Abstract
Intanza®/IDflu® (Sanofi Pasteur, Lyon, France) is an intradermal inactivated trivalent influenza vaccine developed as an alternative to intramuscular influenza vaccine. The objective of this study was to confirm the immunogenicity and safety of Intanza/IDflu in South Korean adults. In a phase IV multicenter trial, South Korean adults 18–59 y old (n = 120) and ≥ 60 y old (n = 120) were randomized 1:1 to receive a single dose of Intanza/IDflu (9 µg for 18–59 y, 15 µg for ≥ 60 y) or trivalent intramuscular vaccine (Vaxigrip® 15 µg, Sanofi Pasteur, Lyon, France). Blood was collected on pre-vaccination (day 0) and on day 21. Hemagglutination inhibition titers, seroprotection rates and seroconversion rates were determined on day 21. Geometric mean titers, seroprotection and seroconversion rates were similar between the intradermal and intramuscular vaccines in both age groups for all three vaccine strains (A/H1N1, A/H3N2 and B). Both vaccines met Committee for Medicinal Products for Human Use criteria for all three strains. Solicited systemic reactions of the intradermal groups were generally mild, transient, and similar to those of the intramuscular groups. Solicited injection site reactions were more frequent in the intradermal groups but were mostly mild, transient, and consisted mainly of pain, erythema, and pruritus. No treatment-related serious adverse events or other safety concerns were reported. These results confirm that Intanza/IDflu is an effective and well-tolerated alternative to IM influenza vaccination. (Clinicaltrials.gov NCT ID: NCT01215669)
Keywords: Influenza vaccine, intradermal, intramuscular, immunogenicity, safety
Introduction
Seasonal influenza causes up to 500,000 deaths per year worldwide in non-pandemic periods.1 Annual vaccination against influenza virus is the primary strategy for preventing these seasonal infections and their related severe complications. Pregnant women, elderly adults, young children, and patients with chronic medical conditions are at high risk for severe complications of influenza such as pneumonia, hospitalization, and death, and the World Health Organization (WHO) recommends seasonal influenza vaccination for all these high-risk groups.2-4 Nevertheless, global influenza vaccination coverage rates remain below WHO target levels for many of these populations.5 In addition, many older people do not respond well to influenza vaccines because their aging immune systems are prone to immunosenescence. In a meta-analysis of antibody responses to influenza vaccines, seroprotection rates in the elderly were found to be 50% to 75% lower than those of younger adults.6 Thus, elderly adults have an urgent medical need for alternative influenza vaccines or vaccination strategies able to induce antibody responses beyond those of standard vaccines.
Although influenza vaccines have been traditionally administered by intramuscular (IM) vaccination, the ID route is now known to be an effective alternative route of vaccination.7-9 Skin is a major immunocompetent organ and is easily accessible, making it an ideal site for vaccination.10 The dermis is particularly rich in dendritic cells, which can efficiently generate innate and adaptive immune responses.11 The density of lymphatic vessels and blood capillaries in the dermis is also very high, which favors rapid cellular and fluid exchanges.12 In addition, ID vaccination has been shown to be effective for influenza, rabies, and hepatitis B virus vaccines.13-16
Intanza®/IDflu® (Sanofi Pasteur, Lyon, France) is an ID trivalent, inactivated influenza vaccine delivered using the Soluvia™ microinjection system.17,18 It has been available in Europe since 2009 as a 9 µg formulation for adults 18−59 y of age and as a 15 µg formulation for adults ≥ 60 y of age.17,19 In a phase III clinical study, the immunogenicity of 9 µg Intanza in 18–60 y-old subjects was non-inferior to that of a 15 µg intramuscular (IM) influenza vaccine (Vaxigrip®).7 Also, in a phase III clinical study in elderly adults (≥ 60 y-old), the immunogenicity of the higher dose of Intanza (15 µg) was superior to that of the 15 µg IM influenza vaccine.8
Intanza/IDflu has not been previously evaluated in South Korea or Asia. Here, we report the results of a phase IV study performed as a post-registration commitment to confirm the immunogenicity and safety of two strengths of Intanza/IDflu in South Korean adults.
