Abstract
The restoration of pluripotency circuits by the reactivation of endogenous stemness factors, such as SOX2, may provide a new paradigm in cancer development. The tumoral stem cell reprogramming hypothesis, i.e., the ability of stemness factors to redirect normal and differentiated tumor cells toward a less-differentiated and stem-like state, adds new layers of complexity to cancer biology, because the effects of such reprogramming may remain dormant until engaged later in response to (epi)genetic and/or (micro)environmental events. To test this hypothesis, we utilized an in vitro model of a SOX2-overexpressing cancer stem cell (CSC)-like cellular state that was recently developed in our laboratory by employing Yamanaka’s nuclear reprogramming technology in the estrogen receptor α (ERα)-positive MCF-7 breast cancer cell line. Despite the acquisition of distinct molecular features that were compatible with a breast CSC-like cellular state, such as strong aldehyde dehydrogenase activity, as detected by ALDEFLUOR, and overexpression of the SSEA-4 and CD44 breast CSC markers, the tumor growth-initiating ability of SOX2-overexpressing CSC-like MCF-7 cells solely occurred in female nude mice supplemented with estradiol when compared with MCF-7 parental cells. Ser118 phosphorylation of estrogen receptor α (ERα), which is a pivotal integrator of the genomic and nongenomic E2/ERα signaling pathways, drastically accumulated in nuclear speckles in the interphase nuclei of SOX2-driven CSC-like cell populations. Moreover, SOX2-positive CSC-like cells accumulated significantly higher numbers of actively dividing cells, and the highest levels of phospho-Ser118-ERα occurred when chromosomes lined up on a metaphase plate. The previously unrecognized link between E2/ERα signaling and SOX2-driven stem cell circuitry may significantly impact our current understanding of breast cancer initiation and progression, i.e., SOX2 can promote non-genomic E2 signaling that leads to nuclear phospho-Ser118-ERα, which ultimately exacerbates genomic ER signaling in response to E2. Because E2 stimulation has been recently shown to enhance breast tumor-initiating cell survival by downregulating miR-140, which targets SOX2, the establishment of a bidirectional cross-talk interaction between the stem cell self-renewal regulator, SOX2, and the local and systemic ability of E2 to increase breast CSC activity may have profound implications for the development of new CSC-directed strategies for breast cancer prevention and therapy.
Keywords: breast cancer, cancer stem cells, SOX2, estrogen receptor, estradiol
The ability of normal and differentiated tumor cells to convert to cancer stem cell (CSC) states necessarily implies an intrinsic plasticity and, consequently, a dynamic equilibrium between differentiation and oncogenic reprogramming during the establishment, maintenance, and evolution of cellular hierarchies in human malignancies. Acquisition of the self-renewal and tumor-initiating abilities of CSCs may arise from some of the molecular mechanisms underlying cellular reprogramming to pluripotency; i.e., spontaneous dedifferentiation and initiation of tumorigenesis in vivo may involve the reactivation of one or more pluripotency-associated factors.1-4 In this regard, SOX2, a member of the SRY-related HMG-box family of transcription factors, has mainly been studied in embryonic stem cells and in reprogramming of adult somatic cells to a pluripotent stem cell state, and it has been recently recognized to promote aberrant stem cell self-renewal signaling in breast cancer.5-7 First, normal breast tissues express low levels of SOX2, but SOX2 in early-stage breast carcinomas is differentially reactivated in the ductal areas of tumors that still show intact ductal structures, which strongly suggests that SOX2 may be expressed during the initial phases of tumorigenesis. However, SOX2 is lost as the tumor progresses toward advanced stages. Second, SOX2 expression is induced during mammosphere formation, which is a stem-like functional assay that allows for the propagation of mammary epithelial and breast cancer cells in an undifferentiated state based on their ability to proliferate in suspension. Moreover, SOX2 activation is sufficient and necessary to induce the mammosphere stem-like feature. Third, SOX2 appears to determine the in vivo tumor-initiating capacity of heterogeneous breast cancer populations, because a marked reduction in the size of tumors can be observed in SOX2-silenced MCF-7 breast cancer cells inoculated into mouse xenograft models.
