Abstract
One-month Phase II trial was conducted in 43 sputum smear-positive patients with pulmonary tuberculosis randomized into treatment (n = 22) and placebo (n = 21) arms to investigate the safety and efficacy of an orally-administered therapeutic TB vaccine (V7) containing 10 μg of heat-killed Mycobacterium vaccae provided by Longcom company. Immunotherapy and control groups comprised 8 newly diagnosed (1stDx TB; 18.6%), 6 re-treated (RTB; 14%), and 29 multidrug-resistant (MDR-TB; 67.4%) cases distributed at 5:4:13 and 3:2:16 ratios, respectively. Both arms received conventional TB drugs administered under directly observed therapy. The average weight gain in V7 arm was modest, but statistically significant (0.6 kg; p = 0.004), while placebo patients lost 0.1 kg (p = 0.77). Except defervescence and increased lymphocyte percentage, other secondary endpoints such as erythrocyte sedimentation rate (ESR), leukocyte counts and hemoglobin content were not significantly affected. In control patients only one secondary endpoint, ESR, has improved. After one month mycobacterial clearance in sputum smears was observed in 31.8% (p = 0.03) and 9.5% (p = 0.83) of patients on V7 and placebo. However, the difference between outcomes in two arms was below significance threshold (p = 0.07). Thus, larger population of patients with prolonged follow-up is required to support these preliminary findings.
Keywords: inflammation, mycobacterium tuberculosis, drug-resistant TB, therapeutic vaccine, immunomodulator, mucosa, DOT, imm02, NCT01380119
Introduction
TB is a public health problem, especially in developing countries due to poverty, HIV co-infection, and drug-resistant TB, especially MDR-TB. The cost of treating MDR-TB is 100 times more expensive than drug-susceptible strains, with the treatment time at least two to three times longer, and with more severe side effects. Despite the availability of TB drugs the situation is far from ideal and it is clear that better, faster-acting, less toxic and affordable therapeutic interventions are urgently needed to curtail the current crisis.1
Immune-based interventions could be an indispensable strategy to complement mycobacterial chemotherapy.2-5 Today, the most familiar TB immunotherapy is heat-inactivated Mycobacterium vaccae preparation, the use of which has been initiated by John Stanford and colleagues almost 30 y ago.6 M. vaccae, discovered in 1964 by Bonicke and Juhasz, is a non-pathogenic environmental saprophyte belonging to the same genus as Mycobacterium tuberculosis.7 It has been investigated extensively as a preventive TB vaccine or immunomodulatory agent for tuberculosis and leprosy and many unrelated diseases such as cancer, autoimmune and mood disorders.8-10 Killed M. vaccae is safe: three decades of clinical studies in more than 20 countries failed to reveal any adverse side-effects except local reaction at the site of injection. Injectable M. vaccae (Vaccae®) was approved in 2001 for sale in China as adjunct immunotherapy to TB drugs.11 This intervention usually resulted in better outcome than chemotherapy alone. In Luo et al., study negative sputum conversion seen in MDR-TB patients after 6 mo was 43% vs. 21% on TB drugs alone.12 Nevertheless, M. vaccae appeared to produce a measurable improvement in some geographical regions, but not in others, this inconsistency has led some researchers to doubt its efficacy.13-15
The capsulated version of M. vaccae administered orally has been first tried-out in 2010 by Dlugovitzky et al., as an alternative to parenteral delivery.16 The results of this ten-patient study in Argentina were highly encouraging—this prompted us to test tableted M. vaccae. For this project two strains of mycobacteria were procured from independent GMP sources—one from China and another from Europe. The objective of this study was to evaluate whether a vaccine delivered per os is safe and produces better outcome than placebo with primary endpoint being sputum smear clearance after one month. The findings from the initial trial of oral formulation based on Chinese preparation of M. vaccae are described in this paper.