Results
Demographic and baseline characteristics
This descriptive study included 120 subjects 18−59 y of age and 120 subjects ≥ 60 y of age. In each age group, equal numbers of subjects (n = 60) were vaccinated with the ID vaccine or the IM vaccine. Within each age group, the mean ages, sex ratios, and vaccination histories of the two vaccination groups were similar, although the 18−59-y-old subjects who received the ID vaccine included the fewest subjects who had received a previous influenza vaccination (Table 1). All subjects completed the study.
Table 1. Baseline characteristics of study participants.
| 18−59 y | ≥ 60 y | |||
|---|---|---|---|---|
| 9 µg ID (n = 60) |
15 µg IM (n = 60) |
15 µg ID (n = 60) |
15 µg IM (n = 60) |
|
| Mean age, years ± SD | 33.0 ± 9.2 | 34.5 ± 10.2 | 64.9 ± 3.6 | 64.5 ± 3.8 |
| Sex, male (%) | 20 (33.3) | 18 (30.0) | 25 (41.7) | 21 (35.0) |
| Previously vaccinated for seasonal influenza (%) |
13 (21.7) | 23 (38.3) | 27 (45.0) | 26 (43.3) |
| Received 2009/2010 seasonal influenza vaccine (%) |
11 (13.3) | 21 (35.0) | 23 (38.3) | 23 (38.3) |
Values are numbers of subjects (percent). ID, intradermal vaccine; IM, intramuscular vaccine; SD, standard deviation.
Immunogenicity
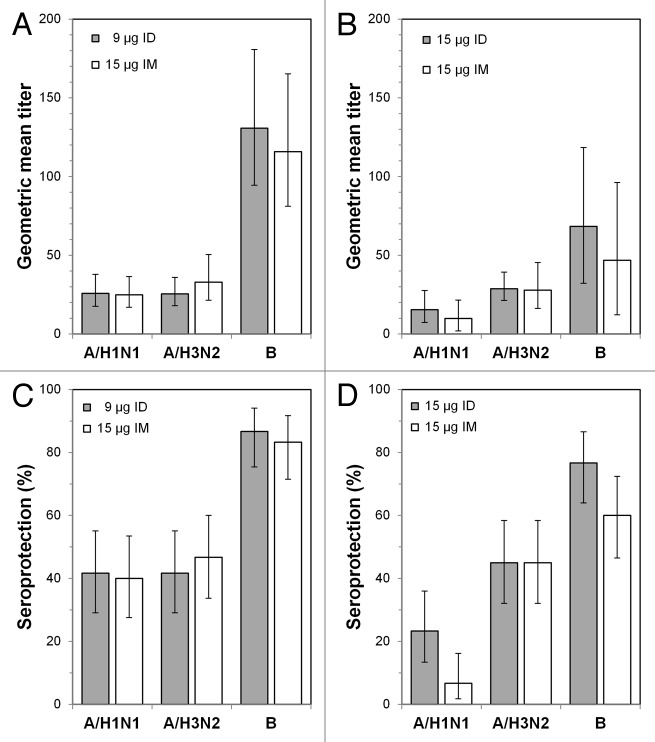
Prior to vaccination, geometric mean titers (GMTs) of antibodies against the two A strains were between 24.9 and 32.9, and seroprotection rates were approximately 40% for the two 18−59-y-old subject groups (Fig. 1A and C). For the B strain, GMTs were 130.7 (ID) and 115.8 (IM), and seroprotection rates were approximately 85%. Pre-vaccination GMTs and seroprotection rates were similar for these two treatment groups despite the presence of fewer subjects with a history of previous influenza vaccination in the ID vaccine group (Table 1). Pre-vaccination GMTs for the two A strains were between 9.9 and 28.8, and seroprotection rates were below 25% for the ≥ 60-y-old subject groups (Fig. 1B and D). For the B strain, GMTs were 68.4 (ID) and 46.8 (IM), and seroprotection rates were ≥ 60%.
Figure 1. Prevaccination geometric mean titers and seroprotection rates. (A) Geometric mean titers (GMTs) for the 18−59 y age groups at day 0. (B) GMTs for the ≥ 60 y age groups at day 0. (C) Seroprotection rates for the 18−59 y age groups at day 0. (D) Seroprotection rates for the ≥ 60 y age groups at day 0. ID: intradermal vaccine; IM: intramuscular vaccine.
