Abstract
We evaluated the immunogenicity and efficacy of Vaxfectin® adjuvanted SIV DNA vaccines in mice and macaques. Vaccination of mice with Vaxfectin® adjuvanted SIV gag DNA induced higher humoral immune responses than administration of unadjuvanted DNA, whereas similar levels of cellular immunity were elicited. Vaxfectin® adjuvanted SIVmac251 gag and env DNA immunization of rhesus macaques was used to examine magnitude, durability, and efficacy of humoral immunity. Vaccinated macaques elicited potent neutralizing antibodies able to cross-neutralize the heterologous SIVsmE660 Env. We found remarkable durability of Gag and Env humoral responses, sustained during ~2 y of follow-up. The Env-specific antibody responses induced by Vaxfectin® adjuvanted env DNA vaccination disseminated into mucosal tissues, as demonstrated by their presence in saliva, including responses to the V1-V2 region, and rectal fluids. The efficacy of the immune responses was evaluated upon intrarectal challenge with low repeated dose SIVmac251. Although 2 of the 3 vaccinees became infected, these animals showed significantly lower peak virus loads and lower chronic viremia than non-immunized infected controls. Thus, Vaxfectin® adjuvanted DNA is a promising vaccine approach for inducing potent immune responses able to control the highly pathogenic SIVmac251.
Keywords: HIV, Rhesus macaques, SIVmac239, SIVsmE660, V1 and V2 antibodies, adjuvant, antibody, avidity, mucosal immunity, neutralizing antibody, rectal fluid, saliva, systemic immunity
Introduction
Almost 30 y after the discovery of the HIV-1, the development of an effective vaccine able to contain the AIDS pandemic still remains a major challenge. Modest success in protection from HIV-1 infection (RV144 clinical trial in Thailand) was reported1 using a combination vaccine consisting of recombinant canarypox ALVAC®-HIV together with gp120 Env protein (AIDSVAX® B/E) but no evidence of vaccine-induced virus control was found in the infected individuals.
The macaque vaccine model using repeated low dose SIV virus challenge has shown delay of virus acquisition by different vaccine regimens2-7 and reduction of viremia.2,3,5,7,8 DNA-based vaccines are a promising approach to induce potent immune responses against HIV/SIV and other diseases (reviewed in ref. 9). We and others have previously reported that macaques immunized with SIV/HIV DNA alone administered by needle and syringe via the intramuscular route (IM) induced potent cellular and humoral immune responses against SIV and SHIV able to reduce viremia upon infection10-23 and established the effectiveness of cellular immune responses by DNA vaccine, although the magnitude of the responses has been relatively low. A significant improvement in magnitude and quality of immunogenicity induced by DNA vaccines was found using in vivo electroporation (EP) as a delivery method (reviewed in refs. 24, 25). IM/EP delivery of SIV/HIV DNA resulted in induction of robust and durable cellular and humoral immune responses7,8,16,21,23,26-31 able to significantly reduce SIV viremia in macaques.7,8,21,23,27,29,31 A recent phase I trial in which an HIV DNA vaccine delivered via IM/EP together with IL-12 DNA as adjuvant resulted in higher frequency of responders and higher longer-lasting immunity than needle/syringe delivery,32 corroborating findings from the macaque model.
Although DNA vaccines induce robust immunity, these responses have not provided sterilizing protection, and therefore, alternative DNA delivery methods are being explored. Vaxfectin® is a cationic lipid-based formulation that has been shown to effectively act as an adjuvant for both DNA and protein.33 Several studies have established that Vaxfectin® adjuvanted DNA vaccines induce significantly higher antibody responses than DNA-only.34-37 A preclinical evaluation of a prophylactic DNA vaccine adjuvanted with Vaxfectin® against cytomegalovirus established that this vaccine platform was immunogenic and well-tolerated in mice and rabbits and showed a favorable safety profile.38 A Vaxfectin® adjuvanted HSV-2 DNA vaccine was shown to be effective in the guinea pig model of genital herpes for both prophylactic and therapeutic use.39 A recent report demonstrated that a Vaxfectin® adjuvanted DNA vaccine encoding the measles virus proteins elicited protective immunity against challenge in macaques.40 A phase 1 clinical trial with Vaxfectin® adjuvanted plasmid DNA encoding influenza A virus H5 hemagglutinin has shown to be well-tolerated and immunogenic.41
In this report, we evaluate the immunogenicity of Vaxfectin® adjuvanted SIV DNA vaccine in mice and macaques. We demonstrate induction of high and persistent levels of humoral responses, including Env-specific responses disseminating to mucosal tissues. In support of the protective ability of this vaccine method, we found a trend in delay in virus acquisition and a significant control of pathogenic SIVmac251 viremia after challenge of vaccinated macaques.
