Abstract
In vivo electroporation (EP) has been shown to be a highly efficient non-viral method for enhancing DNA vaccine delivery and immunogenicity, when the site of immunization is the skin or muscle of animals and humans. However, the route of entry for many microbial pathogens is via the mucosal surfaces of the human body. We have previously reported on minimally invasive, surface and contactless EP devices for enhanced DNA delivery to dermal tissue. Robust antibody responses were induced following vaccine delivery in several tested animal models using these devices. Here, we investigated extending the modality of the surface device to efficiently deliver DNA vaccines to mucosal tissue. Initially, we demonstrated reporter gene expression in the epithelial layer of buccal mucosa in a guinea pig model. There was minimal tissue damage in guinea pig mucosal tissue resulting from EP. Delivery of a DNA vaccine encoding influenza virus nucleoprotein (NP) of influenza H1N1 elicited robust and sustained systemic IgG antibody responses following EP-enhanced delivery in the mucosa. Upon further analysis, IgA antibody responses were detected in vaginal washes and sustained cellular immune responses were detected in animals immunized at the oral mucosa with the surface EP device. This data confirms that DNA delivery and EP targeting mucosal tissue directly results in both robust and sustainable humoral as well as cellular immune responses without tissue damage. These responses are seen both in the mucosa and systemically in the blood. Direct DNA vaccine delivery enhanced by EP in mucosa may have important clinical applications for delivery of prophylactic and therapeutic DNA vaccines against diseases such as HIV, HPV and pneumonia that enter at mucosal sites and require both cellular and humoral immune responses for protection.
Keywords: direct mucosal, intradermal, DNA vaccine, electroporation
Introduction
The route of entry for many microbial pathogens, such as influenza, HIV, and the bacteria causing pneumonia, is via the mucosal surfaces of the human body. As such, there is a growing interest in developing mucosal-targeted vaccines that can elicit functional, long-lived mucosal immune responses, providing a frontline defense and thus effectively preventing systemic infections. A possible advantage of direct mucosal delivery might be the induction of tissue relevant cellular and humoral immune responses, and more effective generation of immunity against specific disease targets invading the mucosa.1 This prompted us to investigate the possibility of developing a novel methodology to facilitate DNA delivery to mucosal tissue resulting in high transfection rates and robust immune responses.
Due to their ability to generate both humoral and cellular responses, DNA vaccines are predicted to play a major role in future therapeutic and prophylactic immunization schedules for a variety of diseases which currently have no available vaccine, most notably HIV.2,3 However, the delivery of naked DNA through a standard intramuscular (IM) injection is notoriously inefficient outside of rodent models, and vaccination with naked DNA in large mammals and humans has often failed to achieve robust immune responses.3,4 Therefore, an efficacious way to deliver these vaccines to the appropriate target tissue will be an absolute requirement for clinical success. Novel devices and strategies have been used to aid in DNA delivery, such as electroporation, ballistic devices and viral vectors.2 DNA vaccination in combination with in vivo electroporation has been shown to quantitatively enhance immune responses, increasing the breadth of those immune responses as well as improving the efficiency of dose.5 Electroporation assists in the delivery of plasmid DNA by generating an electrical field at the site of immunization that allows the DNA to passage into the cell more efficiently.6-8 In addition, it also causes a transient inflammatory milieu that has an adjuvant effect In addition to recruiting cells involved in antigen presentation, EP provides adjuvant-like properties through moderate tissue injury and generation of a pro-inflammatory context with cytokine release that enhances the immune response.9,10 Protocols involving skin and muscle electroporation to aid in the delivery of DNA vaccines have been extensively described in pre-clinical and clinical trials.11-13
Several studies have addressed the effect of inducing mucosal immunity through DNA delivery to muscle enhanced by EP.14 However, DNA vaccine studies describing the delivery of DNA vaccines directly at the mucosa in the presence of electroporation are scarce. A previous study by Kanazawa and colleagues indicated that effective DNA vaccination administered through the vaginal tract by electroporation was possible, but that the menstrual stage of the mice was critical to the success of the EP procedure.15 Other studies in which DNA vaccination alone at the mucosa was performed reported only moderate efficacy.16
In this study we chose to target the buccal mucosa in the oral cavity of the guinea pig, rabbit and mouse. This region was chosen based on the accessibility and availability of tissue. The buccal mucosa refers to the inside lining of the cheeks which is a non-keratinized stratified squamous epithelium. Other examples of stratified squamous epithelium are the outermost layer of the skin, esophagus, anus and vagina. This type of epithelia is highly suited to areas of the body prone to abrasion as the upper layers of the tissue can be sequentially sloughed off and replaced. In this study, the EP was performed using a modified minimally invasive surface device to deliver the DNA vaccine in an efficient manner.17 This surface EP (SEP) device operates under low voltage conditions and we believe represents a highly tolerable device for delivery to the skin. Other examples of dermal devices which operate in a non- or minimally invasive (MID) fashion are the multi-electrode array,18,19 calipers,20 plate electrodes21 and meander electrodes.22 Since the SEP device and other low voltage MID devices generate shallow electric fields, they do not activate deep nerves or skeletal muscle, which we believe will translate into a more tolerable experience for patients. Diehl et al.23 compared the tolerability of a MID skin EP device to an intramuscular (IM) platform in healthy volunteers. Mean visual analog pain scores (VAS) did not exceed 7 out of 10 for the IM platform and 3 out of 10 for the ID device.
We report that direct mucosa EP is well tolerated, and using our treatment parameters results in minimal tissue damage. Most importantly, immune responses generated following mucosal DNA vaccine delivery in the presence of EP were shown to significantly improve over mucosal delivery alone. These results suggest that direct DNA vaccine delivery enhanced by EP in the mucosa is feasible and may confer benefit over immunization in the periphery for generation of mucosal targeted immune responses.
