Abstract
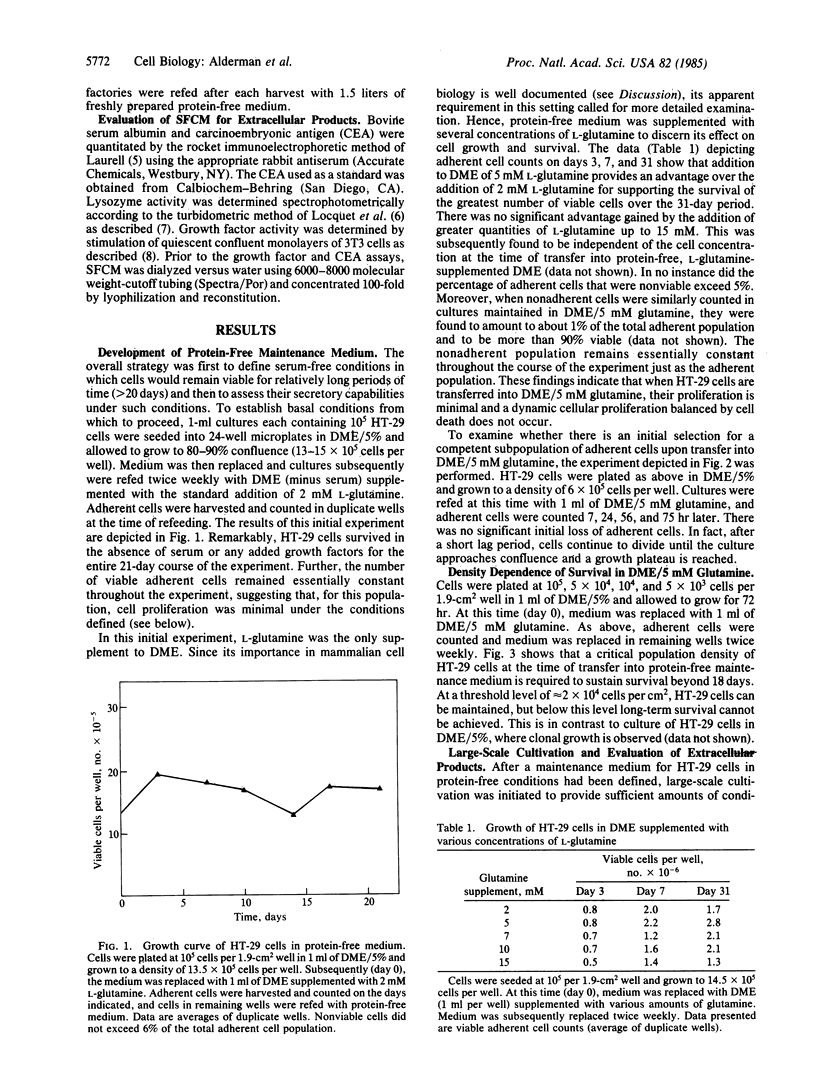
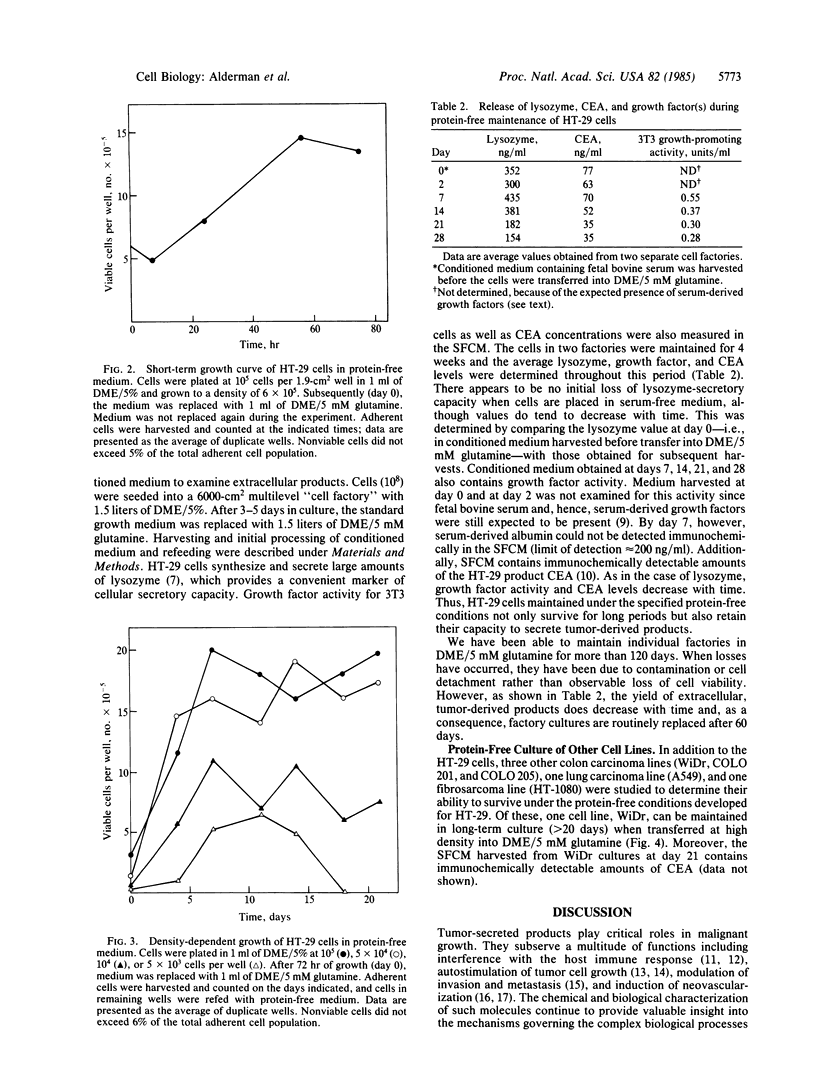
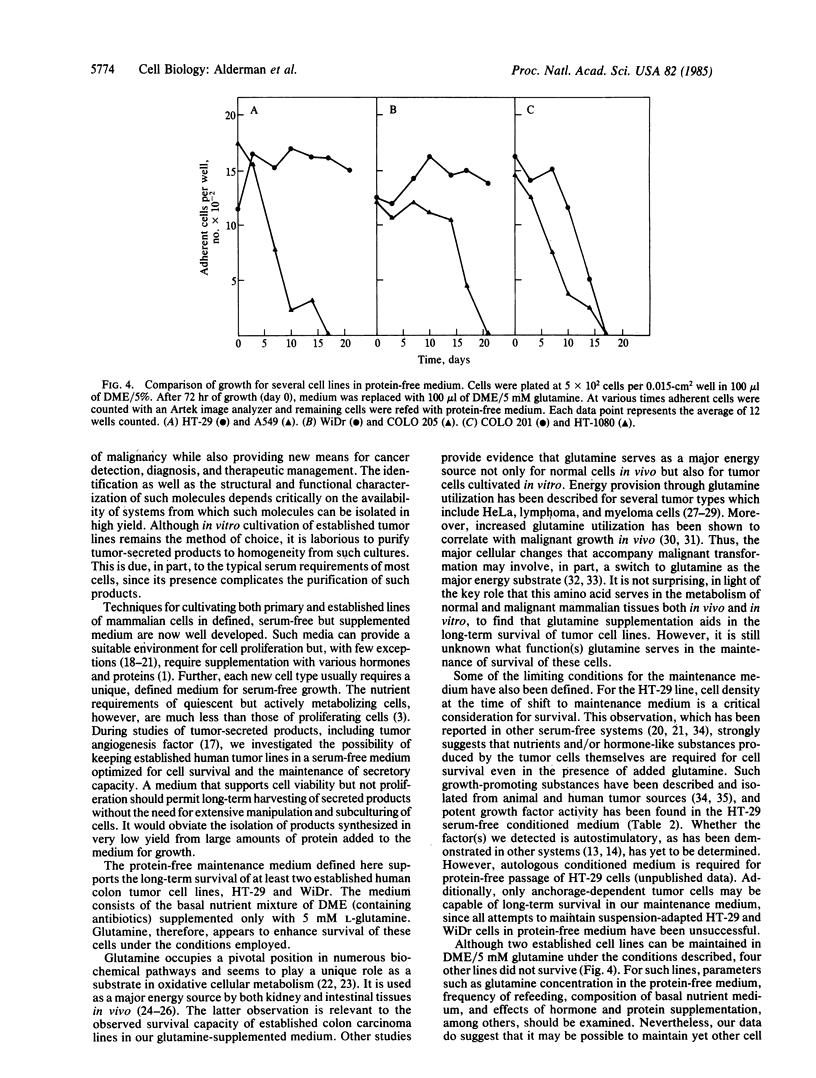
A protein-free synthetic cell-growth medium has been defined that permits long-term survival (greater than 120 days) of an established human colon tumor cell line, HT-29. Viability is dependent upon both the concentration of L-glutamine in the medium and the cell density at the time of initial transfer into it. Cell proliferation is minimal, thus obviating the necessity for subculturing. HT-29 adenocarcinoma cells maintained in large-scale culture with this medium continue to secrete the established colon tumor marker carcinoembryonic antigen as well as growth factors and lysozyme. These and, potentially, other important tumor-derived products can therefore be generated continuously in such cultures so that they can be isolated from a conditioned medium free of contaminating serum and protein supplements.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agy P. C., Shipley G. D., Ham R. G. Protein-free medium for C-1300 mouse neuroblastoma cells. In Vitro. 1981 Aug;17(8):671–680. doi: 10.1007/BF02628402. [DOI] [PubMed] [Google Scholar]
- Antoniades H. N., Stathakos D., Scher C. D. Isolation of a cationic polypeptide from human serum that stimulates proliferation of 3T3 cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2635–2639. doi: 10.1073/pnas.72.7.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Morris H. P., Weber G. Regulatory properties and behavior of activity of carbamoyl phosphate synthetase II (glutamine-hydrolyzing) in normal and proliferating tissues. J Biol Chem. 1982 Jan 10;257(1):432–438. [PubMed] [Google Scholar]