By placing the tumor-initiating event in any cell along the axis of breast differentiation and in any cell within the established tumor, the aforementioned observations suggest that the mode of action of endogenous stemness factors such as SOX2 may not be homogenous throughout the cancer cell population; i.e., the ability of SOX2 to program the malignant state could take place at the CSC status but might not be required within differentiated malignant cells and/or in more advanced cancer stages.8-10 However, the existence of this process may be extremely difficult to demonstrate in human cancers, because to confirm the lack of homogeneity in the mode of action of SOX2 throughout the biological history of tumors, it would be necessary to dissect and isolate the function that SOX2 is playing at the earliest stages of the disease, i.e., at the level of the cell of origin. Moreover, such a system would need to find a way of restricting the expression of SOX2 to the cancer compartment with CSC-like properties and tumor-initiating capabilities. To investigate this issue and to preliminarily elucidate if breast cancer is a stem cell/SOX2-driven tissue, we utilized an in vitro model of de novo generation of CSC-like states through the nuclear reprogramming of the established estrogen receptor α (ERα)-positive MCF-7 breast cancer cell line. When using Yamanaka’s stem cell technology in an attempt to create stable CSC research cell lines, we generated SOX2-overexpressing MCF-7/Rep cells that acquired distinct molecular characteristics compatible with a breast CSC-like cellular state.4 Thus, well-accepted breast CSC markers, such as SSEA-4, ALDEFLUOR, and CD44, were significantly overrepresented in the CSC-like SOX2-overexpressing MCF-7 cells.
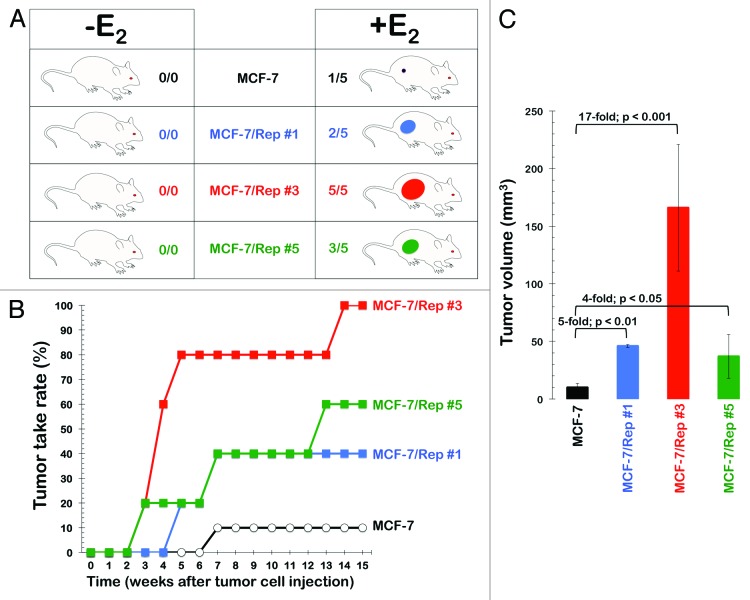
We recently decided to profile the in vivo tumorigenicity of the CSC-like SOX2-overexpressing MCF-7/Rep cells by monitoring their engraftment and growth in the absence or presence or circulating estradiol (E2) in comparison to MCF-7 parental cells (Fig. 1A). Two days before cell transplantation, athymic nude mice were divided into 2 groups with 20 mice per group. A 0.72-mg 17β-estradiol 60-d release pellet (E2 pellet) was implanted subcutaneously (s.c.) in the dorsal interscapular regions of the first group of animals. Cells were injected without the implantation of the E2 pellet in the second group of animals. MCF-7 cells (1 × 106; n = 5 per group) or SOX2-overexpressing MCF-7/Rep cells (1 × 106; n = 5 per clone and group) were then injected s.c. in the dorsal flank region of E2 and non-E2 mice in the absence of admixed Matrigel, which is a well-known enhancer of tumorigenicity of MCF-7 breast cancer cells. The number of tumors that grew to a size ≥10 mm3 in each group by the end of the experiment on day 112 is indicated in Figure 1A. The time-dependent incidence of xenografts with volumes ≥10 mm3 is shown in Figure 1B. Xenografts from SOX2-overexpressing MCF-7/Rep clones grew to ≥10 mm3 in up to 100% of the mice (clone #3) by approximately 4 mo after tumor cell implantation and E2 supplementation. The median volume of the tumors that grew ≥10 mm3 is shown in Figure 1C. It was obvious that SOX2 overexpression in CSC-like cell populations substantially and significantly enhanced s.c. xenograft success, because it drastically increased tumor incidence when compared with MCF-7 parental cells, which were unable to form tumors in E2-supplemented mice in the absence of co-injected Matrigel. Importantly, SOX2 overexpression failed to promote tumor initiation in mice without E2 supplementation.