Results
Effect on mycobacterial clearance
After one month seven (31.8%) of V7-treated patients became sputum negative, which was significant (p = 0.03). Among converted patients, four (57.1%) had MDR-TB, two (28.6%) RTB and only one (14.3%) newly diagnosed TB. In placebo arm two patients, one with MDR-TB and another with 1st Dx TB, had converted (Fig. 1). The difference in outcomes between treatment arms as calculated by two-tailed Fisher's exact test was not, however, significant (p = 0.07). Patients in V7 arm had higher mean bacillary load than control patients, i.e., 1.4 ± 0.8 vs 1 ± 0.29 (p = 0.02) and after one month this value had decreased 2-fold to 0.77 ± 0.69 vs. 0.87 ± 0.46 (p = 0.58; unpaired t-test).
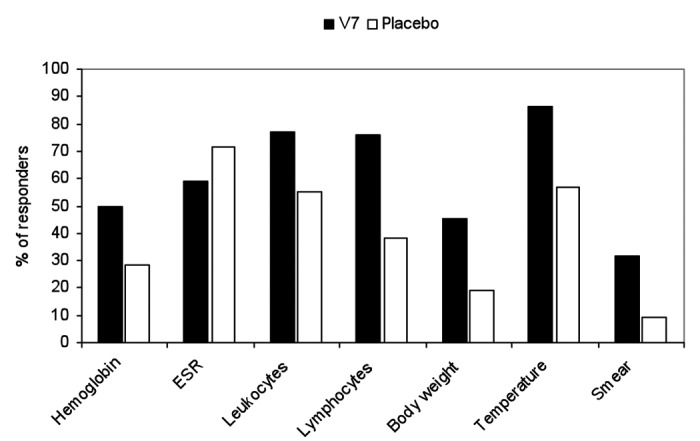
Figure 1. The proportion of patients who responded favorably in two treatment arms.
Lack of adverse reactions
During the entire duration of the follow-up no adverse events neither mild, moderate, severe, life-threatening or unexpected attributable to V7 were identified. No re-activation of TB was noted either. Quite contrary patients who were receiving chemotherapy along with V7 appeared to fare better. The quantitative endpoints detailed below indicate that the addition of V7 to anti-tuberculosis therapy (ATT) was safe and produced better clinical outcome.
Effect on axillary body temperature
The average ± SD (median) baseline body temperature values in V7 and placebo arms were relatively low, i.e., 37.1°C ± 0.5 (37°C) vs. 37.4°C ± 0.6 (37.8°C) (p = 0.03). The proportion of patients in V7 arm who had low-grade fever was smaller as well, e.g., 54.5% vs. 66.7% in placebo, but the inter-group difference was not statistically significant (p = 0.31). After one month the proportion of V7 recipients who had normalized temperature of 37°C or less increased to 86.4% as opposed to placebo, among whom slightly over half (57.1%) had achieved normal body temperature (Fig. 1).
Effect on hematology parameters
The effect of V7 and placebo on white blood cells and hematology parameters are shown in Table 1 and Figure 1. Except slight but significant increase in relative lymphocyte count from 27.9% ± 7.7 to 30.9% ± 6.2 (p = 0.006) no other significant changes were observed including before and after leukocyte counts, erythrocyte sedimentation rate (ESR), and hemoglobin levels (Table 1). Among patients on placebo only ESR has been reduced in a significant manner (p = 0.001). Out of 21 patients 15 (71.4%) had experienced modest decrease in ESR (range 2–12 mm/h); 2 had unchanged levels (9.5%); and remaining 4 (19%) had increased erythrocyte sedimentation rate. In V7 arm 13 out of 22 (59.1%) had decreased ESR, while the rest (40.9%) experienced increased ESR (range 1–24 mm/h).
Table 1. Summary of baseline and outcome data from one-month trial of oral M. vaccae.
