sativa, infested by the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae) Appl Entomol Zool (Jpn) 2012;47:27–34. doi: 10.1007/s13355-011-0085-3. [DOI] [Google Scholar]
- 8.Deng H, Liu H, Li X, Xiao J, Wang S. A CCCH-type zinc finger nucleic acid-binding protein quantitatively confers resistance against rice bacterial blight disease. Plant Physiol. 2012;158:876–89. doi: 10.1104/pp.111.191379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F. Network properties of robust immunity in plants. PLoS Genet. 2009;5:e1000772. doi: 10.1371/journal.pgen.1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimono M, Sugano S, Nakayama A, Jiang CJ, Ono K, Toki S, et al. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell. 2007;19:2064–76. doi: 10.1105/tpc.106.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal GK, Rakwal R, Jwa NS. Rice (Oryza sativa L.) OsPR1b gene is phytohormonally regulated in close interaction with light signals. Biochem Biophys Res Commun. 2000;278:290–8. doi: 10.1006/bbrc.2000.3781. [DOI] [PubMed] [Google Scholar]
- 12.Shimono M, Koga H, Akagi A, Hayashi N, Goto S, Sawada M, et al. Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol Plant Pathol. 2012;13:83–94. doi: 10.1111/j.1364-3703.2011.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raskin I. Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:439–63. doi: 10.1146/annurev.pp.43.060192.002255. [DOI] [Google Scholar]
- 14.Wakuta S, Suzuki E, Saburi W, Matsuura H, Nabeta K, Imai R, et al. OsJAR1 and OsJAR2 are jasmonyl-L-isoleucine synthases involved in wound- and pathogen-induced jasmonic acid signalling. Biochem Biophys Res Commun. 2011;409:634–9. doi: 10.1016/j.bbrc.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 15.Gomi K, Ogawa D, Katou S, Kamada H, Nakajima N, Saji H, et al. A mitogen-activated protein kinase NtMPK4 activated by SIPKK is required for jasmonic acid signaling and involved in ozone tolerance via stomatal movement in tobacco. Plant Cell Physiol. 2005;46:1902–14. doi: 10.1093/pcp/pci211. [DOI] [PubMed] [Google Scholar]