Figure 1. Incidence of MCF-7- and SOX2-overexpressing MCF-7/Rep xenografts. Detection of tumors with volume ≥10 mm3 that grew progressively to distinguish from local inflammation following the injection of 1 × 106 cells in the absence of Matrigel in nude mice. The number of tumors is shown as a rate in (A), and the incidence of tumors is shown as a function of time in (B). The volume of evident tumors (columns, means; bars, SE) on day 112 is shown in (C). Fold and P values indicate comparison of tumor volume with the control group of MCF-7 parental cells.
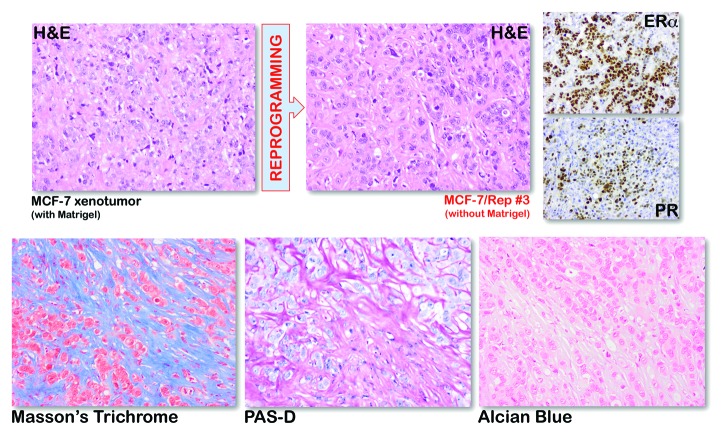
When compared with MCF-7 xenografts that were obtained upon co-injection with Matrigel, histological examination of Matrigel-independent SOX2-overexpressing MCF-7/Rep xenografts revealed ER- and progesterone receptor (PR)-positive solid tumors that resembled highly aggressive and poorly differentiated invasive breast carcinomas without any evidence of higher organization or differentiation (Fig. 2, top). To initially examine reactive stroma, SOX2-overexpressing tumors were stained with Masson Trichrome, which differentially stains stromal components and is useful in distinguishing smooth muscle cells from collagen fibers and other stromal cell types. This analysis showed a mixture of red-staining cancer cells and blue-staining collagen fibers (Fig. 2, bottom). Although isolated smooth muscle cells were present in some cases, the blue staining pattern was consistently associated with regions of cancer and suggested a loss of typical smooth muscle cells plus elevated collagen in SOX2-overexpressing breast cancer stroma. Periodic acid-Schiff with diastase (PAS-D) staining, a sensitive histochemical method that differentiates glycogen from other PAS-positive elements, such as mucins, which may be present in the tissue sample, strongly suggested the absence of mucins in tissues from SOX2-overexpressing xenotumors (Fig. 2, bottom). Furthermore, the absence of metachromatic staining of sulfated acid mucopolysaccharides with alcian blue confirmed the absence of metaplastic carcinomas of the matrix-producing type (Fig. 2, bottom), which tend to contain cartilage or osseous elements as their heterologous component.
Figure 2. Immunohistochemical analysis of MCF-7 and SOX2-overexpressing MCF-7/Rep xenotumors. Top: H&E, ERα, and PR immunohistochemical staining in MCF-7 and SOX2-overexpressing MCF-7/Rep (clone #3) xenotumor tissues. Bottom: the Masson trichrome stain was used to distinguish collagen from smooth muscle and to identify an increase in collagenous tissue in xenograft specimens (muscle fibers-red, collagen-blue, fibrin-pink, cytoplasm-red, and nuclei-blue/black). The combination of PAS and PAS with diastase (PASD) was used for demonstration of glycogen content. PAS stains magenta the glycogen stores, but it also stains with the same color additional tissue elements (neutral mucosubstances, epithelial sulfomucins, and sialomucins). PASD reaction digests glycogen with a pretreatment of the tissue with α-amylase (depolymerizes glycogen), and as a result of this, any magenta staining is solely attributed to the other tissue elements described above but not to glycogen. The Alcian Blue stain was used for the demonstration of neutral and acidic mucosubstances. Alcian Blue imparts a blue color to the acidic mucins and other carboxylated or weakly sulphated acid mucosubstances. Because the PAS reaction stains basement membranes, glycogen, and neutral mucosubstances pink to red, mixtures of neutral and acidic mucosubstances will appear purple due to positive reactions with both Alcian Blue and PAS. Original magnification, ×200.