| Arms | No. | Sex F/M |
Age | Dx | Sputum smear (+/−) |
Axillary temperature (Co) |
Body weight (kg) |
BMI (kg/m2) |
Hemoglobin (g/L) |
ESR (mm/h) |
Leukocytes (x109L) |
Lymphocytes (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| before | after | before | after | before | after | before | after | before | after | before | after | before | after | before | after | ||||||||
| M. vaccae | 22 | 7/15 | 41.9 ± 10 (39) |
1stDx = 5 RTB = 4 MDR = 13 |
22/0 | 15/7 | 37.1 ± 0.5 |
36.8 ± 0.5 |
55.9 ± 9.8 |
56.5 ± 9.4 |
19.3 ± 2.6 |
19.5 ± 2.6 |
128.4 ± 17.7 |
127.4 ± 13.5 |
18.5 ± 14.1 |
18.3 ± 11.6 |
8.5 ± 2.6 |
7.9 ± 2.2 |
27.9 ± 7.7 |
30.9 ± 6.2 |
|||
| 31.8% converted p = 0.03 |
-0.3 p = 0.05 |
+0.6 p = 0.004 |
+0.2 p = 0.003 |
-1 p = 0.59 |
-0.2 p = 0.92 |
-0.6 p = 0.15 |
+3 p = 0.006 |
||||||||||||||||
| Placebo | 21 | 4/17 | 44.3 ± 11 (44) |
1stDx = 3 RTB = 2 MDR = 16 |
21/0 | 19/2 | 37.4 ± 0.6 |
37.2 ± 0.7 |
63.9 ± 12.6 |
63.8 ± 12.9 |
22.01 ± 4.8 |
21.99 ± 4.9 |
105 ± 18.8 |
106.2 ± 18.7 |
22.4 ± 12.3 |
18.6 ± 8.9 |
7.5 ± 3.5 |
7.5 ± 2.8 |
27.2 ± 8.6 |
27.1 ± 5.5 |
|||
| 9.5% converted p = 0.83 |
-0.2 p = 0.12 |
-0.1 p = 0.77 |
-0.02 p = 0.65 |
+1.2 p = 0.26 |
-3.8 p = 0.001 |
0 p = 0.98 |
-0.1 p = 0.93 |
||||||||||||||||
| Difference between arms (P) |
0.27 | 0.65 | 0.14 | 1.0 | 0.07 | 0.08 | 0.12 | 0.03 | 0.04 | 0.03 | 0.04 | 0.001 | 0.001 | 0.34 | 0.92 | 0.29 | 0.6 | 0.78 | 0.04 | ||||
Effect on body weight and body mass index (BMI)
According to BMI almost half (45.5%) of enrolled patients in V7 arm had entry level values below normal 18.5 kg/m2 and the rest were in the normal range between 18.6–25.2 kg/m2. The mean/median baseline weight and body mass index were 55.9 ± 9.8/53.5 kg and 19.3 ± 2.6/18.8 kg/m2 respectively. After one month one patient (4.5%) lost 1 kg, 11 patients (50%) retained the same weight and in remaining 10 (45.5%) patients the increase in body mass ranging between 0.5–2 kg, with average (median) body weight accrual equal to 0.6 ± 0.9 (0) kg (p = 0.004) (Fig. 1). Changes in body mass index mirrored the variations in the absolute bodyweight.
In control group four patients (19%) lost weight (range 1–2 kg), 13 patients (61.9%) retained the same body mass, and remaining 4 (19%) gained 1 kg each, with average (median) loss equal to 0.1 ± 0.74 (0) kg. Nevertheless, the weight loss in the control group was not statistically significant (p = 0.77). According to BMI the patients on placebo were better off at baseline since only 5 (23.8%) had entry level values below normal 18.5 kg/m2 and the rest (76.2%) were in the normal range between 19–31.2 kg/m2. Among patients on placebo the proportion of underweight to normal BMI has not changed at study conclusion.
Discussion
The oral administration of M. vaccae was safe and produced statistically significant sputum smear conversion and improved secondary endpoints such as weight gain, body mass index, lymphocyte counts and deffervescence. Other secondary endpoints such ESR, leukocyte counts and hemoglobin content were not affected. Although the proportion of smear converted patients in V7 arm was 3-fold higher than in the control, i.e., 31.8% vs. 9.5%, the inter-group difference was not statistically significant (p = 0.07). It is thus, apparent that the effect was suboptimal since targeted sample size, i.e., 20 patients per arm, which was sufficient in our prior immunotherapy trials, was not large enough to attain a satisfactory level of statistical power. If required this needs to be explored further by recruiting larger population of patients and following them for longer periods of time. As a hindsight we now can estimate the minimum sample size, i.e., at least 38 subjects per arm, in order to attain robust statistical power with C.I. level 0.95 with SD = 1.