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Baruch S. B., Eun C. K., MacLeod M., Pitts R. F. Renal CO2 production from glutamine and lactate as a function of arterial perfusion pressure in dog. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4235–4238. doi: 10.1073/pnas.73.11.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P. S., Lavietes B. B. Membrane cholesterol, tumorigenesis, and the biochemical phenotype of neoplasia. CRC Crit Rev Biochem. 1981;11(4):341–393. doi: 10.1080/10409238109104421. [DOI] [PubMed] [Google Scholar]
- Egan M. L., Todd C. W. Carcinoembryonic antigen: synthesis by a continuous line of adenocarcinoma cells. J Natl Cancer Inst. 1972 Sep;49(3):887–889. [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Vallee B. L., Artymiuk P. J., Collett S., Phillips D. C., Dobson C. M., Redfield C. Lysozyme: a major secretory product of a human colon carcinoma cell line. Biochemistry. 1985 Feb 12;24(4):965–975. doi: 10.1021/bi00325a024. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1974;19(0):331–358. doi: 10.1016/s0065-230x(08)60058-5. [DOI] [PubMed] [Google Scholar]
- HEALY G. M., FISHER D. C., PARKER R. C. Nutrition of animal cells in tissue culture. X. Synthetic medium No. 858. Proc Soc Exp Biol Med. 1955 May;89(1):71–77. doi: 10.3181/00379727-89-21719. [DOI] [PubMed] [Google Scholar]
- Higuchi K. Cultivation of animal cells in chemically defined media, a review. Adv Appl Microbiol. 1973;16:111–136. doi: 10.1016/s0065-2164(08)70025-x. [DOI] [PubMed] [Google Scholar]