Results
Vaccination with SIV gag DNA adjuvanted in Vaxfectin® induces higher humoral immune responses in mice
First, we evaluated the immunogenicity of Vaxfectin® adjuvanted SIV DNA in BALB/c mice. Animals were vaccinated with 100 µg of gag DNA formulated with Vaxfectin® (n = 10) or PBS (n = 10), respectively, at week 0 and week 4 (Fig. 1A). The gag plasmid expressed a fusion of Gag to the monocyte chemoattractant protein 3 (MCP-3) chemokine having the myristoylation signal replaced with the complete MCP-3; this protein is actively secreted and chemotactically attracts antigen presenting cells.22 Two weeks after the 2nd vaccination, splenocytes and plasma were collected for the analysis of cellular and humoral immune responses. Anti-p27gag antibodies were measured in plasma from individual mice (Fig. 1B). Mice immunized with Vaxfectin® adjuvanted DNA developed significantly higher titers (p = 0.0052) of anti-p27gag antibodies compared with mice immunized with DNA formulated in PBS. Cellular immune responses were measured by IFN-γ ELISPOT assay from splenocytes stimulated with the Gag peptide pool, and responses were reported as spot forming cells (SFC) per million of splenocytes (Fig. 1C). Splenocytes cultured in medium without peptide or stimulated with phorbol myristate acetate (PMA) and calcium ionophore were used as negative and positive controls, respectively. Both groups of mice had similar levels of cellular Gag-specific immune responses with a median of ~300 and ~400 SFC per million splenocytes, respectively. Thus, in comparison to immunization with DNA in PBS, Vaxfectin® adjuvanted SIV gag DNA vaccination induced higher humoral and similar levels of cellular immune responses.
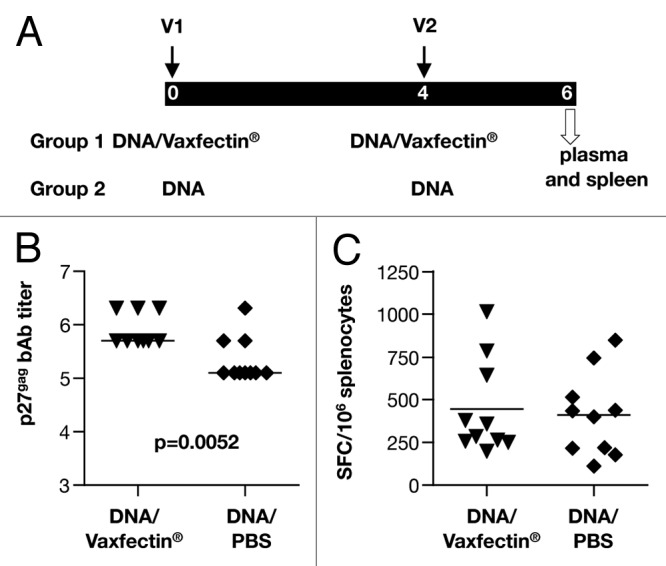
Figure 1. Vaccination with SIV gag DNA formulated with Vaxfectin® induces higher humoral immune responses in mice. (A) BALB/c mice (n = 10/group) were vaccinated at 0 and 4 weeks with SIV gag DNA formulated with Vaxfectin® or PBS, and were sacrificed 2 weeks after the 2nd vaccination. (B) Reciprocal endpoint titers of the Gag-specific binding antibodies from all the individual mice are shown in log. (C) Splenocytes were stimulated with a Gag peptide pool and the IFN-γ producing T cells were measured by ELISPOT. Statistical analysis was performed using non-parametric t test.
