“For every action, there is an equal and opposite reaction.” This axiom is true not only for physical processes, but also for biological activation events, where inhibitory mechanisms are in place to reestablish equilibrium. Nowhere is this more evident than during acute inflammation, where cytokines or pathogen-associated stimuli ramp up a rapid and powerful inflammatory cascade, followed by an equally important phase of resolution. Keeping inflammation in check avoids collateral tissue damage, ultimately preventing autoimmune and hyperproliferative disorders.
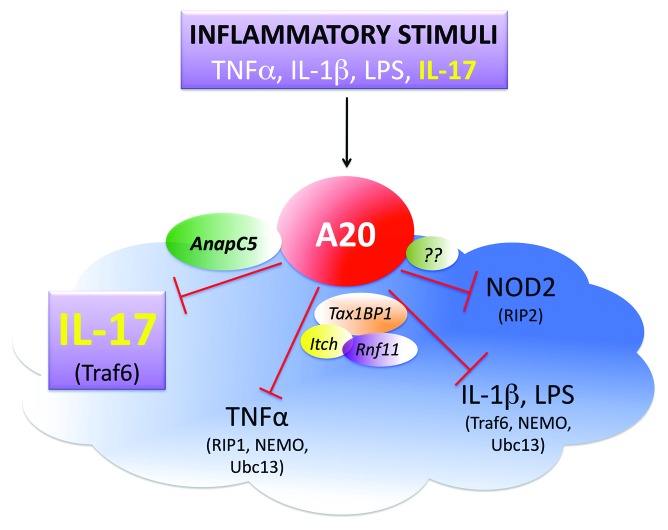
A20 is a vital inhibitor of inflammation encoded by the gene TNFα-induced protein 3 (TNFAIP3). The TNFAIP3 locus is linked to autoimmune diseases and B-cell lymphomas.1 A20 is a deubiquitinating enzyme that reverses Lysine (K)63-linked ubiquitination. A20 was initially described as a negative feedback inhibitor of TNFα-induced signaling, where it acts principally by targeting RIP1, and thereby terminating activation of the NFκB transcription factor. A20 also inhibits the TLR, IL-1R, and Nod-like receptor pathways, where it targets other signaling intermediates such as TRAF6, NEMO, and Ubc13 (Fig. 1).2 Mathematical modeling of the TNFα pathway led to the insight that A20 restricts activation of the second, not the first phase of classical NFκB activation. Moreover, due to its long protein half-life, steady-state levels of A20 dictate the magnitude of inflammatory signaling. Notably, induction of A20 by any stimulus is predicted to exert a regulatory impact on the global response; thus, A20 is considered a “rheostat” that maintains equilibrium following NFκB activation.3
Figure 1. Schematic diagram depicting A20-mediated regulation of inflammatory pathways. The model represents the two phases of feedback inhibition of inflammation with A20 (tnfaip3) as a central player. In the first phase, several stimuli such as TNFα, IL-1β, and IL-17 induce A20 mRNA expression along with other inflammatory proteins. A20 induction is essential as it acts as a feedback inhibitor of its inducing inflammatory pathways. This regulation happens in the second phase where A20 functions as deubiquitinase enzyme and targets adaptor proteins such as Traf6 and RIP1, resulting in downregulation of inflammatory gene expression. These targets vary by the pathway being regulated and have been depicted in parentheses next to the stimulus being targeted. Further, several proteins such as Tax1BP1, Itch and RNF11 assist A20 in its role as a watchdog of inflammatory pathways. However, in case of IL-17, A20 does not require these adaptors and instead associates with Anapc5.