While our outcome appears to be modest the results of available to us clinical reports of injectable Vaccae® are in line with our findings. For example, according to Wang et al., sputum conversion rate after one month was 31% in adjunct M. vaccae group, while it was 23% in drug-sensitive control patients on 2HRZS/4HR regimen (n = 35), but only 3% in MDR-TB group.17 In Luo et al., study of MDR-TB patients divided into immunotherapy (n = 28) and control (n = 28) groups the sputum conversion rates at 6 mo were 43% and 21%.12 In another Luo et al., trial involving re-treated TB patients the bacillary clearance rates after one month in immunotherapy (n = 171) and control (n = 171) populations were 36.8% vs. 19.9% respectively. However, the statistical difference between two groups at the end of 6 mo was negligible, i.e., 99.4% vs. 98.8% respectively.18 In a separate randomized trial of unrelated M. vaccae strain, the sputum culture conversion after 1 mo was 35% in the M. vaccae group and only 14% in the placebo group.19 Thus, in these studies the addition of M. vaccae resulted in one-month conversion rates, which were very similar, i.e., 31%; 36.8%; and 35% vs. 31.8% in our study.
Nevertheless, such an outcome is somewhat inferior to immune therapies we and others have evaluated in the past.20 For example, in the latest trial of Immunoxel, a multiherbal immunomodulator from Ukraine, the conversion rate after one month stood at 84.1% vs. 19% in controls.21 In randomized, double-blind, placebo-controlled Phase IIb trial of V5 in first-diagnosed, relapsed and MDR-TB we had 88.7% vs. 14.8% patients converted after one month.22 In both studies no statistical difference was observed when first-diagnosed, drug-sensitive TB and MDR-TB subsets of patients were compared in regard to smear conversion rate.
There appear to be major distinctions which set apart clinical features produced by Chinese version of M. vaccae from two above interventions. Except defervescence other inflammation markers such as elevated leukocyte counts and ESR were not affected. Prior to enrollment both groups had mean body temperature above cut-off normal value 37°C. At study conclusion the mean values normalized in V7 group, i.e., less than 37°C, but not in placebo recipients. In Stanford et al., studies, which employed “rough” version of M. vaccae strain at much higher 1 mg dose, there was a clear anti-inflammatory effect as evidenced by ESR decline.6 The negligible effect of oral Vaccae® on inflammation biomarkers correlates with suboptimal sputum conversion, which supports our hypothesis that effective TB immunotherapy, must possess anti-inflammatory properties instead of exacerbating already present immune reaction against mycobacteria.20 However, this does not mean that the immune response needs to be shut down.
In prior immunotherapy studies we have seen very clear increase in CD4-positive lymphocyte population, but unfortunately we have little information regarding their subtype. This needs to be established in studies addressing the immune mechanism of V7 action that could identify the long-sought immune correlates, such as cytokine profiles and immune cell subtypes, associated with positive clinical outcome. The only inflammation related improvement we have seen in placebo patients is reduction in ESR. This has been seen in our other studies as well, indicating that some forms of ATT might display the anti-inflammatory activity in addition to direct antimicrobial effect. This indeed has been noted by McConkey and Situnayake in their 1988 study of patients with rheumatoid arthritis who surprisingly responded favorably to rifampicin and isoniazid combination even though they had no TB.23
It is commonly held that difficult-to-treat cases such as MDR-TB or RTB respond poorly to M. vaccae therapy. However, truly effective immune intervention, which targets the host response, should not discriminate drug-sensitive strains from species of M. tuberculosis refractory to TB drugs.2-5 Almost every drug-resistant TB patient in this study has been on palliative therapy consisting of two drugs only, i.e., H and R, to which by definition MDR-TB is not responsive. These patients are unique as majority of them have no signs of inflammation. Such individuals respond poorly even to V5 therapy since one-month conversion rate in this population was in 50–62.5% range.24,25
There are several limitations to our study, which were primarily due to lack of funds. First, the follow-up period was one month only, which is not sufficient to see long-term effects, such as recurrence and conversion rate on follow-up; second, no mechanistic studies were performed to identify immune correlates, e.g., particular cytokines or phenotypes of cells involved; third, the optimal antigen load is ought to be determined if further studies of Vaccae® are warranted. Alternatively, other sources of M. vaccae need to be evaluated as well as an effort to find optimally effective dose. Our manuscript describing the outcome of clinical trial of V7 formulation based on “rough” strain of M. vaccae used by Stanford et al., is now submitted to another journal.