Vaccination of macaques with SIV gag DNA formulated in Vaxfectin® induces robust and long-lasting humoral immune responses
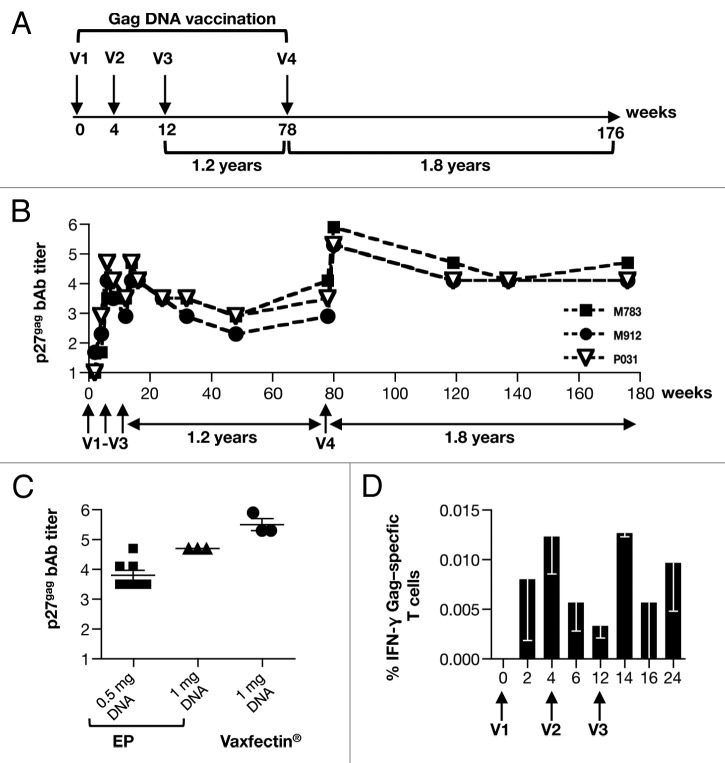
Based on the encouraging results from the mouse study, we tested the immunogenicity of Vaxfectin® adjuvanted SIV DNA in rhesus macaques. Three animals were immunized sequentially with Vaxfectin® adjuvanted SIV DNAs expressing Gag (V1-V4), Env (V5-V7), and lastly by a simultaneous vaccination with a combination of both DNAs (V8-V10) given at separate sites, as outlined in Figure 2. The vaccination schedule allowed the monitoring of the induced immune responses upon individual (V1-V4, gag DNA; V5-V7, env DNA) or simultaneous (V8–10, gag and env DNAs) vaccine administration as well as the longevity of the Gag and Env-specific immune responses (1.8 and 1.6 y of follow-up respectively).
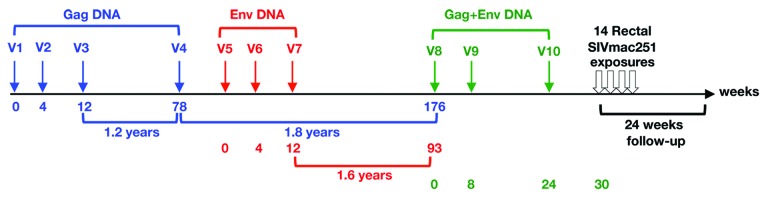
Figure 2. Study outline of macaques vaccinated with Vaxfectin® adjuvanted SIV DNAs. Indian rhesus macaques (n = 3) were sequentially vaccinated with SIV gag and env DNA, followed by simultaneous vaccination with both DNAs. Six weeks following the last vaccination (V10), the animals were challenged by sequential intrarectal exposure to a low dose of a previously titrated SIVmac251 viral stock, and were monitored for 24 weeks.
First, the animals were vaccinated with gag DNA (V1-V3, Figure 3A) which showed induction of robust Gag humoral immune responses with peak titers after V2 of ~4–5 logs (Fig. 3B). Thus, 2 vaccinations were sufficient to induce maximal immune responses using this regimen. We also compared the peak antibody titers to those obtained upon IM/EP (28 and our unpublished observation) delivery of gag DNA using 0.5 mg (n = 8) and 1 mg (n = 3), respectively (Fig. 3C). Comparing Ab titers at 2 weeks post 4th vaccination, we observed that the Vaxfectin® adjuvanted gag DNA elicited slightly higher responses.