Results
Prototype development of a novel concept mucosal EP device
In a bid to enhance delivery to the immunologically important mucosal tissue, we adapted our minimally invasive surface EP device17 by lengthening the electrodes and altering the electrode numbers. The skin EP device had electrode lengths of 3 mm. The length was adjusted to 8 mm for the mucosal device (Fig. 1A). This adaptation allowed improved access to the mucosal cavity. While the 4 × 4-electrode array was suitable for use in the buccal cavity of larger animals (rabbits and guinea pigs), the array size (approximately 5mm2) proved too cumbersome for mouse work. To address this issue, a smaller 2 × 2-electrode array was manufactured (Fig. 1B). This provided a suitably sized array for use in the oral cavity of mice. Both these prototype devices were built with an attachment cord for linkage to the ELGEN 1000 pulse generator (Fig. 1C).24 A photographic depiction of the injection and EP procedure in rabbit buccal mucosa is shown in Figure 1D.
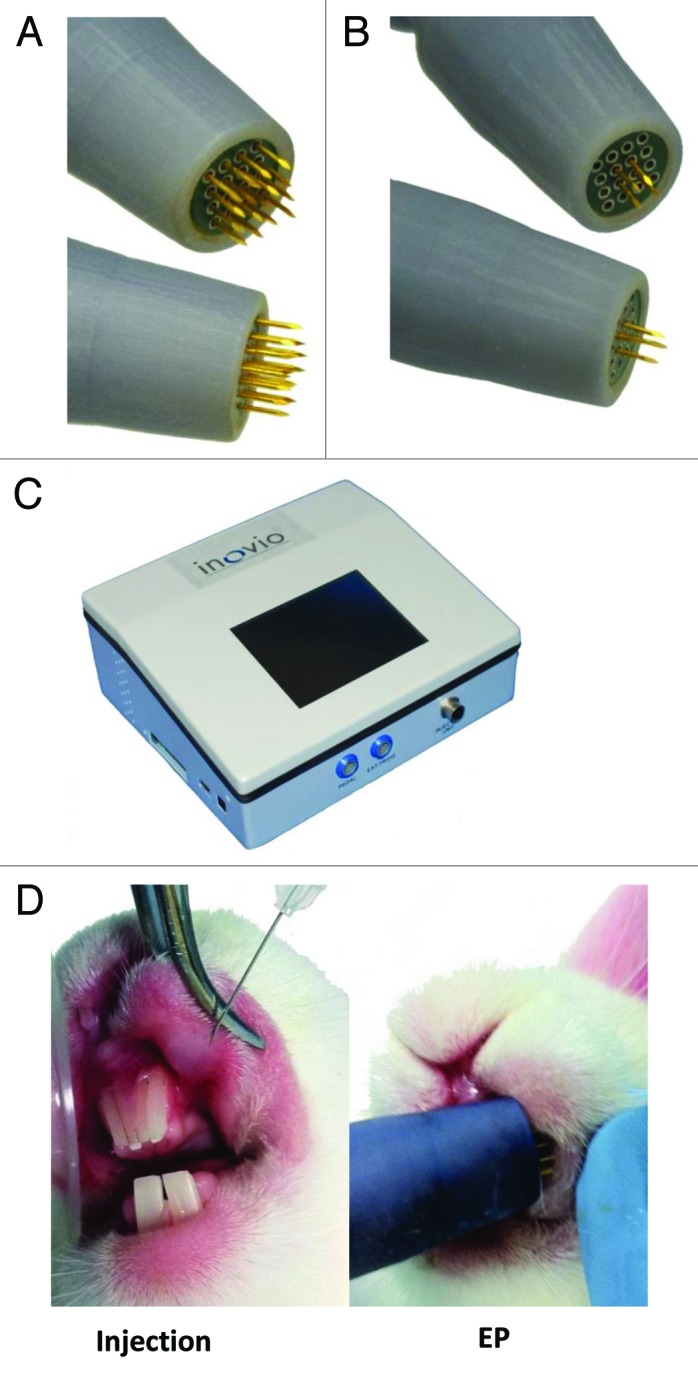
Figure 1. Photographic illustration of the devices and techniques involved in mucosal electroporation. Minimally invasive surface EP array modified to incorporate lengthened electrodes for EP of the rabbit and guinea pig buccal mucosa (A), and a smaller 2 x 2 electrode pattern for use in the oral cavity of mice (B). The ELGEN 1000 pulse generator (b). Plasmid injection and EP procedure at the rabbit buccal mucosa (D).
Electroporation in mucosal tissue enhances reporter gene expression and cellular infiltration to the site
To determine if direct EP-enhanced delivery of plasmid to buccal mucosal tissue was feasible, separate cheek sites on guinea pigs were injected with plasmid encoding GFP (50 µl of 1 mg/ml) and immediately electroporated with the surface EP device. Gross imaging revealed robust and reproducible GFP expression in the guinea pig mucosal tissue that was visible 8 h following EP treatment and peaked at 72 h (Fig. 2B). Minimal GFP expression was detectable following GFP plasmid injection alone (Fig. 2A). To address the cellular localization of the reporter gene expression, cheek tissue was sectioned and viewed under a fluorescence microscope. The majority of GFP expression 48 h after treatment was observed in the upper layers of the epidermis (Fig. 2C and D – right panels).