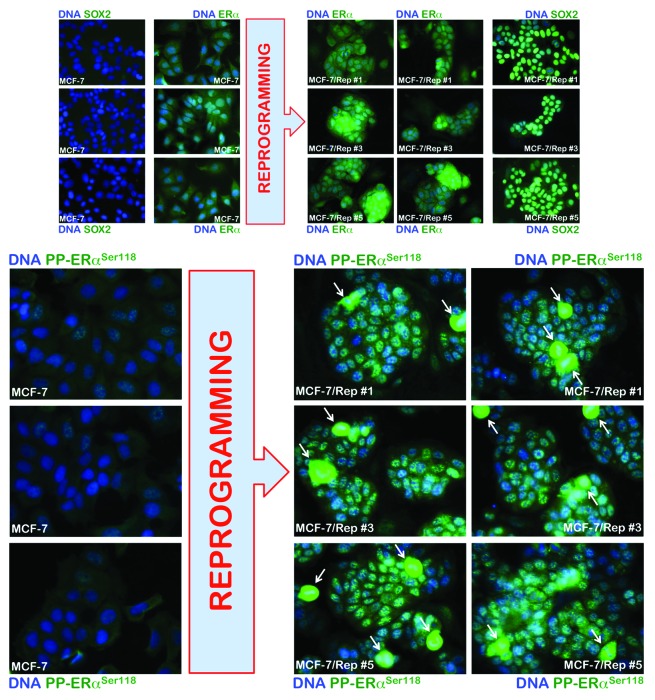
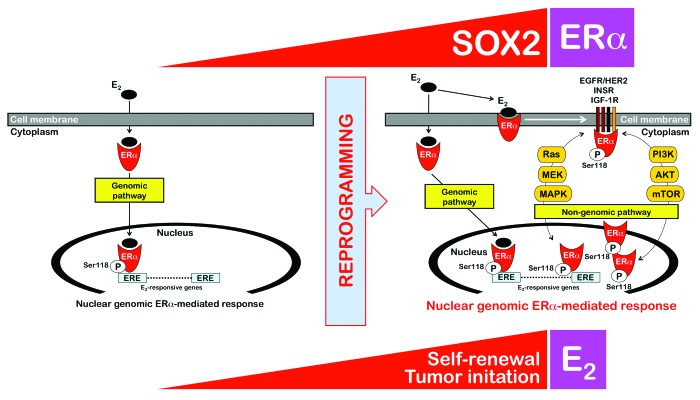
The abovementioned findings showing that SOX2-overexpressing MCF-7/Rep cells were engrafted with significantly higher efficacy and formed tumors at a faster rate than their MCF-7 parental counterparts exclusively in nude mice subcutaneously implanted with E2 pellets strongly suggested that SOX2 might facilitate tumor initiation in an E2-dependent manner. Because it has been previously shown that ERα signaling can regulate breast CSC frequency,11-13 with ERα stimulation resulting in increased numbers of CSCs, we investigated if the enhanced breast cancer-initiating capability of SOX2-overexpressing CSC-like MCF-7/Rep cells might be due to changes in E2 signaling activation via non-genomic pathways. Serine phosphorylation is required for full activation of ERα, and phosphorylation of ERα on Ser118 activates the N-terminal transcriptional activation function (AF-1) that renders ERα hypersensitive to E2; i.e., Ser118-ERα indicates an intact E2-dependent ERα pathway in primary breast tumors in vivo. Ser118 of ERα is a major phosphorylation site that results from activation of the Ras/Raf/MAPK pathway, and this process can occur in a ligand-independent manner. Indeed, E2 and growth factors/growth factor receptors can result in phosphorylation of ERα at Ser118 by MAPK, thus linking rapid, membrane-initiated, non-genomic ERα responses with later bona fide ERα genomic actions.14-19 Because the genomic and non-genomic actions of ERα are complementary and even synergistic via cross-regulatory interactions that are mediated through cross-talk with other growth factor and cellular signal transduction pathways, the fact that the SOX2-driven reprogramming of breast CSC-like states was involved in the derepression of the phospho-Ser118-ERα-activating insulin receptor/mTOR pathway4 strongly suggested that the E2-hypersensitive phenotype of the SOX2-overexpressing MCF-7/Rep xenotumors might be explained in terms of increased phospho-Ser118-ERα. When testing the hypothesis that phospho-Ser118-ERα levels might correspond to enhanced estrogenic responses and tumor-initiating ability in SOX2-overexpressing MCF-7/Rep cells, we first observed that the basal protein expression of ERα, which was diffusely distributed throughout the cytoplasm and nucleus, was increased in SOX2-overexpressing CSC-like MCF-7/Rep cells compared with MCF-7 parental cells (Fig. 3, top). Importantly, basal phospho-Ser188-ERα was drastically higher in SOX2-overexpressing MCF-7/Rep cells when compared with MCF-7 parental cells, which displayed significantly lower levels of nuclear phospho-Ser188-ERα. Phospho-Ser188-ERα was almost exclusively nuclear in SOX2-overexpressing MCF-7/Rep cells, and remarkably, it showed an apparent accumulation as extremely bright, nuclear punctate “speckles” in interphase cell nuclei (Fig. 3, bottom). We also noted that the intensity of