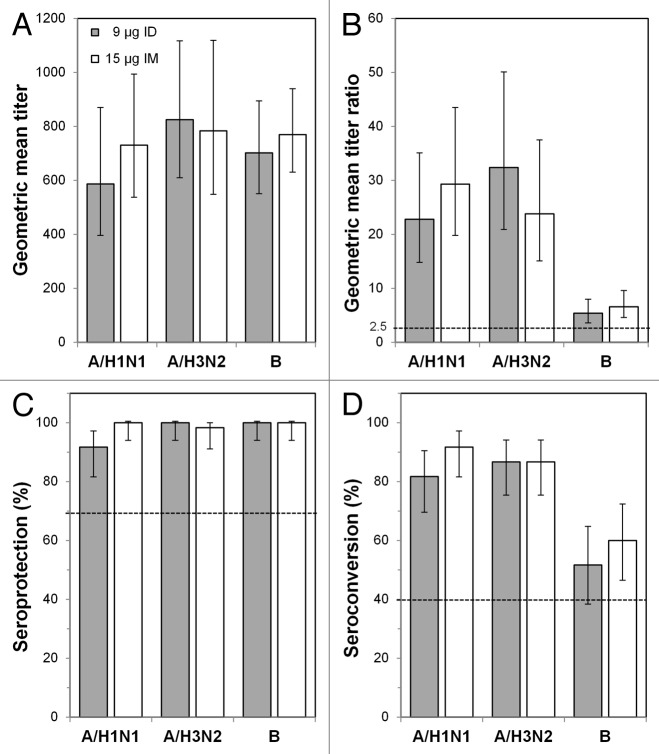
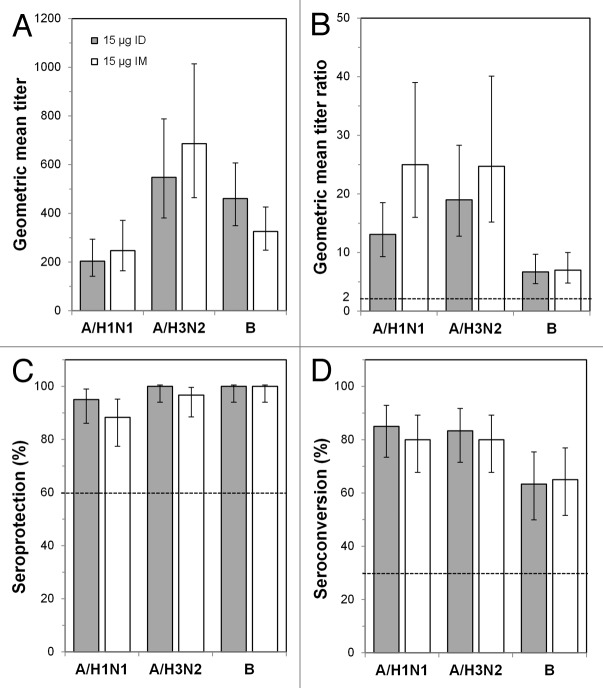
By post-vaccination day 21, GMTs against the A/H1N1 and A/H3N2 strains increased between 20- and 35-fold in subjects 18−59 y of age (Fig. 2A and B) and between 13- and 25-fold in subjects ≥ 60 y of age (Fig. 3A and B). GMT increases against the B strain were lower for all groups: 5.4-fold (ID) and 6.6-fold (IM) in subjects 18−59 y of age and 6.7-fold (ID) and 7.0-fold (IM) in subjects ≥ 60 y of age. Although GMTs for the A/H1N1 and A/H3N2 strains in subjects ≥ 60 y of age were slightly higher with the IM vaccine than with the ID vaccine, GMTs and day 21/day 0 GMT ratios in both age groups were not significantly different between subjects receiving the ID or IM vaccine for any strain. GMT ratios for all vaccines and all strains were above the Committee for Medicinal Products for Human Use (CHMP) requirements (Figs. 2B and 3B). Seroprotection was nearly 100% in both age groups for all three strains (Figs. 2C and 3C). The seroconversion rates in both age groups were between 80% and 95% for the H1N1 and H3N2 strains for both the ID and IM vaccines and between 50% and 70% for the B strain (Figs. 2D and 3D). Thus, the ID and IM vaccines met all CHMP criteria for all three strains in both age groups. The lower limits of the 95% confidence intervals (CIs) of the immunogenicity measures also met CHMP thresholds in all cases except for seroconversion for the B strain in subjects 18–59 y of age receiving the ID vaccine.