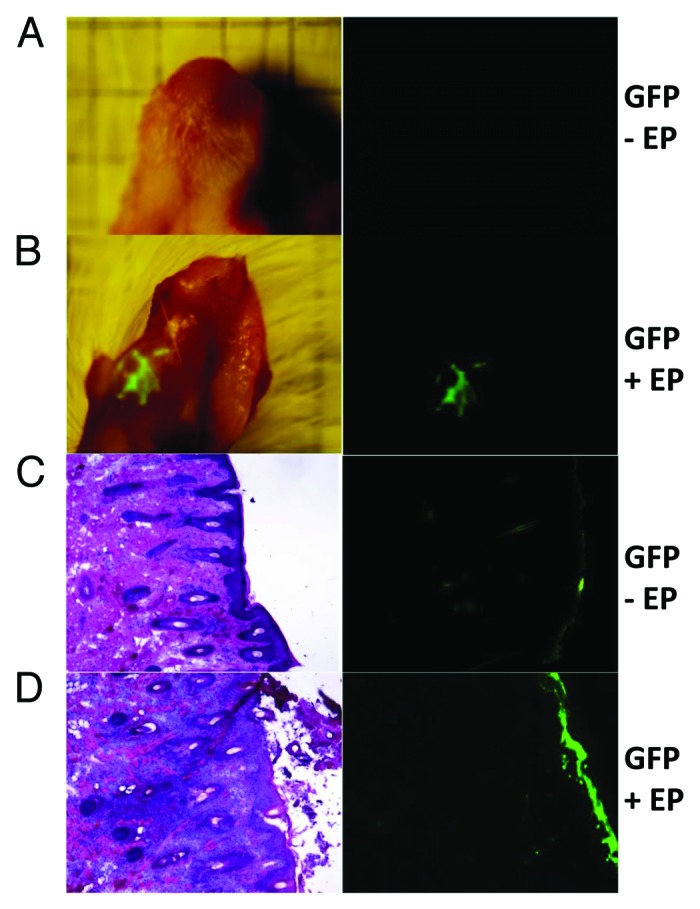
Figure 2. Efficient GFP (reporter gene) transfection in guinea pig mucosal tissue (cheek) following electroporation by a minimally invasive device. Animals were injected with 50 μl of pgWIZ-GFP at the buccal mucosa under anesthetia in the absence (A and C) or presence of electroporation (B and D). Electroporation was performed using a minimally invasive device (30 V, 3 pulses). Animals were euthanized and the cheek harvested. (A and B) GFP expression was visualized under natural light (left) and by fluorescent microscopy at 72 h (right). (C and D) Sectional histological analysis, hemotoxylin and eosin stain (left), and fluorescent microscopy (right) at 48 h.
Immediately following EP treatment (data not shown) and upon sacrifice of the animals 48 h later, we observed the direct effect of EP parameters on buccal mucosa. We observed each animal for redness, swelling and necrosis at the treatment site resulting from the application of EP. Some erythema was detected immediately following EP treatment although this resolved in 24 h. No visible tissue damage was subsequently observed prior to sacrifice (48 h) following EP (data not shown). In addition, we assessed cellular infiltration by sectioning the tissue and performing an H&E stain (Fig. 2C and D – left panels). H&E staining revealed moderate immune cell trafficking of macrophages and neutrophils to the site of injection after administration of GFP plasmid alone at the mucosa (Fig. 2C left). However, injection in combination with EP elicited the strongest infiltration indicated by the high density of cells stained at the site of injection (Fig. 2D left).
Mucosal electroporation induces robust humoral responses at both distal mucosal sites and systemically in the blood
The ability of intramuscular electroporation to enhance delivery of DNA vaccines to induce both IgG and IgA responses has been previously demonstrated.25 Here, we investigated the humoral immune responses elicited by immunization with a vaccine plasmid encoding influenza nucleoprotein (NP)26 by direct mucosal electroporation. The guinea pig model was useful to assess gene expression in the mucosa and histology associated with EP delivery. However, due to the paucity of molecular reagents available for guinea pigs, we were limited to assessing only the IgG responses in this model, and we noted strong IgG responses in the serum following direct mucosal EP (data not shown). To investigate whether mucosal IgA responses could also be induced following mucosal vaccination with DNA-EP, we moved our immunogenicity studies to a rabbit model where molecular tools are available for the assessment of IgA responses. Buccal mucosal injection and EP resulted in robust IgA responses to NP antigen, a 9-fold increase in mean endpoint titers, compared with injection in the absence of EP (450 and 50, respectively), at the distal mucosal site of the vagina (Fig. 3). The IgA response generated in the presence of EP by the direct mucosal delivery was also superior to plasmid delivered intradermally (mean end point titer 450 of vs. 180, respectively). Due to technical issues with saliva collections, we were unable to evaluate responses at the proximal mucosal site. Mucosal EP-enhanced delivery also facilitated the induction of an IgG response in the blood comparable to the peripheral delivery with EP (Fig. 3). This demonstrates that EP-enhanced delivery of DNA vaccines at the mucosa is capable of inducing both potent systemic humoral immunity as well as at mucosal sites.
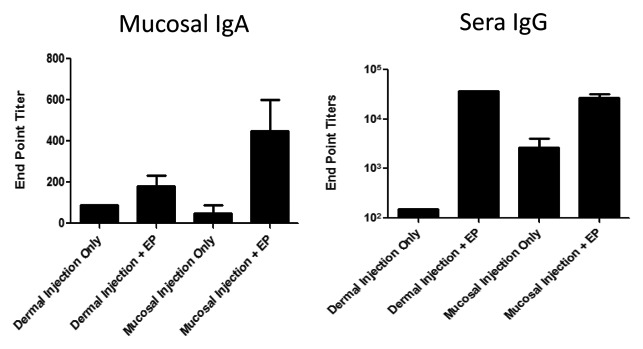
Figure 3. DNA vaccination combined with EP at the mucosa elicits antigen specific mucosal IgA and blood IgG responses in a rabbit model. New Zealand Rabbits were vaccinated with a influenza plasmid (vaccine construct encodes nucleoprotein sequence derived from A/Puerto Rico/8 (H1N1) strain of influenza (100 μg/plasmid)) in a volume of 50 μl diluted in PBS. Animals were immunized at the dermis or buccal mucosa, either followed immediately with EP or not. Immunizations were performed on days 1, 14 and 28. Blood or vaginal washes were collected from animals on day 42, and NP-specific sera IgG and vaginal mucosal IgA were detected by ELISA.