Confirming the importance of A20 in restricting inflammation, A20−/− mice die prematurely of multi-organ inflammation and cachexia. Surprisingly, despite the prominent role of A20 in tempering TNFα signaling, inflammation in A20−/− mice was not alleviated by crossing to mice lacking the TNF receptor. Subsequent in vivo studies revealed that the baseline inflammation in A20-deficient mice is due to signals from commensal microbiota via TLR/MyD88 signaling. A number of subsequent studies examined cell type-specific effects of A20 using conditionally targeted mice, demonstrating important activities for this protein in numerous immune subtypes.1
In recent years IL-17 has come to the limelight due the recognition of Th17 cells and their connection to autoimmune disease. Clinically, antibodies against IL-17 or its receptor are showing promise in treating a variety of other conditions.4 The IL-17R belongs to a distinct subfamily of receptors, with many unique structural and functional properties. Nonetheless, the IL-17 signaling pathway shares many downstream adaptors and transcription factors with the TNFα, IL-1, and TLR pathways.5 Given these commonalities, we predicted that A20 might also serve as an inhibitor of IL-17 signaling. Indeed, IL-17 induced expression of the TNFAIP3 gene in an NFκB-dependent manner. A20 deficiency upon siRNA silencing or in knockout cells led to enhanced IL-17-dependent expression of inflammatory genes. Mechanistically, A20 binds to and dequbitinates TRAF6, ultimately inactivating IL-17-mediated NF-κB and MAPK (particularly JNK) pathways.6
Although the mechanism used by A20 to inhibit IL-17 signaling has parallels with the IL-1R pathway, there are striking differences. In other pathways, A20 interacts with accessory proteins such as Tax1bp1 and Itch to efficiently hydrolyze K63-linked substrates. Deletion of these proteins results in a phenotype similar to the lack of A20, though less severe.2 Unexpectedly, Tax1bp1 and Itch were dispensable for A20s activity in IL-17 signaling, as siRNA-mediated silencing of Tax1bp1 or Itch did not affect IL-17-dependent gene expression.7 This finding raised the possibility that A20 either functions solo or uses alternate adaptor molecules. Accordingly, we found that A20 associates with AnapC5 (APC5), a subunit of a multi-protein E3 ubiquitin ligase complex known as the Anaphase Promoting Complex (APC). APC subunits have not been previously linked with the regulation of inflammatory signaling, suggesting a novel role for AnapC5 outside of regulating the cell cycle (Fig. 1).
In analyzing how A20 is recruited to the IL-17R signaling complex, we found that A20 associates directly with the IL-17RA subunit.6 Notably, A20 binds to a C-terminal domain of IL-17RA that was previously shown to downmodulate IL-17 signaling.8 To our knowledge, this is the first demonstration that A20 associates with an inducing receptor. A20 interaction with receptors may also occur in other systems, potentially serving as a mechanism to recruit this inhibitor after a signaling event is triggered.
These discoveries about A20 function in the context of IL-17 suggest there are distinct mechanisms by which A20 restricts inflammatory pathways. It will be key to define the contribution of A20 in regulating IL-17 in vivo. As noted, SNPs in the TNFAIP3 gene locus are associated with autoimmune diseases that are linked to IL-17 activity. Therefore, it would not be surprising if patients with TNFAIP3 polymorphisms are more likely to be responsive to anti-IL-17 therapy, as has been demonstrated for TNF inhibitors. To date, therapeutic strategies to block IL-17 have mainly focused on biologic drugs that target Th17 cells (anti-IL-6 or anti-IL-12/23) or directly neutralize this cytokine (anti-IL-17, anti-IL-17RA). Development of small-molecule inhibitors will be aided by a better understanding of how IL-17 pathways are regulated in nature.
Garg AV, et al. Sci Signal. 2013;6:ra44. doi: 10.1126/scisignal.2003699.
Note
We apologize to those authors whose work could not be cited due to space constraints.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/26699
References
- 1.Ma A, et al. Nat Rev Immunol. 2012;12:774–85. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shembade N, et al. Cell Mol Immunol. 2012;9:123–30. doi: 10.1038/cmi.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basak S, et al. Immunol Rev. 2012;246:221–38. doi: 10.1111/j.1600-065X.2011.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel DD, et al. Ann Rheum Dis. 2013;72(Suppl 2):116–23. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- 5.Gaffen SL. Nat Rev Immunol. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg AV, et al. Sci Signal. 2013;6:ra44–55. doi: 10.1126/scisignal.2003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho AW, et al. PLoS One. 2013;8:e70168. doi: 10.1371/journal.pone.0070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maitra A, et al. Proc Natl Acad Sci U S A. 2007;104:7506–11. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]