Figure 2. Immunogenicity outcomes after intradermal (ID) or intramuscular (IM) influenza vaccination in the 18−59 y age group. Subjects were vaccinated with 9 μg ID vaccine or with 15 μg IM vaccine, and immunogenicity characteristics were determined 21 d later. (A) Geometric mean titers (GMTs) at day 21. (B) GMT ratios (GMTRs), which were the geometric means of the day 21 hemagglutinin inhibition (HI) titer divided by the pre-vaccination (day 0) HI titer for each subject. (C) Seroprotection rates. Error bars indicate the 95% confidence intervals, and the horizontal bars indicate the Committee for Medicinal Products for Human Use criteria for adults 18–59 y old.
Figure 3. Immunogenicity outcomes after intradermal (ID) or intramuscular (IM) influenza vaccination in the ≥ 60 y age group. Subjects were vaccinated with 15 μg ID vaccine or with 15 μg IM vaccine, and immunogenicity characteristics were determined 21 d later. (A) Geometric mean titers (GMTs) at day 21. (B) GMT ratios (GMTRs), which were the geometric means of the day 21 hemagglutinin inhibition (HI) titer divided by the pre-vaccination (day 0) HI titer for each subject. (C) Seroprotection rates. (D) Seroconversion rates. Error bars indicate the 95% confidence intervals, and the horizontal bars indicate the Committee for Medicinal Products for Human Use criteria for adults ≥ 60 y old.
Safety
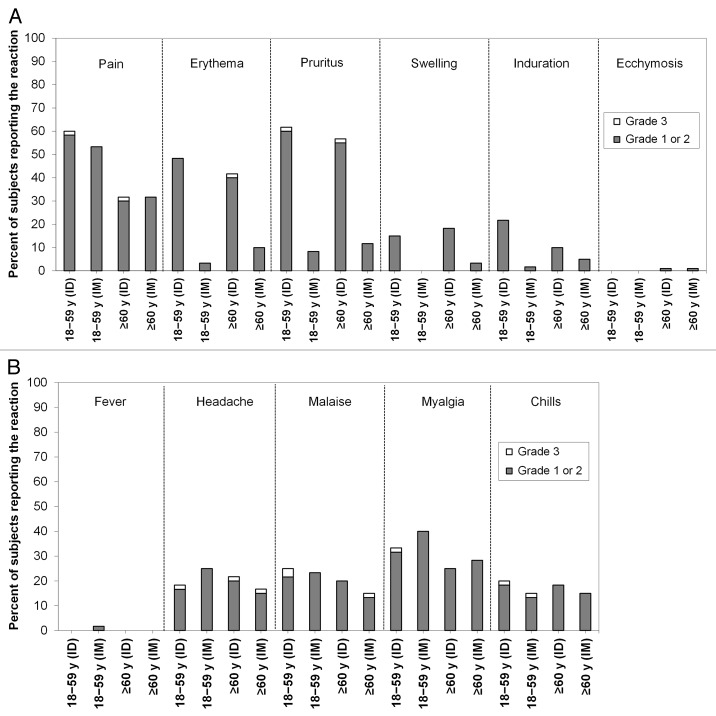
In both age groups, the frequencies of solicited injection site reactions except ecchymosis were higher in the ID groups than in the IM groups (Fig. 4A). The most common injection site reaction was pain, followed by pruritus and erythema. Most solicited injection site reactions resolved without treatment within 3 d and were of mild (grade 1) intensity, and grade 3 reactions were rare. The frequencies of solicited systemic reactions in both age groups were similar between subjects receiving the ID and IM vaccines (Fig. 4B). Most solicited systemic reactions were mild in intensity and resolved without treatment within 3 d. Severe (grade 3) solicited systemic reactions were infrequent and occurred in ≤ 3.3% of the subjects in all treatment groups. Frequencies of unsolicited AEs and CHMP-defined clinical reactions were also similar between the ID and IM groups (Table 2 and Table 3). Only two to four subjects in each group (3.3% to 6.7%) experienced an unsolicited AE considered treatment-related, all of which were mild or moderate in intensity. No immediate unsolicited adverse events (AEs), no serious adverse events (SAEs), no AEs leading to study discontinuation, and no deaths were reported.