Cellular responses are augmented by mucosal electroporation
Many disease targets require both a humoral and cellular response to afford protection from disease. Due to a lack of available tools to measure cellular immunity in guinea pigs and rabbits, this section of our studies was performed in a mouse model. We assessed the cellular and humoral responses to NP following direct mucosal injection and EP. BALB/c mice were injected with NP plasmid at days 1 and 14 at the buccal mucosa in the presence or absence of EP. Serum IgG responses were measured at day 21. Figure 4A shows electroporation significantly enhanced NP-specific IgG titers in the sera of vaccinated mice by 10-fold (p = 0.0407, mean end point titer of 8110 vs. 825, respectively). To assess the cellular immune response, spleens were harvested on day 21 and ELISpot analysis of CD4+ and CD8+ T cell IFN-γ responses against NP-derived peptides were determined. Figure 4B demonstrates that CD4+ IFN-γ+ responses to NP peptide 55 were significantly higher in the vaccine + EP group compared with the vaccine only group (p < 0.001, mean of 503 vs. 230 spots / 106 cells, respectively). Also, CD8+ IFN-γ+ responses to NP peptide 147 were significantly higher in the vaccine + EP group compared with the vaccine only group (p < 0.001, mean of 1168 vs. 357 spots/106 cells), respectively (Fig. 4C). Building on previous dermal and muscle EP studies, these results demonstrate that electroporation strongly potentiates both the humoral and cellular arms of the immune response generated by DNA vaccination at the mucosa.
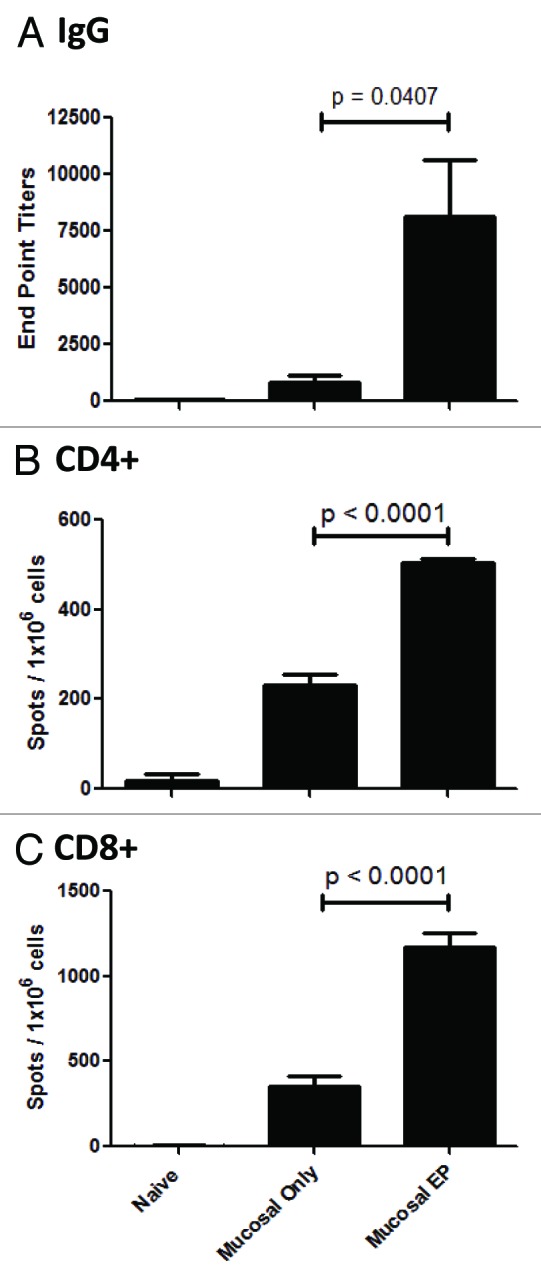
Figure 4. DNA vaccination combined with EP at the mucosa elicits antigen specific humoral and cellular immune responses in a mouse model. BALB/c mice were vaccinated with 2 μg of an influenza plasmid (vaccine construct encodes nucleoprotein sequence derived from A/Puerto Rico/8 (H1N1) strain of influenza). The plasmid was directly injected into the buccal mucosa and either followed immediately with EP or not. Immunizations were performed on days 1 and 14. Blood and spleens collected on day 21, and sera NP-specific IgG titers measured by ELISA (A), and splenocyte CD4+ (B) and CD8+ (C) T cell IFN-γ responses to NP peptide were detected by ELISpot.
Discussion
The initial attachment and subsequent entry of pathogens into the host cell regularly occurs at mucosal sites. Diseases such as HIV/AIDS, tuberculosis and influenza specifically target the mucosa as their portal of entry. Generation of antigen specific protective mucosal immunity has become the goal of many current vaccination strategies. Here we demonstrate that electroporation-enhanced DNA vaccination at the mucosa induces strong cellular and humoral immunity.
DNA vaccine technology offers an attractive mucosal immunization strategy, posing many advantages over conventional vaccination platforms. In comparison to live attenuated and inactivated virus vaccines, the simplicity and efficiency of DNA manufacturing, along with the ability to generate both humoral and cellular immunity makes genetic-based vaccines an appealing alternative. However, to harness the power of this technology, a strategy is required to achieve maximal DNA delivery and robust immune responses. Enhanced gene delivery can be achieved through multiple platform technologies, such as liposomes, ultrasound and ballistic particles.27 Our particular modality of interest is enhanced DNA delivery through the physical process of electroporation. Electroporation has successfully aided in enhancing the delivery of DNA vaccines, leading to the generation of stronger immune responses (10–100-fold) compared with naked DNA vaccine delivery alone to the muscle or the skin.5 However, little is known concerning the role of electroporation in enhancing direct mucosal DNA delivery.
The ability of DNA plasmids to efficiently enter tissue resident cells is a critical requirement for genetic immunization in all tissues, including the mucosa. The mucosa however, has the added complication of multiple physical and chemical barriers that could impede DNA transfection, including mucolytic enzymes, endonucleases, low pH and the ciliated epithelium. Therefore a critical requirement for successful DNA delivery in this tissue would be an optimized and efficient delivery modality. We have investigated delivering a DNA vaccine directly into the mucosal tissue in the presence of low energy electroporation. The SEP device used for this study operates with electrical power between 0.35 and 0.45 Watts.