Figure 3. Vaccination of macaques with Vaxfectin® adjuvanted SIV gag DNA induces high and sustained p27gag binding antibody titers. (A) Outline of vaccination study of macaques vaccinated with Vaxfectin® adjuvanted SIV gag DNA. (B) Reciprocal endpoint of SIV p27gag bAb titers measured overtime are shown in log. (C) Comparison of p27gag endpoint bAb titers to data reported after 4 vaccinations with gag DNA using IM/EP (0.5 mg and 1 mg, respectively28 and our unpublished observation). Mean and SEM are given. (D) PBMCs were stimulated with the SIV Gag peptide pool and the frequency of the IFN-γ producing T cells was determined by intracellular cytokine staining followed by flow cytometry.
Monitoring the macaques that received the Vaxfectin® adjuvanted gag DNA over a rest period of 1.2 y, we found that these Ab titers declined by ~0.6–1.2 logs (Fig. 3B). After an additional vaccination (V4), the Gag Ab titers rapidly increased in all animals (~1.5–2 logs), reaching overall similar levels as measured after V3. The animals were monitored for another 1.8 y during which the Gag antibody titers declined by ~1.2 to 1.8 logs which occurred during the first 60 weeks of follow-up. Measurements during these two long-term follow-up periods (V3 to V4; of 1.2 y and V4 to V8 of 1.8 y) demonstrated that this vaccine regimen induced high and long-lasting Gag-specific humoral responses. Thus, the Vaxfectin® adjuvanted SIV DNA delivery represents a potent vaccine approach.
We also measured Gag-specific cellular responses during the initial phase of the vaccine study by intracellular cytokine staining followed by flow cytometry. The animals developed cellular immune responses reaching ~0.02% of IFN-γ producing T cells (Fig. 3D). These Gag-specific T-cell responses are lower (~10–50x) than those previously reported using IM/EP DNA delivery7,28 but are comparable to those obtained in macaques vaccinated upon IM DNA delivery using needle/syringe.22 We noted that these responses were barely detectable after subsequent IM delivery of the Vaxfectin® adjuvanted DNA using needle/syringe vaccinations, pointing to a difference among the vaccine delivery systems (Biojector vs. needle/syringe).
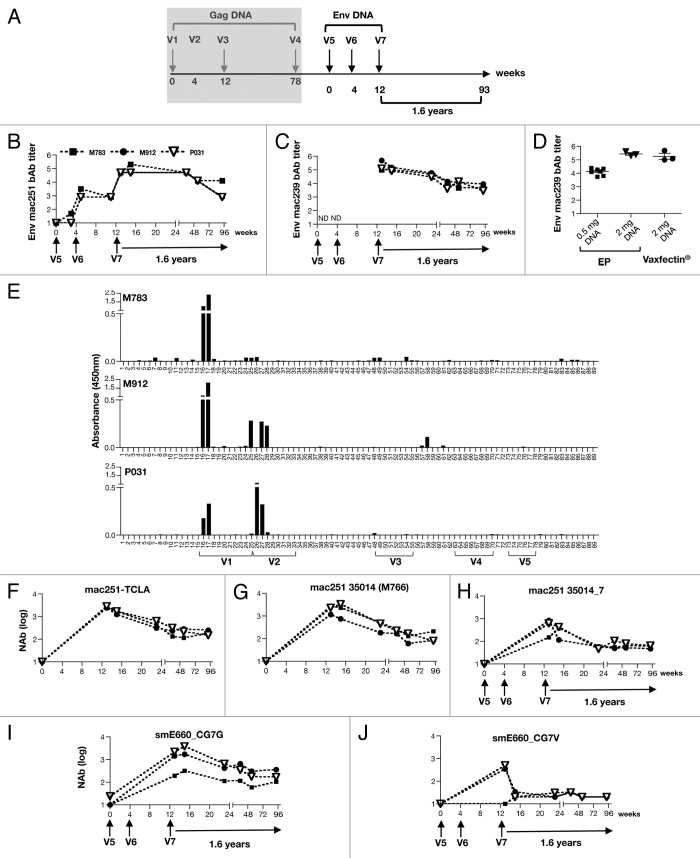
Vaxfectin® adjuvanted env DNA induces long-lasting antibody responses with cross-reactivity against tier 1A-like and tier 1B-like Env
Subsequently, the animals received 3 vaccinations with SIV env DNAs (V5-V7), followed by a rest period of 1.6 y (Fig. 4A). SIVmac251 bAb were measured after each immunization (Fig. 4B) and reached peak levels after the 3rd vaccination (V7) of ~4.7–5.3 logs. Similarly, robust bAb titers (~5–6 log) to SIVmac239 were obtained (Fig. 4C). We further compared the SIVmac239 Env bAb titers at 2 weeks post 3rd env DNA vaccination to those obtained from macaques vaccinated via IM/EP with 0.5 mg of SIVmac239 DNA (n = 8) or with 2 mg (n = 3) of the same env DNAs (ref. 28 and unpublished data). We found overall similar titers elicited by the Vaxfectin® adjuvanted env DNA (Fig. 4D). Thus, Vaxfectin® adjuvanted DNA induced robust humoral responses. We also monitored the durability of the humoral responses. The Env bAb titers declined by ~1 log during a the first year of follow-up (~0.6 log for mac251 and ~1.3–1.7 log for mac239) and then persisted during the remaining follow-up period. These data demonstrated that Vaxfectin® adjuvanted SIV env DNA induced robust and long-lasting Env antibody responses.