- Kaighn M. E., Kirk D., Szalay M., Lechner J. F. Growth control of prostatic carcinoma cells in serum-free media: interrelationship of hormone response, cell density, and nutrient media. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5673–5676. doi: 10.1073/pnas.78.9.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. L., Anderson M., Ozanne B. Transforming growth factor(s) production enables cells to grow in the absence of serum: an autocrine system. Proc Natl Acad Sci U S A. 1982 Jan;79(2):485–489. doi: 10.1073/pnas.79.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacević Z., Morris H. P. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 1972 Feb;32(2):326–333. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Lavietes B. B., Regan D. H., Demopoulos H. B. Glutamate oxidation of 6C3HED lymphoma: effects of L-asparaginase on sensitive and resistant lines. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3993–3997. doi: 10.1073/pnas.71.10.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. R., Fett J. W. Purification of two distinct growth factors from bovine neural tissue by heparin affinity chromatography. Biochemistry. 1984 Dec 18;23(26):6295–6299. doi: 10.1021/bi00321a001. [DOI] [PubMed] [Google Scholar]
- Locquet J. P., Saint-Blancard J., Jollès P. Apparent affinity constants of lysozymes from different origins forMicrococcus lysodeikticus cells. Biochim Biophys Acta. 1968 Aug 27;167(1):150–153. doi: 10.1016/0005-2744(68)90284-2. [DOI] [PubMed] [Google Scholar]
- MERCHANT D. J., HELLMAN K. B. Growth of L-M strain mouse cells in a chemically defined medium. Proc Soc Exp Biol Med. 1962 May;110:194–198. doi: 10.3181/00379727-110-27464. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L. Glycolysis, glutaminolysis and cell proliferation. Cell Biol Int Rep. 1982 Jul;6(7):635–650. doi: 10.1016/0309-1651(82)90125-4. [DOI] [PubMed] [Google Scholar]