Figure 4. Solicited injection site and systemic reaction profiles. (A) Solicited injection site reactions. (B) Solicited systemic reactions. Data are the percentages of subjects within each group who experienced a grade 1 or 2 reaction or a grade 3 reaction through post-vaccination day 7. ID: intradermal vaccine; IM: intramuscular vaccine.
Table 2. Unsolicited adverse events.
| 18−59 y | ≥ 60 y | |||
|---|---|---|---|---|
| 9 µg ID (n = 60) |
15 µg IM (n = 60) |
15 µg ID (n = 60) |
15 µg IM (n = 60) |
|
| All unsolicited AEs (within 21 d) | 16 (26.7) | 15 (25.0) | 14 (23.3) | 15 (25.0) |
| Grade 3 | 4 (6.7) | 2 (3.3) | 1 (1.7) | 1 (1.7) |
| Treatment-related | 4 (6.7) | 2 (3.3) | 2 (3.3) | 2 (3.3) |
| Grade 3 treatment-related | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| SAEs | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Values are numbers of subjects experiencing at least one reaction, with percent in brackets. AE, adverse event; ID, intradermal vaccine; IM, intramuscular vaccine; SAE, serious adverse event.
Table 3. Committee for Medicinal Products for Human Use-defined solicited reactions.
| 18–59 y | ≥ 60 y | |||
|---|---|---|---|---|
| 9 µg ID (n = 60) |
15 µg IM (n = 60) |
15 µg ID (n = 60) |
15 µg IM (n = 60) |
|
| Injection site induration ≥ 50 mm for ≥ 3 d | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Injection site ecchymosis > 0 mm | 2 (3.3) | 2 (3.3) | 1a (1.7) | 2a (3.3) |
| Pyrexia (> 38°C) for ≥ 1 d | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Malaise | 14 (23.3) | 14 (23.3) | 12 (20.0) | 8 (13.3) |
| Shivering | 11 (18.3) | 7 (11.7) | 11 (18.3) | 8 (13.3) |
Except for injection site induration, all were reactions occurring within 3 d of vaccination. Values are numbers of subjects experiencing at least one reaction, with percent in brackets. ID, intradermal vaccine; IM, intramuscular vaccine. a One of these subjects reported grade 1 ecchymosis (≥ 2.5 mm).
Discussion
ID vaccination with Intanza/IDflu was developed as an alternative to IM vaccination and was approved in 2009 for the prevention of seasonal influenza in adults. This phase IV study, performed in Korean adults, confirmed that Intanza/IDflu was well tolerated and met CHMP criteria for all three vaccine strains. The study also showed that Intanza/IDflu was at least as immunogenic as the IM vaccine for all three vaccine strains.
The lower limits of the 95% CIs for the GMT ratios, seroconversion rates, and seroprotection rates also met the CHMP criteria in all cases except for the seroconversion rate against strain B in adults 18−59 y of age who received the ID vaccine. In that case, the lower limit of the 95% CI was slightly below the CHMP-recommended threshold of 40%. This was likely due to the high pre-vaccination titers against strain B. Indeed, the pre-vaccination seroprotection rates against strain B in all groups were at or well above CHMP criteria. These high pre-vaccination titers might have been due to the fact that the 2009/2010 seasonal influenza vaccine contained the same B strain as the 2010/2011 vaccines used in this study. Despite this high pre-existing immunity to the B strain, ID vaccination substantially increased the seroprotection rates in both age groups. Although the subjects sometimes had substantial pre-existing immunity to the A/H1N1 and A/H3N2 strains, all groups also attained similarly high seroprotection rates against both A-type strains.