Figure 4. Vaccination of macaques with Vaxfectin® adjuvanted SIV env DNAs induces high sustained binding and neutralizing antibody titers. (A) Outline of the Vaxfectin® adjuvanted SIV env DNAs vaccination in macaques. (B, C) Reciprocal endpoint binding Ab titers to SIVmac251 (B) and SIVmac239 (C) Envs are shown in log. (D) Comparison of peak bAb responses (2 weeks after 3 SIV env DNA vaccinations) to SIVmac239 Env obtained upon Vaxfectin® adjuvanted env DNA delivery and IM/EP env DNA delivery (0.5 or 2 mg env DNA; reported previously28 and our unpublished observation). Mean and SEM are given. (E) Analysis of linear peptide responses at 2 weeks post V7 from the plasma samples of the vaccinated macaques using 20-mer overlapping by 14 AA. (F-L) Neutralizing antibody titers against SIVmac251-TCLA measured in M7-Luc cells (F). Neutralizing antibody titers against pseudotyped viruses having the following Env: transmitted SIVmac251 Env 35014 (M766) (G) and 35014_7 (H), the heterologous SIVsmE660 Env CG7G (I) and CG7V (J) were measured using the TZM-bl assay. Arrows indicate env DNA vaccinations (V5-V7). Neutralization titers shown are the log of the reciprocal dilution of sample that reduced the signal by 50% compared with virus in the absence of sample.
We also performed peptide scan analysis to further understand the specificity of antibody responses to linear peptides covering gp120 of SIVmac251. We found that Ab recognized linear epitopes lie uniquely within the V1 and V2 regions of gp120 (Fig. 4E). Since the recognition of the V1/V2 region correlated with protection from infection42-44 in the RV144 Thai trial, these data suggest that Vaxfectin® adjuvanted DNA induced responses may be useful in protection against infection.
The SIVmac239 bAb were further tested for their avidity and showed an avidity index with a range of 10–15% at one month and 4–18% at three month after the 3rd vaccination (V7) (Table 1). For one of the animals (macaque M912), the avidity index remained at similar level during the 80-week of follow-up, whereas the other animals showed persistence over 3–6 mo. We previously reported median peak avidity index of 33–39% obtained upon vaccination with unadjuvanted and IL-12 adjuvanted SIVmac239 env DNA, respectively, upon in vivo IM/EP28 which persisted over 7 mo of follow-up. The avidity index of non-coding DNA injected animals or pre-samples were below detection limit (assigned to 0.1%). DNA vaccination was able to induce bAb with good avidity that is stable over several months.
Table 1. Avidity of SIVmac239 Env binding Ab.
| Avidity measurements after Env DNA vaccination (V7) | ||||||
|---|---|---|---|---|---|---|
| V7wk2 | V7wk4 | V7wk12 | V7wk25 | V7wk41 | V7wk80 | |
| M783 | 2.6 | 15.1 | 18.0 | 30.3 | ND | ND |
| M912 | 3.1 | 9.6 | 12.6 | 25.5 | 28.0 | 28.7 |
| P031 | 15.1 | 11.6 | 3.5 | ND | ND | ND |
ND, not determined
We further examined the neutralizing activity of the vaccine-induced antibodies against a panel of SIV variants including the homologous T cell line-adapted (TCLA) SIVmac251 (Fig. 4F), and two SIVmac251 Env isolated early after transmission: 35014 (M766) (Fig. 4G) and 35014_7 (Fig. 4H). Plasma samples from different time points (week 2, 4, 12, 25, 41 and 93) after the 3rd env DNA vaccination (V7) were compared. NAb to TCLA-SIVmac251 showed a median peak titer of ~3.5 log. Similarly, robust levels of NAb were generated against the two transmitted SIVmac251 Env with median peak titers of 3.5 and 3 logs, respectively. Despite the high antibody titers induced by the Vaxfectin® adjuvanted DNA vaccine, no neutralizing activity against the two very difficult to neutralize Env SIVmac239 and mac251_1562 was detected in plasma samples from any of the immunized macaques.