In this study, due to the ease of accessibility we choose the inner lining of the mouth (buccal mucosa) to be the site of immunization and electroporation. The mucosal tissue interface is a stratified squamous epithelium consisting of squamous (flattened) epithelial cells arranged in layers upon a basement membrane. Only one layer is in contact with the basement membrane; the other layers adhere to one another to maintain structural integrity. This type of epithelium is well suited to areas in the body subject to constant abrasion, as it is the thickest and layers can be sequentially sloughed off and replaced before the basement membrane is exposed. It forms the outermost layer of the skin and the inner lining of the mouth, esophagus and vagina. These are all regions of the body susceptible to the invasion of pathogens. In this study, we first demonstrated that expression of the reporter gene GFP is strongly enhanced in the mucosal tissue when delivered in the presence of electroporation (Fig. 2). Both gross and cellular localization images confirmed enhanced expression of our reporter gene when mucosal delivery was performed in the presence of EP. The majority of the transfection was detected in the upper stratified squamous epidermal levels of the buccal mucosa. We previously reported on DNA vaccine delivery using a related device and identical EP parameters to the epidermal layers of the skin.17 Consistent with that study, the positioning of the GFP expression in both skin and mucosa was localized in the upper layers of the tissue and the duration of GFP expression persisted for approximately a week. In future studies, we aim to identify the cell populations directly transfected within the mucosa. This will aid in the elucidation of the important antigen presenting mechanisms that operate at this site. Such information can then be transferred to allow for the generation of optimal immunity depending on the target of interest. The most common cell type in the epithelial layer of buccal mucosa is the keratinocyte. The nature of gene delivery by an electroporation platform dictates that we transfect these cells in the majority, however it is also possible that we are directly transfecting the antigen presenting Langerhan cells. It has previously been shown that upon antigen capture, the buccal mucosal dendritic cells migrate to crevico-mandibular lymph nodes to present their antigen to the immune system.28 Future studies will involve the investigation of direct antigen presenting cell transfection and their subsequent trafficking to draining lymph nodes.
Data generated in this study indicates that increased plasmid DNA expression in the presence of EP was associated with an augmented systemic immune response at both the humoral and cellular level (Figs. Three and 4). Importantly, we demonstrated the induction of IgA together with IgG responses (Fig. 3). In contrast to the dominance of the IgG responses of the systemic immune system, IgA production and secretion dominates humoral mucosal immunity.29 This alteration in isotype distribution defines the distinct nature of mucosal immunology and demonstrates the functional role that IgA plays as high-affinity neutralizing antibody for multiple pathogenic microbes and toxins, including HIV.30,31 Therefore, the ability of our vaccination regimen to induce enhanced IgA titers in the distal mucosal sites is very encouraging, and the induction of vaginal IgA may be relevant for targeting diseases spread through sexual intercourse.32
We believe electroporation enhances the immunity of genetic vaccines through two important pathways - increased plasmid DNA delivery into the target tissue, as well as the induction of an inflammatory milieu at the site of immunization. The mucosal environment is rich in nucleases, so the ability of EP to accelerate DNA delivery in the mucosa is of paramount importance. The second important attribute of the vaccination regimen is that the immune tolerance mechanisms that operate at the mucosa could impede the generation of productive immune responses to vaccine antigens. As expected, the direct administration of naked DNA vaccines to the mucosa was associated with the generation of only moderate immunity (Figs. Three and 4).33 This is in agreement with previous studies investigating the effect of DNA delivery of plasmid expressing influenza antigens at respiratory mucosal sites.16,34 These studies demonstrated that limited antigen specific responses were detectable following this route of administration, but only limited effects on morbidity were observed. However, both studies documented the benefits of a mucosal adjuvant. It is possible that in addition to inefficient delivery, naked DNA vaccines (in the absence of a strong adjuvant) failed to overcome the local tolerance mechanisms mediated by regulatory cells or induce sufficient co-stimulatory signals and/or cytokines to kick start a productive immune response. Electroporation of the muscle has been shown to create an inflammatory milieu and augment the recruitment of professional antigen presenting cells to the site of immunization,35 therefore creating favorable conditions to overcome local tolerance mechanisms. Here we demonstrate an influx of immune cells to the mucosal treatment site following electroporation (Fig. 2). Our results suggest that EP at the mucosa creates a favorable environment to aid in the generation of a robust immune response.
Additionally, DNA vaccination in combination with EP at the buccal mucosa drives the induction of cellular immunity (Fig. 4). Such immunity is considered essential in protection against many diseases, including HIV, and virally induced tumors, such as mucosal cells expressing HPV oncoproteins.36,37 In this study, we observed that our modified minimally invasive surface device (Fig. 1A and B) and EP parameters were well tolerated in the species (mice, guinea pig and rabbit) treated, with minimal transient damage of the mucosal surface at the vaccination site (Fig. 1D). The lengthening of the EP electrodes facilitated improved contact with the buccal tissue in all species, allowing appropriate access to the cheek region. Importantly, we demonstrated that our vaccination regimen at the buccal mucosa elicits a response that leads to increased IgA levels in the vagina, a distal mucosal site (Fig. 3). This finding was very encouraging due to the importance of the generation of local protective immunity (for example, anti-HIV responses at the rectal and vaginal regions). Since there are obvious practical difficulties involved with rectal and vaginal vaccination, a less invasive, distal mucosal site might be preferred. In future studies, it will be important to determine if the responses generated at distal mucosal sites are protective in challenge models. In conclusion we believe direct administration of DNA vaccine delivery enhanced by EP at accessible sites of the mucosa may have important clinical implications in the prevention or treatment of diseases that enter at mucosal sites and require both cellular and humoral immune responses.