phospho-Ser118-ERα was highest in the prometaphase and metaphase stages of mitosis (arrows in Fig. 3, bottom). Thus, SOX2-overexpressing MCF-7/Rep cells accumulated significantly higher numbers of mitotic cells, and these cells appeared to have higher levels of phospho-Ser118-ERα around the mitotic plate. The accumulation of nuclear phospho-Ser118-ERα in the S and G2/M phases of the cell cycle indicate how SOX2 overexpression can link E2 hypersensitivity to cell cycle progression in CSC-like breast cancer cells, i.e., SOX2 promoted non-genomic E2-signaling likely by activating the insulin/Ras/MAPK pathway, which led to nuclear phospho-Ser118-ERα and, thus, exacerbated genomic ER signaling in response to E2 (Fig. 4).
Figure 3. Immunofluorescence analysis of ERα and phospho-ERαSer118 in CSC-like SOX2-overexpressing MCF-7/Rep cells vs. MCF-7 parental cells. Immunofluorescence data show that CSC-like SOX2-overexpressing MCF7/Rep cells have constitutively elevated ERα phosphorylation at Ser118 in nucleoplasmic speckles compared with MCF-7 parental cells. White arrows indicate mitotic cells with very high levels of phospho-ERαSer118.
Figure 4. Reprogramming of non-genomic estrogen signaling by SOX2: A new driver of breast cancer stem-like cellular states with tumor-initiating capacity. SOX2 overexpression confers a tumor-initiating advantage to ERα-positive MCF-7 breast cancer cells in an estrogen-dependent manner, i.e. when s.c. injected into ovariectomized nude mice implanted with estrogen pellets, SOX2-overexpressing MCF-7/Rep cells formed more and larger tumors than MCF-7 parental cells, which mostly failed to produce tumor outgrowths in the absence of Matrigel. The previously unrecognized ability of the stemness factor SOX2 to promote estrogen-dependent tumorigenesis of ERα-positive breast cancer cells appears to depend on the activation of non-genomic estrogen signaling. E2 activates nuclear ERα (genomic pathway) and ERα in or near the membrane (non-genomic pathway). Membrane-associated ERα associates with growth factor signaling components (e.g., EGFR/HER2, INSR, IGF-1R), thus allowing E2 to activate the growth factor signaling by activating key molecules such as PI3K or Ras and downstream molecules such as AKT, mTOR, MEK, and MAPK. The crosstalk between the non-genomic estrogen signaling and the growth factor signaling is bidirectional, because a variety of kinases including MAPKs, AKT, and mTOR coordinately regulate the phosphorylation of specific sites of the ERα, leading to ligand (E2)-independent ERα activation. Moreover, phosphorylation of ERα co-regulatory proteins by growth factor kinases regulates the ERα signaling pathway to enhance the nuclear genomic ERα-mediated response. Our findings strongly suggest that reprogramming of non-genomic estrogen signaling by the stemness factor SOX2 is a key molecular feature that determines the tumor-initiating capacity of breast cancer stem-like cellular states.