In all cases, post-vaccination GMTs in the subjects ≥ 60 y of age were lower than those in the 18−59-y-old subjects. Although the study was not designed to compare vaccine immunogenicity between age groups or between vaccination routes, this trend is likely due to immunosenescence in the older subject group, and further suggests that elderly adults need vaccines that are more highly immunogenic standard vaccines. Possible methods of increasing immunogenicity are to use vaccines containing adjuvants20 or higher doses of antigen21 or to administer vaccines by the ID route.9 This study was not designed to assess the immunogenic superiority of ID vaccination but other larger studies in elderly adults ≥ 65 y of age have shown ID vaccination to be superior to IM vaccination for equivalent amounts of antigen16 and comparable to IM vaccination with an adjuvanted vaccine.22
As reported previously, injection site reactions were more common with ID vaccination than with IM vaccination, and the most common of these were pruritus, pain and erythema.23,24 Also, as previously reported, most solicited reactions were mild and resolved without treatment within 3 d. Most importantly, the ID vaccine was well-tolerated as indicated by a lack of SAEs, severe treatment-related AEs, and dropouts due to AEs. This finding is consistent with those of other studies that also found ID Intanza/IDflu vaccination to be well-tolerated.25-28
Although this was a relatively small study, we were able to confirm that the vaccine met all CHMP criteria. However, due to the limited number of subjects in the study, we did not compare age or treatment groups nor perform any multivariate analyses or analyses according to stratified subgroups. Thus, it is possible that uncontrolled differences between treatment groups may have been present and may have influenced our findings. Nevertheless, differences in previous influenza vaccination history did not appear to have factored in these results as pre-vaccination GMTs and seroprotection rates for the 18−59 y ID and IM vaccine groups were very similar. As with other studies comparing ID and IM vaccination, it was not possible to blind the subjects or investigators, which might have influenced the reporting of subjective reactions or AEs, such as injection site pain or myalgia. Despite these limitations, our results agreed with those of other several clinical studies on Intanza/IDflu showing that Intanza/IDflu meets CHMP criteria and is well-tolerated in both non-elderly and elderly adults.7,8,23,24 With the added convenience of the microinjection system and the high level of acceptance by vaccinees and health care providers,25 Intanza/IDflu should be an effective alternative to IM influenza vaccination and might help improve influenza vaccination coverage rates.
Patients and Methods
Study design
This was a descriptive phase IV open-label randomized multicenter controlled trial performed as a Korean Ministry of Food and Drug Safety post-registration commitment to confirm the immunogenicity and safety of ID vaccination with Intanza/IDflu in Korean adults (ClinicalTrials.gov NCT ID: NCT01215669). The primary objectives were to determine whether the ID vaccines meet CHMP criteria for immunogenicity and to describe the safety of the vaccines.29 The study was performed in six university-affiliated hospitals in South Korea between October 2010 and February 2011. Subjects were vaccinated on day 0 (visit 1) and blood samples collected on day 0 and on post-vaccination day 21 (visit 2). All participating hospitals received approval for this study from their local institutional review boards. The study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Manufacturing Practice and Good Clinical Practice. All subjects provided written informed consent for participation in the trial.
Subjects
Equal numbers of subjects 18−59 and ≥ 60 y of age were enrolled. Women of childbearing potential had to be using an effective method of contraception or to abstain from sexual activity for the four weeks prior to vaccination through the four weeks after vaccination. Individuals were excluded from the study for the following reasons: systemic hypersensitivity to eggs, chicken proteins, or any of the vaccine components; a history of a life-threatening reaction to any vaccine used in the trial or to a vaccine containing any of the same substances; pregnant or had a positive urine pregnancy test; currently breastfeeding a child; received either a seasonal or pandemic influenza vaccine within the previous 12 mo or any other vaccine within the four weeks preceding the trial; an underlying chronic disease including end-stage renal disease requiring dialysis, chronic liver disease, active neoplastic disease or hematologic malignancy, received immunosuppressive therapy within the preceding 6 mo; received long-term systemic corticosteroid therapy; a contraindication for IM or other vaccination, including thrombocytopenia or anticoagulant therapy; seropositive for human immunodeficiency virus, hepatitis B or hepatitis C; received blood or blood-derived products that might interfere with assessment of the immune response within the previous 3 mo.
Treatments
All subjects in each age group were randomized 1:1 to receive a single dose of either ID influenza vaccine (Intanza/IDflu) or IM influenza vaccine (Vaxigrip®, Sanofi Pasteur, Lyon, France). Both are inactivated split-virion influenza vaccines. In compliance with the WHO recommendations for the Northern Hemisphere 2010−2011 formulation, both included the A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008 strains. All vaccines were administered into the deltoid region of the arm. The IM vaccine contained 15 µg of hemagglutinin per strain (30 µg/ml; 0.5 ml per injection) and was delivered using a pre-filled standard syringe. The ID vaccine contained 9 µg per strain (90 µg/ml; 0.1 ml per injection) for subjects 18−59 y of age and 15 µg per strain (150 µg/ml; 0.1 ml per injection) for subjects ≥ 60 y of age and was delivered using a pre-filled Soluvia™ microinjection device (BD, Franklin Lakes, New Jersey, USA).9
Immunogenicity assessments
Hemagglutination inhibition (HI) titers were measured as described previously in duplicate using blood samples collected on pre-vaccination day 0 and on post-vaccination day 21 from the arm opposite the vaccination.22 The serum HI titer was defined as the highest dilution factor (e.g., 1:10) that completely inhibited hemagglutination. The value for each subject was the geometric mean of the duplicate titers. Seroconversion at day 21 was defined as either a day 0 HI titer < 1:10 and a day 21 HI titer ≥ 1:40 or a day 0 HI titer ≥ 1:10 and a day 21 HI titer that was ≥ 4-fold higher than the day 0 HI titer. Seroprotection was defined as a day 21 HI titer ≥ 1:40. The geometric mean titer ratio for each subject was the day 21 HI titer divided by the day 0 HI titer.