Materials and Methods
Animals
Animals were housed at Biotox Sciences. All procedures complied with the Animal Welfare Act (AWA) and Institutional Animal Care and Use Committees (IACUC). Female Hartley guinea pigs (Charles River) weighing between 350–400 g and female New Zealand White Rabbits (Western Oregon) weighing about 1900 g were allowed to acclimate for 2 weeks prior to the study. Female BALB/c mice (Charles River) approximately 6 weeks of age were allowed to acclimate for one week prior to the study.
Plasmids
Plasmids used in this study were the SynConTM vaccine construct that encodes a consensus sequence of Influenza Nucleoprotein (Inovio Pharmaceuticals) and pgWIZ-GFP plasmid (Aldevron N.D.). Plasmids were diluted with PBS.
Devices
CELLECTRA® 3P is a three pronged array that delivers a current of 0.2 A in 2 sets of 2 pulses that are 52 ms long and 52 ms apart with 1s between sets. The electrode arrays are comprised of 25 gauge solid stainless steel needles set in an isosceles triangle formation and have a 3mm depth of penetration.
The 4 × 4 array was designed to contact the skin or buccal mucosa without penetrating the tissue, and is a minimally invasive surface electrode device. The array is comprised of 16 gold-plated stainless steel needle electrodes with trocar grinds at 1.5 mm spacing in a 4 × 4 configuration.17 Due to the size of the mouse buccal cavity, adjustments to the 4 × 4 array were made by removing needle electrodes resulting in a 2 × 2 configuration without affecting the integrity of the applicator or its parameters. 4 × 4 and 2 × 2 arrays were used in conjunction with the ELGEN1000 (Inovio Pharmaceuticals, San Diego, CA, USA) pulse generator. The device parameter is set to deliver three 25 or 30V pulses of 100 ms duration with 100 ms delay between pulses.
Treatment
All injections were performed using the Mantoux injection technique with a 29 gauge tuberculin syringe. EP treatments were conducted immediately following injection by placing either the CELLECTRA® 3P or ELGEN 4 × 4 or 2 × 2 on top of the bleb created by the injection and applying minimal pressure during the entire EP procedure to ensure good contact and electrotransfer.
Guinea Pigs
Two guinea pigs received a 100 µg injection of gWiz-GFP on each buccal mucosa (cheek) resulting in two treatment sites per guinea pig. Only the left cheek of each animal received an EP treatment using the ELGEN 4 × 4 at 30V immediately after injection.
Rabbits
Rabbits were divided into 4 groups (n = 4/group). Treatments were performed every 2 weeks at days 0, 14, and 28. Treatment regimen was as follows: group 1 received two 50 µl injections of 50 µg pNP on the flank with no EP; group 2 received two 50 µl injections of 50 µg pNP in the buccal mucosa with no EP; group 3 received two 50 µl injections of 50 µg pNP on the flank, with each injection being immediately followed by EP treatment using the CELLECTRA® 3P; group 4 received two 50 µl injections of 50 µg pNP in the buccal mucosa, with each injection being immediately followed by EP treatment using the ELGEN 4x4 at 25V.
Mice
The mice were divided up into 3 groups of 5 and received treatments on days 0 and 14. Treatment regimen was as follows: group 1 received a 10 µl injection of 2 µg of pNP into the buccal mucosa with no EP, group 2 received a 10 µl injection of 2 µg of pNP into the buccal mucosa immediately followed by EP using the ELGEN 2 × 2 at 25V, group 3 was untreated.
Sample Collection
Peripheral Blood
Rabbit blood collection was conducted every 3 weeks beginning at day 0 and up to week 12. Collection consisted of inserting a 22 gauge catheter into the central auricular artery and allowing the blood to flow into a 5 ml serum separation tube. The blood was then centrifuged (Rotanta 460) at 3540 rcf for 10 min. The serum was transferred into a 1.7 mL microcentrifuge tube and placed at -20°C until testing.
Mouse blood (250 µl) was collected via retro orbital bleeding at weeks 0, 2, and 3. After centrifugation for 5 min at a rate of 10,600 rcf, the serum was transferred into microcentrifuge tube and placed at -20°C until testing.
Vaginal Wash
Rabbit vaginal washes were conducted using 500 µl of protease inhibitor PBS solution flushed in and out of the vaginal canal multiple times. The vaginal washes were centrifuged at a rate of 10,600 rcf for 10 min and supernatant was stored at -20°C until testing.
Tissue Collection
Guinea pig cheeks were excised 48 or 72 h post treatment. Cheeks collected at 72 h were stored at -20°C for gross imaging using Olympus OV100 imaging system (AntiCancer Inc., San Diego, CA) at 480 nm. The 48 h cheek samples were fixed in 10% neutral buffered formalin and placed at 4°C overnight. The next day, the samples were buffered in a 15% sucrose solution at 4°C and stored until sectioning.
Mice spleens were harvested 21 d post initial immunization and single cell suspensions were prepared for ELISpot analysis.
Histopathology
Guinea Pig Cheek samples were embedded in OCT Compound (Sakura) and sectioned at thickness of 20 µm using an OTF Bright Cryostat. The sections were then hematoxylin and eosin (H&E) stained and viewed with a Zeiss Axioplan microscope using a 20x objective.
ELISA
Antibody responses against NP were evaluated by ELISA using sera or vaginal washes obtained from rabbits and mice. 96-well plates (Costar) were coated with 0.3 µg/ml of Influenza A, NP, Recombinant Protein (Imgenex) at 4°C overnight. The next day, using Skanwash 96 well automated plate washer, the plates were washed 3 times. The plates were then blocked with 200 µl of nonspecific binding solution (1xPBS with 0.5% BSA) and incubated for one hour at 37°C. After incubation, the washing step was repeated. Samples were run in duplicates and added to row A, sera at a 1:50 dilution and vaginal wash at a 1:10 dilution, using a dilution buffer solution (PBS with 0.2% BSA and 0.05% Tween-20). From row A, 50uL of sample was taken and serially diluted 1:3 in the corresponding rows up to G; row H was used as a negative control background measurement. After two hours of incubation at 37°C, the plates were washed and 100 µL of either goat anti-mouse IgG-HRP (Sigma) diluted at 1:10,000, goat anti-rabbit IgG-HRP (Sigma) diluted at 1:10,000 or goat anti-rabbit IgA-HRP (Pierce) at a 1:5,000 using dilution buffer solution was added to each well and incubated for one hour at 37°C. The plates washed 3 times and 100 µl of TMB 2-component Microwell Peroxidase System (KPL) was added and developed for 8 min at room temperature. Development was stopped by adding 50 µl of TMB Stop Solution (KPL). The plates were read on a Molecular Devices SpectraMax 384 plate reader at an OD of 450nm. A positive titer was calculated by subtracting 2 times the average background OD from the average sample OD. Positive titers were plotted as end-point titers.