Despite persisting doubt concerning the efficacy of M. vaccae as an immune adjunct it has been, nevertheless, approved in China for TB indication. According to recent review articles Vaccae® has been evaluated in over 50 clinical trials in China, almost all of which reported positive clinical outcome.11,26 These data are hard to dismiss and it is imperative to allocate additional resources to reveal the benefit of M. vaccae that can be administered by simpler delivery route.
In conclusion, our study of tableted M. vaccae shows that this formulation is well-tolerated without any observed adverse effects and has a potential as add-on option to the chemotherapy of drug-resistant TB capable of producing significantly improved sputum conversion at faster rate than conventional chemotherapy regimens. In addition, oral M. vaccae needs be investigated as a prophylactic intervention since the parenteral version has been demonstrated to prevent both de novo infection and activation of latent TB in high risk individuals including those infected with HIV.8,26
Materials and Methods
Vaccine preparation
V7 vaccine preparation was made from bulk of GMP grade M. vaccae (Vaccae®; Batch No. M20111124 courtesy of Longcom company (Anhui Longcom Biologic Pharmacy Co., Ltd., 93 Kexue Avenue, National New High-Tech Industrial Development Zone, Hefei, Anhui Province 230088, China). Vaccae® was approved by Chinese FDA in 2001 for adjunct therapy of tuberculosis. The vaccine contains 22.5 μg of antigen per dose and is administered intramuscularly once per week or every two weeks for 6 mo. M. vaccae was grown on solid agar media, harvested; cells were broken with a press and autoclaved as per Longcom procedure. The obtained antigenic material was used for making tableted vaccines. The antigen quantity from the bulk source was adjusted to an equivalent of 10 μg of total mycobacterial protein per tablet. Placebo pills had the same appearance and ingredients except M. vaccae.
Subjects
The subject population consisted of 43 sputum smear-positive patients, of which two third (n = 29; 67.4%) had MDR-TB diagnosis and remaining patients had 1st diagnosed TB (n = 8; 18.6%) and re-treated TB (n = 6; 14%). The distribution of three categories of TB among treatment and placebo arms was 13:4:5 and 16:2:3 ratios respectively (p = 0.14). All MDR-TB patients, except one, were on palliative care consisting of daily dose of Isoniazid (H) and Rifampicin (R) only. The average/median age was 43.1/41 y (range 28–71) with 11 females and 32 males recruited at three TB dispensaries in Eastern Ukraine and computer-randomized into two groups. Randomization has not produced significant baseline bias in demographic features as discrepancies in age and gender were not different with p = 0.65 and p = 0.27, respectively. Inflammation markers in both groups such as erythrocyte sedimentation rate (ESR) and leukocyte counts were similar with p = 0.34 and p = 0.29. Subjects in V7 arm had slightly lower axillary body temperature, i.e., 37.1°C ± 0.5 vs. 37.4°C ± 0.6 (p = 0.08) and significantly lower body weight and body mass index (BMI), i.e., 55.91 kg vs 63.86 kg (p = 0.03) and 19.3 vs 22 (p = 0.03), respectively. Mean elapsed time since TB diagnosis in V7 arm was 4.6 vs. 6.2 y in control group (p = 0.3). Overall, subject populations in two groups were not distinct and treatment outcomes were unlikely to be biased by baseline heterogeneity. All study subjects presented with active form of pulmonary TB. Most common symptoms were prolonged cough, pain in the chest, low-grade fever, night sweats, fatigue, dyspnea, hemoptysis, and loss of weight and appetite. Active pulmonary tuberculosis was certified by a medical history and clinical findings compatible with tuberculosis, a chest X-ray showing lung involvement and positive sputum smear for acid-fast bacilli (AFB). All subjects signed the consent form with understanding that they were free to withdraw from the study at any time. The conduct of the trial (imm02) was approved by IRB of Lisichansk TB Dispensary and registered at ClinicalTrials.gov site under NCT01380119 identifier.