Safety assessments
Solicited systemic reactions (fever, headache, malaise, myalgia, and shivering) and injection site reactions (pain, erythema, swelling, induration, ecchymosis, and pruritus) were recorded for seven days following vaccination (i.e., days 0−7). Subjects were provided with a diary card, digital thermometer, and flexible centimeter ruler, and were instructed how to measure and record all solicited injection site and systemic reactions, including any treatment action taken, on the dairy card. On days 8−10, subjects received a telephone call from a study site member to review and collect safety information. Unsolicited AEs were also recorded by subjects on the diary cards for 21 d following vaccination. At the day 21 visit, the study site investigator or an authorized designee interviewed the subject to collect information for any solicited reactions and unsolicited AEs recorded on the diary card and attempted to clarify anything that was incomplete or unclear. Erythema, swelling, induration and ecchymosis were considered to be grade 1 if they were ≥ 25 and ≤ 50 mm in diameter, grade 2 if they were ≥ 51 and ≤ 100 mm and grade 3 if they were > 100 mm.30 Fever was considered to be grade 1 if ≥ 38.0°C and ≤ 38.4°C, grade 2 if ≥ 38.5°C and ≤ 38.9°C, and grade 3 if ≥ 39°C. AEs and all other reactions were considered to be grade 1 if they did not interfere with daily activities, grade 2 if there was some interference with activities, and grade 3 if they prevented daily activity. AEs and SAEs were defined according to the International Conference on Harmonization Guideline for Clinical Safety Data Management.
Statistical analysis
The sample size of the study was based on the CHMP recommendation that at least 50 subjects are included per age group to assess the immunogenicity and safety of influenza vaccines. Given the risk of losses to follow-up and protocol deviations, 60 subjects were planned for each group. Means and 95% CIs were first calculated from the log10 transformations of the measurements using standard methods and assuming a normal distribution of the log10 values. Antilog transformations were applied to the results of these calculations to determine the geometric means and their 95% CIs. The Clopper-Pearson method was used to calculate the 95% CIs.31 As this was a small, descriptive study designed to evaluate only whether these vaccines comply with CHMP guidelines, the study was not sufficiently powered to allow any statistically valid multivariate analyses or comparisons to be made between treatment groups or subgroups.
Acknowledgments
The authors thank Drs. Kurt Liittschwager and Phillip Leventhal (4Clinics, Paris, France) for medical writing support.