ELISpot
Mouse IFN-gamma ELISpots were performed as previously described.38 Briefly, 96-well PVDF ELISpot microplates were coated with anti-mouse interferon (IFN)-γ monoclonal antibodies and incubated overnight at 4°C. In accordance with the protocol, wells were washed and blocked. Pooled splenocytes from each group were added to triplicate wells at 2 x 105 cells/well and incubated overnight with 10 µg/ml of either NP147-CD8 peptide (TYQRTRALV Biosynthesis Inc.), NP55-CD4 peptide (RLIQNSLTIERNVLS, Biosynthesis Inc.), Concanavalin A (Sigma), or media. The plates were then washed and developed in accordance with the protocol. The spots were counted and analyzed using CTL Analyzer and Immunospot SC software suite (Shaker Heights, OH).
Statistical analysis
Data presented as the mean ± s.d. Statistical differences between groups was assessed using a two-tailed, paired Student’s t-test that yielded a specific P value for each test. Comparisons between samples with a P value < 0.05 were considered to be statistically significant.
Author Disclosure Statement
All authors are employees of Inovio Pharmaceutical’s and as such receive salary and stock options.
Acknowledgments
We would like to thank Chris Knott for technical help; Kimberly A. Kraynyak for manuscript assistance; Maria Yang, Philip Armendi and Jenna Robles for plasmid preparation; Xuefei Shen and Feng Lin for assistance with ELISPOT and Steve Kemmerrer for support of the EP device.
Glossary
Abbreviations:
- EP
electroporation
- i.d.
intradermal
- NP
nucleoprotein
- MID
Minimally invasive device
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25272
References
- 1.Belyakov IM, Ahlers JD. Mucosal immunity and HIV-1 infection: applications for mucosal AIDS vaccine development. Curr Top Microbiol Immunol. 2012;354:157–79. doi: 10.1007/82_2010_119. [DOI] [PubMed] [Google Scholar]
- 2.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239:62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 3.Weiner DB. DNA vaccines: crossing a line in the sand. Introduction to special issue. Vaccine. 2008;26:5073–4. doi: 10.1016/j.vaccine.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–48. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 5.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23:421–9. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugar IP, Neumann E. Stochastic model for electric field-induced membrane pores. Electroporation. Biophys Chem. 1984;19:211–25. doi: 10.1016/0301-4622(84)87003-9. [DOI] [PubMed] [Google Scholar]
- 7.Sugar IP, Förster W, Neumann E. Model of cell electrofusion. Membrane electroporation, pore coalescence and percolation. Biophys Chem. 1987;26:321–35. doi: 10.1016/0301-4622(87)80033-9. [DOI] [PubMed] [Google Scholar]
- 8.Wolf H, Rols MP, Boldt E, Neumann E, Teissié J. Control by pulse parameters of electric field-mediated gene transfer in mammalian cells. Biophys J. 1994;66:524–31. doi: 10.1016/S0006-3495(94)80805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiarella P, Massi E, De Robertis M, Sibilio A, Parrella P, Fazio VM, et al. Electroporation of skeletal muscle induces danger signal release and antigen-presenting cell recruitment independently of DNA vaccine administration. Expert Opin Biol Ther. 2008;8:1645–57. doi: 10.1517/14712598.8.11.1645. [DOI] [PubMed] [Google Scholar]
- 10.Ahlén G, Söderholm J, Tjelle T, Kjeken R, Frelin L, Höglund U, et al. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J Immunol. 2007;179:4741–53. doi: 10.4049/jimmunol.179.7.4741. [DOI] [PubMed] [Google Scholar]
- 11.Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000;164:4635–40. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- 12.Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012;4:ra138. doi: 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zucchelli S, Capone S, Fattori E, Folgori A, Di Marco A, Casimiro D, et al. Enhancing B- and T-cell immune response to a hepatitis C virus E2 DNA vaccine by intramuscular electrical gene transfer. J Virol. 2000;74:11598–607. doi: 10.1128/JVI.74.24.11598-11607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Drunen Littel-van den Hurk S, Lawman Z, Wilson D, Luxembourg A, Ellefsen B, van den Hurk JV, et al. Electroporation enhances immune responses and protection induced by a bovine viral diarrhea virus DNA vaccine in newborn calves with maternal antibodies. Vaccine. 2010;28:6445–54. doi: 10.1016/j.vaccine.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa T, Takashima Y, Hirayama S, Okada H. Effects of menstrual cycle on gene transfection through mouse vagina for DNA vaccine. Int J Pharm. 2008;360:164–70. doi: 10.1016/j.ijpharm.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Ban EM, van Ginkel FW, Simecka JW, Kiyono H, Robinson HL, McGhee JR. Mucosal immunization with DNA encoding influenza hemagglutinin. Vaccine. 1997;15:811–3. doi: 10.1016/S0264-410X(96)00263-0. [DOI] [PubMed] [Google Scholar]
- 17.Broderick KE, Shen X, Soderholm J, Lin F, McCoy J, Khan AS, et al. Prototype development and preclinical immunogenicity analysis of a novel minimally invasive electroporation device. Gene Ther. 2011;18:258–65. doi: 10.1038/gt.2010.137. [DOI] [PubMed] [Google Scholar]