Altogether, our findings indicate a potential mechanism by which E2 selectively decreases latency and enhances tumor incidence of subcutaneously injected, SOX2-reprogrammed CSC-like cells in the absence of co-injected Matrigel. In addition, we detected a prominent localization of phospho-Ser118-ERα in the nucleus of actively dividing SOX2-overexpressing MCF-7/Rep cells, which provides a new scenario for understanding the role of estrogen in the initiation of breast cancer by supporting a role for the SOX2/ERα interaction in cell replication. The previously unrecognized survival and growth advantage of breast CSC-like cells is highly dependent on the concurrent presence of the stemness factor, SOX2, and E2 signals to add a new layer of complexity to tumor stem cell reprogramming as a cancer driver. That is, the reprogramming properties of stemness factors, such as SOX2, can solely induce cancer development when engaged in response to contextual signals ([epi]genetic and/or environmental). Because it is suspected that CSCs are already programmed in early breast cancer lesions, such as premalignant DCIS, linking E2 with a SOX2-driven deregulated stem cell circuitry may significantly impact the natural history of an important subset of breast carcinomas. It is reasonable to suggest that a SOX2-driven increase in growth factor (e.g., insulin) and cellular kinase signaling (e.g., MAPK) in CSC-like cellular states potentiates the ERα pathway, which, in turn, reactivates growth factor signaling via genomic and non-genomic activities and results in a stimulatory cycle that intensifies the activity of ERα and SOX2-activated growth factor pathways. If this increased cross-talk makes the pathways more dependent upon one another, SOX2/ERα-positive tumors may become more sensitive to specific inhibitors of these pathways. The opposite may also be true, because enhanced SOX2/ERα cross-talk could neutralize the efficacy of SERMs, because a notably increased phosphorylation status of ERα on Ser118 has been observed in tamoxifen-refractory breast cancer cells, thereby suggesting the possible involvement of such a mechanism in the agonistic activity of tamoxifen and/or the hyper-response to tamoxifen-refractory cells to E2.14-18 Importantly, E2 stimulation has recently been shown to enhance breast tumor-initiating cell survival by downregulating miR-140, which targets SOX2.20 A bidirectional cross-talk interaction between the stem cell self-renewal regulator, SOX2, and the local and systemic ability of E2 to increase breast CSC activity may have profound implications for the development of new CSC-directed strategies for breast cancer prevention and therapy.13,21-23
Acknowledgments
This work was financially supported by the Ministerio de Ciencia e Innovación (SAF2012-38914, Plan Nacional de I+D+I, MICINN, Spain). Alejandro Vazquez-Martin received a Sara Borrell post-doctoral contract (CD08/00283, Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria -FIS-, Spain). Sílvia Cufí received a research fellowship (Formación de Personal Investigador, FPI) from the Ministerio de Ciencia e Innovación (MICINN, Spain).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/26692
REFERENCES
- 1.Menendez JA, Vellon L, Oliveras-Ferraros C, Cufí S, Vazquez-Martin A. mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: a roadmap from energy metabolism to stem cell renewal and aging. Cell Cycle. 2011;10:3658–77. doi: 10.4161/cc.10.21.18128. [DOI] [PubMed] [Google Scholar]
- 2.Vazquez-Martin A, Vellon L, Quirós PM, Cufí S, Ruiz de Galarreta E, Oliveras-Ferraros C, Martin AG, Martin-Castillo B, López-Otín C, Menendez JA. Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle. 2012;11:974–89. doi: 10.4161/cc.11.5.19450. [DOI] [PubMed] [Google Scholar]
- 3.Menendez JA, Joven J, Cufí S, Corominas-Faja B, Oliveras-Ferraros C, Cuyàs E, Martin-Castillo B, López-Bonet E, Alarcón T, Vazquez-Martin A. The Warburg effect version 2.0: metabolic reprogramming of cancer stem cells. Cell Cycle. 2013;12:1166–79. doi: 10.4161/cc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corominas-Faja B, Cufí S, Oliveras-Ferraros C, Cuyàs E, López-Bonet E, Lupu R, Alarcón T, Vellon L, Iglesias JM, Leis O, et al. Nuclear reprogramming of luminal-like breast cancer cells generates Sox2-overexpressing cancer stem-like cellular states harboring transcriptional activation of the mTOR pathway. Cell Cycle. 2013;12:3109–24. doi: 10.4161/cc.26173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Shi L, Zhang L, Li R, Liang J, Yu W, Sun L, Yang X, Wang Y, Zhang Y, et al. The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J Biol Chem. 2008;283:17969–78. doi: 10.1074/jbc.M802917200. [DOI] [PubMed] [Google Scholar]