Glossary
Abbreviations:
- AE
adverse event
- CHMP
Committee for Medicinal Products for Human Use
- CI
confidence interval
- GMT
geometric mean titer
- HI
hemagglutination
- ID
intradermal, IM, intramuscular
- SAE
serious adverse event
- WHO
World Health Organization
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25295
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;87:461–76. [PubMed] [Google Scholar]
- 3.World Health Organization. World Health Organization 56th World Health Assembly. Prevention and control of influenza pandemics and annual epidemics. Geneva, 2003. WHA56.19. [Google Scholar]
- 4.Monto AS. Preventing influenza in healthy adults: the evolving story. JAMA. 2000;284:1699–701. doi: 10.1001/jama.284.13.1699. [DOI] [PubMed] [Google Scholar]
- 5.Monto AS. Seasonal influenza and vaccination coverage. Vaccine. 2010;28(Suppl 4):D33–44. doi: 10.1016/j.vaccine.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 7.Arnou R, Eavis P, Pardo JR, Ambrozaitis A, Kazek MP, Weber F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18-60 years: Randomized, controlled, phase III trial. Hum Vaccin. 2010;6:346–54. doi: 10.4161/hv.6.4.10961. [DOI] [PubMed] [Google Scholar]
- 8.Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–12. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 9.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–8. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 10.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–22. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–83. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Schröder JM, Reich K, Kabashima K, Liu FT, Romani N, Metz M, et al. Who is really in control of skin immunity under physiological circumstances - lymphocytes, dendritic cells or keratinocytes? Exp Dermatol. 2006;15:913–29. doi: 10.1111/j.1600-0625.2006.00506_1.x. [DOI] [PubMed] [Google Scholar]
- 13.Coleman PJ, Shaw FE, Jr., Serovich J, Hadler SC, Margolis HS. Intradermal hepatitis B vaccination in a large hospital employee population. Vaccine. 1991;9:723–7. doi: 10.1016/0264-410X(91)90287-G. [DOI] [PubMed] [Google Scholar]
- 14.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 15.Lang J, Hoa DQ, Gioi NV, Vien NC, Nguyen CV, Rouyrre N, et al. Immunogenicity and safety of low-dose intradermal rabies vaccination given during an Expanded Programme on immunization session in Viet Nam: results of a comparative randomized trial. Trans R Soc Trop Med Hyg. 1999;93:208–13. doi: 10.1016/S0035-9203(99)90309-7. [DOI] [PubMed] [Google Scholar]
- 16.Micozkadioglu H, Zumrutdal A, Torun D, Sezer S, Ozdemir FN, Haberal M. Low dose intradermal vaccination is superior to high dose intramuscular vaccination for hepatitis B in unresponsive hemodialysis patients. Ren Fail. 2007;29:285–8. doi: 10.1080/08860220601166263. [DOI] [PubMed] [Google Scholar]
- 17.Beran J, Ambrozaitis A, Laiskonis A, Mickuviene N, Bacart P, Calozet Y, et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009;7:13. doi: 10.1186/1741-7015-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25:8833–42. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Atmar RL, Patel SM, Keitel WA. Intanza(®): a new intradermal vaccine for seasonal influenza. Expert Rev Vaccines. 2010;9:1399–409. doi: 10.1586/erv.10.134. [DOI] [PubMed] [Google Scholar]
- 20.Van Damme P, Arnou R, Kafeja F, Fiquet A, Richard P, Thomas S, et al. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. BMC Infect Dis. 2010;10:134. doi: 10.1186/1471-2334-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–80. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 22.Zakay-Rones Z. Human influenza vaccines and assessment of immunogenicity. Expert Rev Vaccines. 2010;9:1423–39. doi: 10.1586/erv.10.144. [DOI] [PubMed] [Google Scholar]
- 23.Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine. 2007;25:659–63. doi: 10.1016/j.vaccine.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–94. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 25.Arnou R, Frank M, Hagel T, Prébet A. Willingness to vaccinate or get vaccinated with an intradermal seasonal influenza vaccine: a survey of general practitioners and the general public in France and Germany. Adv Ther. 2011;28:555–65. doi: 10.1007/s12325-011-0035-z. [DOI] [PubMed] [Google Scholar]
- 26.Leroux-Roels I, Vets E, Freese R, Seiberling M, Weber F, Salamand C, et al. Seasonal influenza vaccine delivered by intradermal microinjection: A randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008;26:6614–9. doi: 10.1016/j.vaccine.2008.09.078. [DOI] [PubMed] [Google Scholar]
- 27.Reygrobellet C, Viala-Danten M, Meunier J, Weber F, Nguyen VH. Perception and acceptance of intradermal influenza vaccination: Patient reported outcomes from phase 3 clinical trials. Hum Vaccin. 2010;6:336–45. doi: 10.4161/hv.6.4.10753. [DOI] [PubMed] [Google Scholar]
- 28.Duggan ST, Plosker GL. Intanza 15 microg intradermal seasonal influenza vaccine: in older adults (aged >or=60 years) Drugs Aging. 2010;27:597–605. doi: 10.2165/11203880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.European Medicines Agency (EMA) Committee for Medicinal Products for Human Use. Note for guidance on harmonisation of requirements for influenza vaccines. 1997. CPMP/BWP/214/96.
- 30.Center for Biologics Evaluation and Research. U.S. Food and Drug Administration. Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. 2007. [Google Scholar]
- 31.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–72. doi: 10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]