Treatment regimen
All subjects received either standard TB therapy consisting of orally administered Isoniazid (H; 300 mg); Rifampicin (R; 600 mg); Pyrazinamide (Z; 2,000 mg); Ethambutol (E; 1200 mg); with or without intramuscular injection of Streptomycin (S; 1,000 mg) or were on individualized treatment regimens as decided by the medical staff based on results of drug resistance profile or prior medical history. Accordingly, 28 out of 29 patients with MDR-TB were on palliative care consisting of daily HR due to repeated failure to respond to chemotherapy over the course of years and were thus deemed incurable. The anti-TB drugs were procured through the centralized national supply system of Ukraine. Subjects in immunotherapy and control groups received in addition to ATT once-daily tablet of V7 or placebo; usually 30 min before or after breakfast. As per approved protocol treatment was administered to hospitalized patients for 30 d under directly observed therapy (DOT). V7 is approved in 2011 by the Ministry of Health of Ukraine as an immunomodulating supplement.
Clinical endpoints and exclusion/inclusion criteria
Every hospitalized patient with AFB-positive sputum smear was eligible to enter the study. No restrictive exclusion criteria for study enrollment were set up except disallowing patients who tolerated poorly chemotherapy and were likely to be non-compliant or those who have taken study-unrelated immunomodulatory therapies during study or prior to entry. Pregnant or breast-feeding women were excluded and as well as those with alcohol or substance abuse or mental ineptitude, which in opinion of the local investigator would interfere with adherence to the requirements of the study. The primary endpoint of interest was the effect of prescribed therapy on sputum smear microscopy findings. The main secondary endpoint was absence or presence of adverse effects attributable to V7 administration. The additional secondary endpoints comprised changes in select hematology parameters as well as changes in axillary body temperature and body weight including body mass index (BMI).
Safety monitoring
The clinical protocol included standard GCP criteria of reporting adverse events (AE) as per NIH or ICH guidelines of clinical safety data management.27 These include grading AE from zero to five.
Laboratory evaluation
In addition to clinical assessment a standard microbiology examination of sputum smear staining by Ziehl Neelsen method was conducted prior to study entry and at one month post-treatment. Smears were scored in a blind fashion from 3 to 0 according to the severity of bacterial load. TB drug resistance was determined by readily available commercial kit (Tulip Diagnostics, Goa, India). These tests were supplemented by investigation of hematological parameters such as hemoglobin content, erythrocyte sedimentation rate (ESR), leukocyte and lymphocyte counts.
Statistical analysis
The obtained results were analyzed with statistical software GraphPad, freely available on the internet (GraphPad Software, Inc., La Jolla, CA 92037). The baseline quantitative values relative to the end of study values were evaluated by paired or unpaired Student t-test. Other statistical calculations such as determination of standard deviation, mean and median, were performed with the same software. The non-parametric or categorical values of treatment outcomes were compared by McNemar, Fisher’s exact two-tailed test or Chi-square contingency tables. The Wilcoxon test was used to compare paired before-after nonparametric values such as conversion rates arranged in “yes” or “no” binary manner. All statistical analyses were done on intent-to-treat basis, involving the total number of subjects without subgrouping them into responders and non-responders. The resulting probability values were considered as significant at p ≤ 0.05.