We also analyzed the plasma samples for their ability to neutralize the heterologous SIVsmE660, a virus that differs by ~20% from the SIVmac251 Envs mimicking the heterogeneity found among the HIV-1 clades. We found that the antibodies were able to neutralize the tier 1A-like SIVsmE660_CG7G (Fig. 4I). In addition, the plasma from two of the animals (M912 and P031) were able to neutralize a tier 1B-like SIVsmE660_CG7V, and these antibodies persisted during the 1.6 y of follow-up, albeit at low levels (Fig. 4J). Thus, the Vaxfectin® formulated DNA vaccine induced both robust and durable binding and neutralizing antibodies in macaques.
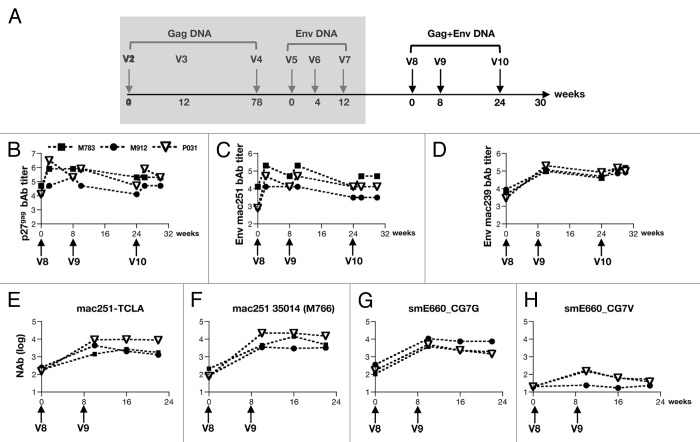
Simultaneous vaccination with Vaxfectin® adjuvanted gag and env DNAs
After sequential immunization with gag and env SIV DNA, the animals had a rest period of 1.6 y (after V7, the last env DNA vaccination) and, subsequently, the animals were immunized with the same gag and env DNAs administered simultaneously at different sites (V8-V10) (Fig. 5A). Development of Gag and Env bAb was monitored after each of the three immunizations. We noted robust increases of both Gag (Fig. 5B) and Env bAb (Fig. 5C and 5D), reaching peak levels similar to those induced by each plasmid administered individually (see Figures 2 and 3). We found similar patterns of linear epitope recognition focusing primarily on V1 and V2 (data not shown). Animal M783 had significant increase in linear peptide recognition throughout gp120. Similarly, we found a rapid increase in NAb to levels similar to those obtained after vaccination with Env DNAs only. The antibodies potently neutralized TCLA-mac251 (Fig. 5E) and the transmitted SIVmac251 35014 (M766) (Fig. 5F) as well as the heterologous tier 1A-like SIVsmE660_CG7G (Fig. 5G) and, to a lesser extent the tier 1B-like SIVsmE660_CG7V (Fig. 5H) Env containing pseudotyped viruses. These data demonstrate that simultaneous vaccination with both SIV gag and env DNAs did not interfere with the development of either binding or neutralizing antibodies.
Figure 5. Simultaneous vaccinations with Vaxfectin® adjuvanted SIV gag and env DNAs. (A) Outline of the DNA vaccination. (B-D) Reciprocal endpoint binding antibody titers for p27gag (B), SIVmac251 Env (C) and SIVmac239 Env (D) are shown in log. (E-H) Reciprocal endpoint neutralizing antibodies titers against the T cell line-adapted SIVmac251-TCLA measured in M7-Luc cells (E) and against the transmitted SIVmac251 clone 35014 (M766) (F) and heterologous SIVsmE660 Env CG7G (G) and CG7V (H) using the TZM-bl assay are shown in log. Arrows indicate env DNA vaccinations. Neutralization titers shown are the log of the reciprocal dilution of sample that reduced the signal by 50% compared with virus in the absence of sample.