- 6.Leis O, Eguiara A, Lopez-Arribillaga E, Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R, Martin AG. Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene. 2012;31:1354–65. doi: 10.1038/onc.2011.338. [DOI] [PubMed] [Google Scholar]
- 7.Lengerke C, Fehm T, Kurth R, Neubauer H, Scheble V, Müller F, Schneider F, Petersen K, Wallwiener D, Kanz L, et al. Expression of the embryonic stem cell marker SOX2 in early-stage breast carcinoma. BMC Cancer. 2011;11:42. doi: 10.1186/1471-2407-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enver T, Pera M, Peterson C, Andrews PW. Stem cell states, fates, and the rules of attraction. Cell Stem Cell. 2009;4:387–97. doi: 10.1016/j.stem.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Abollo-Jiménez F, Jiménez R, Cobaleda C. Physiological cellular reprogramming and cancer. Semin Cancer Biol. 2010;20:98–106. doi: 10.1016/j.semcancer.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Vicente-Dueñas C, Romero-Camarero I, Cobaleda C, Sánchez-García I. Function of oncogenes in cancer development: a changing paradigm. EMBO J. 2013;32:1502–13. doi: 10.1038/emboj.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15:193–7. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, Kuperwasser C. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci U S A. 2010;107:21737–42. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung JW, Park SB, Lee SJ, Seo MS, Trosko JE, Kang KS. Metformin represses self-renewal of the human breast carcinoma stem cells via inhibition of estrogen receptor-mediated OCT4 expression. PLoS One. 2011;6:e28068. doi: 10.1371/journal.pone.0028068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedram A, Razandi M, Aitkenhead M, Hughes CC, Levin ER. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J Biol Chem. 2002;277:50768–75. doi: 10.1074/jbc.M210106200. [DOI] [PubMed] [Google Scholar]
- 15.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–35. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 16.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11:865s–70s. [PubMed] [Google Scholar]
- 17.Massarweh S, Schiff R. Unraveling the mechanisms of endocrine resistance in breast cancer: new therapeutic opportunities. Clin Cancer Res. 2007;13:1950–4. doi: 10.1158/1078-0432.CCR-06-2540. [DOI] [PubMed] [Google Scholar]
- 18.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–33. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudhagar S, Sathya S, Lakshmi BS. Rapid non-genomic signalling by 17β-oestradiol through c-Src involves mTOR-dependent expression of HIF-1α in breast cancer cells. Br J Cancer. 2011;105:953–60. doi: 10.1038/bjc.2011.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Eades G, Yao Y, Li Q, Zhou Q. Estrogen receptor α signaling regulates breast tumor-initiating cells by down-regulating miR-140 which targets the transcription factor SOX2. J Biol Chem. 2012;287:41514–22. doi: 10.1074/jbc.M112.404871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Barco S, Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B, Menendez JA. Metformin: multi-faceted protection against cancer. Oncotarget. 2011;2:896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cufi S, Corominas-Faja B, Vazquez-Martin A, Oliveras-Ferraros C, Dorca J, Bosch-Barrera J, Martin-Castillo B, Menendez JA. Metformin-induced preferential killing of breast cancer initiating CD44+CD24-/low cells is sufficient to overcome primary resistance to trastuzumab in HER2+ human breast cancer xenografts. Oncotarget. 2012;3:395–8. doi: 10.18632/oncotarget.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stolzenburg S, Rots MG, Beltran AS, Rivenbark AG, Yuan X, Qian H, Strahl BD, Blancafort P. Targeted silencing of the oncogenic transcription factor SOX2 in breast cancer. Nucleic Acids Res. 2012;40:6725–40. doi: 10.1093/nar/gks360. [DOI] [PMC free article] [PubMed] [Google Scholar]