Acknowledgments
We thank all volunteers who participated in this study. The wholehearted support of clinicians, nurses and lab personnel who contributed their effort made this study possible. Our gratitude is expressed to many experts in TB and immunology fields who kindly shared with us their opinions and critiques prior to and after this study was completed. We are greatly indebted to Jiang Pu, Feng-li Tao, Shilong Yang, Haiming Wei, Chuanyou Li, Vika Borisova, Alan Reid and Allen Bain for their generosity and tireless assistance in procuring a sample of Vaccae®, which has been used for making the batch of V7 used in this trial.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25280
References
- 1.Lienhardt C, Vernon A, Raviglione MC. New drugs and new regimens for the treatment of tuberculosis: review of the drug development pipeline and implications for national programmes. Curr Opin Pulm Med. 2010;16:186–93. doi: 10.1097/MCP.0b013e328337580c. [DOI] [PubMed] [Google Scholar]
- 2.Churchyard GJ, Kaplan G, Fallows D, Wallis RS, Onyebujoh P, Rook GA. Advances in immunotherapy for tuberculosis treatment. Clin Chest Med. 2009;30:769–82, ix. doi: 10.1016/j.ccm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Dheda K, Schwander SK, Zhu B, van Zyl-Smit RN, Zhang Y. The immunology of tuberculosis: from bench to bedside. Respirology. 2010;15:433–50. doi: 10.1111/j.1440-1843.2010.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doherty TM. Immunotherapy for TB. Immunotherapy. 2012;4:629–47. doi: 10.2217/imt.12.52. [DOI] [PubMed] [Google Scholar]
- 5.Prabowo SA, Gröschel MI, Schmidt EDL, Skrahina A, Mihaescu T, Hastürk S, et al. Targeting multidrug-resistant tuberculosis (MDR-TB) by therapeutic vaccines. Med Microbiol Immunol. 2013;202:95–104. doi: 10.1007/s00430-012-0278-6. [DOI] [PubMed] [Google Scholar]
- 6.Stanford J, Stanford C, Grange J. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis. Front Biosci. 2004;9:1701–19. doi: 10.2741/1292. [DOI] [PubMed] [Google Scholar]
- 7.Boenickse R, Juhasz E. Beschreibung der neuen species Mycobacterium vaccae n.sp. Zentralbl Bakteriol Orig. 1964;192:133–5. [PubMed] [Google Scholar]
- 8.von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, et al. DarDar Study Group Prevention of tuberculosis in Bacille Calmette-Guérin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS. 2010;24:675–85. doi: 10.1097/QAD.0b013e3283350f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalgleish AG. Therapeutic cancer vaccines: why so few randomised phase III studies reflect the initial optimism of phase II studies. Vaccine. 2011;29:8501–5. doi: 10.1016/j.vaccine.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Hale MW, Rook GA, Lowry CA. Pathways underlying afferent signaling of bronchopulmonary immune activation to the central nervous system. Chem Immunol Allergy. 2012;98:118–41. doi: 10.1159/000336505. [DOI] [PubMed] [Google Scholar]
- 11.Yang XY, Chen QF, Li YP, Wu SM. Mycobacterium vaccae as adjuvant therapy to anti-tuberculosis chemotherapy in never-treated tuberculosis patients: a meta-analysis. PLoS One. 2011;6:e23826. doi: 10.1371/journal.pone.0023826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y, Lu S, Guo S. [Immunotherapeutic effect of Mycobacterium vaccae on multi-drug resistant pulmonary tuberculosis] Zhonghua Jie He He Hu Xi Za Zhi. 2000;23:85–8. [PubMed] [Google Scholar]
- 13.de Bruyn G, Garner P. Mycobacterium vaccae immunotherapy for treating tuberculosis. Cochrane Database Syst Rev. 2003;1:CD001166. doi: 10.1002/14651858.CD001166. [DOI] [PubMed] [Google Scholar]