- 18.Heller R, Cruz Y, Heller LC, Gilbert RA, Jaroszeski MJ. Electrically mediated delivery of plasmid DNA to the skin, using a multielectrode array. Hum Gene Ther. 2010;21:357–62. doi: 10.1089/hum.2009.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donate A, Coppola D, Cruz Y, Heller R. Evaluation of a novel non-penetrating electrode for use in DNA vaccination. PLoS One. 2011;6:e19181. doi: 10.1371/journal.pone.0019181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Li L, Hoffmann GA, Hoffman RM. Depth-targeted efficient gene delivery and expression in the skin by pulsed electric fields: an approach to gene therapy of skin aging and other diseases. Biochem Biophys Res Commun. 1996;220:633–6. doi: 10.1006/bbrc.1996.0455. [DOI] [PubMed] [Google Scholar]
- 21.Heller LC, Jaroszeski MJ, Coppola D, McCray AN, Hickey J, Heller R. Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Ther. 2007;14:275–80. doi: 10.1038/sj.gt.3302867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Nolan E, Kreitschitz S, Rabussay DP. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim Biophys Acta. 2002;1572:1–9. doi: 10.1016/S0304-4165(02)00270-2. [DOI] [PubMed] [Google Scholar]
- 23.Diehl MC, Lee JC, Daniels SE, Tebas P, Khan A, Giffear M, et al. Tolerability of Intramuscular and Intradermal Delivery by CELLECTRA® Adaptive Constant Current Electroporation Device in Healthy Volunteers. Hum Vaccin and Immunother. 2013 doi: 10.4161/hv.24702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tjelle TE, Salte R, Mathiesen I, Kjeken R. A novel electroporation device for gene delivery in large animals and humans. Vaccine. 2006;24:4667–70. doi: 10.1016/j.vaccine.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 25.Patel V, Valentin A, Kulkarni V, Rosati M, Bergamaschi C, Jalah R, et al. Long-lasting humoral and cellular immune responses and mucosal dissemination after intramuscular DNA immunization. Vaccine. 2010;28:4827–36. doi: 10.1016/j.vaccine.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen X, Söderholm J, Lin F, Kobinger G, Bello A, Gregg DA, et al. Influenza A vaccines using linear expression cassettes delivered via electroporation afford full protection against challenge in a mouse model. Vaccine. 2012;30:6946–54. doi: 10.1016/j.vaccine.2012.02.071. [DOI] [PubMed] [Google Scholar]
- 27.Locher CP, Putnam D, Langer R, Witt SA, Ashlock BM, Levy JA. Enhancement of a human immunodeficiency virus env DNA vaccine using a novel polycationic nanoparticle formulation. Immunol Lett. 2003;90:67–70. doi: 10.1016/j.imlet.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson K, Ahlfors E, George-Chandy A, Kaiserlian D, Czerkinsky C. Antigen presentation in the murine oral epithelium. Immunology. 1996;88:147–52. doi: 10.1046/j.1365-2567.1996.d01-647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 30.Alfsen A, Iniguez P, Bouguyon E, Bomsel M. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J Immunol. 2001;166:6257–65. doi: 10.4049/jimmunol.166.10.6257. [DOI] [PubMed] [Google Scholar]
- 31.Hocini H, Bomsel M. Infectious human immunodeficiency virus can rapidly penetrate a tight human epithelial barrier by transcytosis in a process impaired by mucosal immunoglobulins. J Infect Dis. 1999;179(Suppl 3):S448–53. doi: 10.1086/314802. [DOI] [PubMed] [Google Scholar]
- 32.Clerici M, Barassi C, Devito C, Pastori C, Piconi S, Trabattoni D, et al. Serum IgA of HIV-exposed uninfected individuals inhibit HIV through recognition of a region within the alpha-helix of gp41. AIDS. 2002;16:1731–41. doi: 10.1097/00002030-200209060-00004. [DOI] [PubMed] [Google Scholar]
- 33.Wang B, Dang K, Agadjanyan MG, Srikantan V, Li F, Ugen KE, et al. Mucosal immunization with a DNA vaccine induces immune responses against HIV-1 at a mucosal site. Vaccine. 1997;15:821–5. doi: 10.1016/S0264-410X(96)00259-9. [DOI] [PubMed] [Google Scholar]
- 34.Fynan EF, Robinson HL, Webster RG. Use of DNA encoding influenza hemagglutinin as an avian influenza vaccine. DNA Cell Biol. 1993;12:785–9. doi: 10.1089/dna.1993.12.785. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Kjeken R, Mathiesen I, Barouch DH. Recruitment of antigen-presenting cells to the site of inoculation and augmentation of human immunodeficiency virus type 1 DNA vaccine immunogenicity by in vivo electroporation. J Virol. 2008;82:5643–9. doi: 10.1128/JVI.02564-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadish AS, Timmins P, Wang Y, Ho GY, Burk RD, Ketz J, et al. Albert Einstein Cervix Dysplasia Clinical Consortium Regression of cervical intraepithelial neoplasia and loss of human papillomavirus (HPV) infection is associated with cell-mediated immune responses to an HPV type 16 E7 peptide. Cancer Epidemiol Biomarkers Prev. 2002;11:483–8. [PubMed] [Google Scholar]
- 37.Riddell SR, Gilbert MJ, Greenberg PD. CD8+ cytotoxic T cell therapy of cytomegalovirus and HIV infection. Curr Opin Immunol. 1993;5:484–91. doi: 10.1016/0952-7915(93)90027-P. [DOI] [PubMed] [Google Scholar]
- 38.Lin F, Shen X, McCoy JR, Mendoza JM, Yan J, Kemmerrer SV, et al. A novel prototype device for electroporation-enhanced DNA vaccine delivery simultaneously to both skin and muscle. Vaccine. 2011;29:6771–80. doi: 10.1016/j.vaccine.2010.12.057. [DOI] [PubMed] [Google Scholar]