- North R. J., Kirstein D. P., Tuttle R. L. Subversion of host defense mechanisms by murine tumors. I. A circulating factor that suppresses macrophage-mediated resistance to infection. J Exp Med. 1976 Mar 1;143(3):559–573. doi: 10.1084/jem.143.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L. J., Wice B. M., Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979 Apr 25;254(8):2669–2676. [PubMed] [Google Scholar]
- Roberts R. S., Hsu H. W., Lin K. D., Yang T. J. Amino acid metabolism of myeloma cells in culture. J Cell Sci. 1976 Aug;21(3):609–615. doi: 10.1242/jcs.21.3.609. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F., Lobb R. R., Higachi N., Fett J. W., Crossley G., Bühler R., Budzik G., Breddam K., Bethune J. L. Tumor-derived angiogenesis factors from rat Walker 256 carcinoma: an experimental investigation and review. Experientia. 1985 Jan 15;41(1):1–15. doi: 10.1007/BF02005853. [DOI] [PubMed] [Google Scholar]
- Veit B. C., Feldman J. D. Altered lymphocyte functions in rats bearing syngeneic Moloney sarcoma tumors. II. Suppressor cells. J Immunol. 1976 Aug;117(2):655–660. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Intestinal metabolism of glutamine and glutamate from the lumen as compared to glutamine from blood. Arch Biochem Biophys. 1975 Dec;171(2):662–672. doi: 10.1016/0003-9861(75)90078-8. [DOI] [PubMed] [Google Scholar]
- Zetterberg A., Engström W. Glutamine and the regulation of DNA replication and cell multiplication in fibroblasts. J Cell Physiol. 1981 Sep;108(3):365–373. doi: 10.1002/jcp.1041080310. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]