- 14.Mwinga A, Nunn A, Ngwira B, Chintu C, Warndorff D, Fine P, et al. LUSKAR collaboration Mycobacterium vaccae (SRL172) immunotherapy as an adjunct to standard antituberculosis treatment in HIV-infected adults with pulmonary tuberculosis: a randomised placebo-controlled trial. Lancet. 2002;360:1050–5. doi: 10.1016/S0140-6736(02)11141-X. [DOI] [PubMed] [Google Scholar]
- 15.Durban Immunotherapy Trial Group Immunotherapy with Mycobacterium vaccae in patients with newly diagnosed pulmonary tuberculosis: a randomised controlled trial. Lancet. 1999;354:116–9. doi: 10.1016/S0140-6736(98)10448-8. [DOI] [PubMed] [Google Scholar]
- 16.Dlugovitzky D, Notario R, Martinel-Lamas D, Fiorenza G, Farroni M, Bogue C, et al. Immunotherapy with oral, heat-killed, Mycobacterium vaccae in patients with moderate to advanced pulmonary tuberculosis. Immunotherapy. 2010;2:159–69. doi: 10.2217/imt.09.90. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Jin G, Ye Y, Xia X, Wang A, Zhuang Y, et al. [A clinical study on vaccine of Mycobacterium vaccae in treating pulmonary tuberculosis] Zhonghua Jie He He Hu Xi Za Zhi. 1999;22:108–10. [PubMed] [Google Scholar]
- 18.Luo Y, National Cooperation Group On Clinical Study Of Mycobacterium Vaccae Vaccine [The immunotherapeutic effect of Mycobacterium vaccae vaccine on initially treated pulmonary tuberculosis] Zhonghua Jie He He Hu Xi Za Zhi. 2001;24:43–7. [PubMed] [Google Scholar]
- 19.Johnson JL, Kamya RM, Okwera A, Loughlin AM, Nyole S, Hom DL, et al. The Uganda-Case Western Reserve University Research Collaboration Randomized controlled trial of Mycobacterium vaccae immunotherapy in non-human immunodeficiency virus-infected ugandan adults with newly diagnosed pulmonary tuberculosis. J Infect Dis. 2000;181:1304–12. doi: 10.1086/315393. [DOI] [PubMed] [Google Scholar]
- 20.Bourinbaiar AS, Mezentseva MV, Butov DA, Nyasulu PS, Efremenko YV, Jirathitikal V, et al. Immune approaches in tuberculosis therapy: a brief overview. Expert Rev Anti Infect Ther. 2012;10:381–9. doi: 10.1586/eri.12.1. [DOI] [PubMed] [Google Scholar]
- 21.Efremenko YV, Arjanova OV, Prihoda ND, Yurchenko LV, Sokolenko NI, Mospan IV, et al. Clinical validation of sublingual formulations of Immunoxel (Dzherelo) as an adjuvant immunotherapy in treatment of TB patients. Immunotherapy. 2012;4:273–82. doi: 10.2217/imt.11.176. [DOI] [PubMed] [Google Scholar]
- 22.Butov DA, Efremenko YV, Prihoda ND, Yurchenko LI, Sokolenko NI, Arjanova OV, et al. Adjunct immune therapy of first-diagnosed TB, relapsed TB, treatment-failed TB, multidrug-resistant TB and TB/HIV. Immunotherapy. 2012;4:687–95. doi: 10.2217/imt.12.59. [DOI] [PubMed] [Google Scholar]
- 23.McConkey B, Situnayake RD. Effects of rifampicin with and without isoniazid in rheumatoid arthritis. J Rheumatol. 1988;15:46–50. [PubMed] [Google Scholar]
- 24.Arjanova OV, Butov DA, Prihoda ND, Zaitzeva SI, Yurchenko LV, Sokolenko NI, et al. Jirathitikal Vichai, Bourinbaiar AS, Frolov VM, Kutsyna GA. One-month immunotherapy trial in treatment-failed TB patients. Open J Immunol. 2011;1:50–5. doi: 10.4236/oji.2011.12006. [DOI] [Google Scholar]
- 25.Arjanova OV, Butov DA, Prihoda ND, Zaitzeva SI, Yurchenko LV, Sokolenko NI, et al. Therapeutic vaccination of treatment-failed TB patients on “palliative” support consisting of isoniazid and rifampicin. Mycobact Dis. 2012:S1–001. [Google Scholar]
- 26.Yang XY, Chen QF, Cui XH, Yu Y, Li YP. Mycobacterium vaccae vaccine to prevent tuberculosis in high risk people: a meta-analysis. J Infect. 2010;60:320–30. doi: 10.1016/j.jinf.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 27.NIH Policy for Data and Safety Monitoring NIH Guide Grants Contracts 1998; 12; http://grants.nih.gov/grants/guide/notice-files/not98-084.htm [Google Scholar]