Vaxfectin® adjuvanted env DNA vaccination elicits durable mucosal immune responses
Mucosal dissemination of humoral immune responses is critical for an HIV/SIV vaccine, since rectal/vaginal mucosa is the route of natural infection. To identify whether the humoral responses induced by the Vaxfectin® adjuvanted env DNA vaccination are able to disseminate to mucosal tissues, we investigated the presence of Env-specific IgG in the saliva (Fig. 6A, B, and D). This analysis revealed that all animals showed responses not only to SIVmac251 (Fig. 6A, left panel), but also to the transmitted SIVmac_M766 (35014) Env (Fig. 6A, middle panel) and to the heterologous E660 CG7V (Fig. 6A, right panel). Remarkably, the antibodies persisted in the saliva during the 17 mo of follow-up (8 weeks post V7 to V8) (Fig. 6B). We further tested rectal fluids collected from rectal washes and found that 2 of the macaques (M783 and P031) showed detectable Env-specific responses to the transmitted SIVmac_M766, even at 8 weeks post V7 (Fig. 6C).
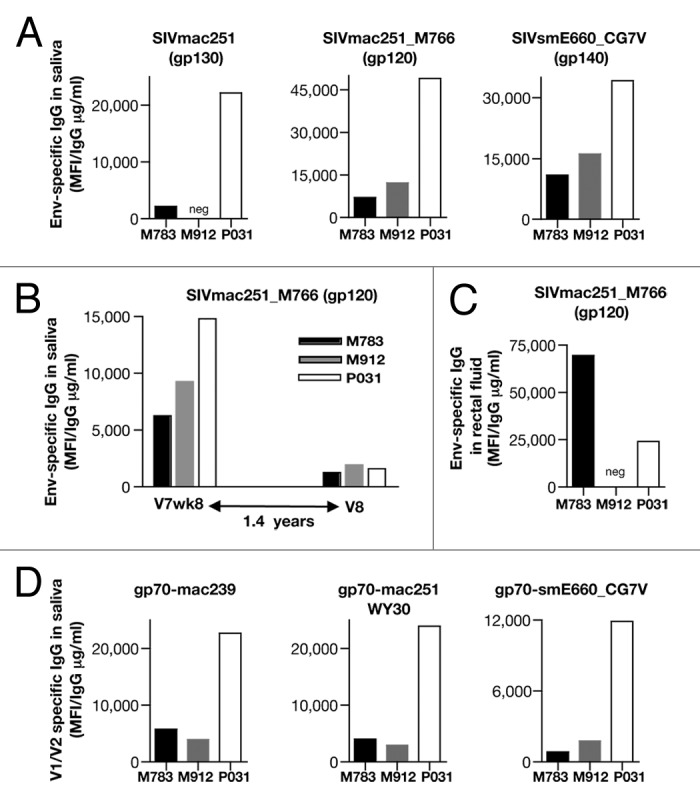
Figure 6. Vaxfectin® adjuvanted SIV env DNA vaccination induced durable mucosal immune responses. (A) Env-specific IgG against a panel of Env (SIVmac251, the transmitted SIVmac M766 and the heterologous SIVsmE660 CG7V were measured at 2 weeks post V10. (B) Durability of the responses against the transmitted SIVmac M766 are shown between V7wk8 and V8, spanning 1.4 y. (C) SIVmac251_M766Env-specific IgG were measured in rectal washes 8 weeks post V7. (D) V1/V2-specific responses were measured using a panel of scaffolded-V1/V2 gp70 proteins at 2 weeks post V10.
The recent RV144 trial conducted in Thailand1 revealed a critical role of humoral responses in preventing infection and anti-Env bAb, especially IgG antibodies against the V1/V2 region correlated with protection from infection.42-44 For this reason, we investigated whether Vaxfectin® adjuvanted env DNA vaccination could induce mucosal responses targeting this Env region. This analysis demonstrated that all 3 macaques showed responses to the scaffolded-V1/V2 gp60 recombinant protein containing SIVmac251, mac239 as well as the heterologous SIVsm E660_CG7V V1-V2 regions (Fig. 6D). Thus, the Vaxfectin® adjuvanted env DNA vaccination is able to induce potent systemic as well as mucosal humoral responses with impressive durability.
Vaccinated macaques control SIVmac251 challenge
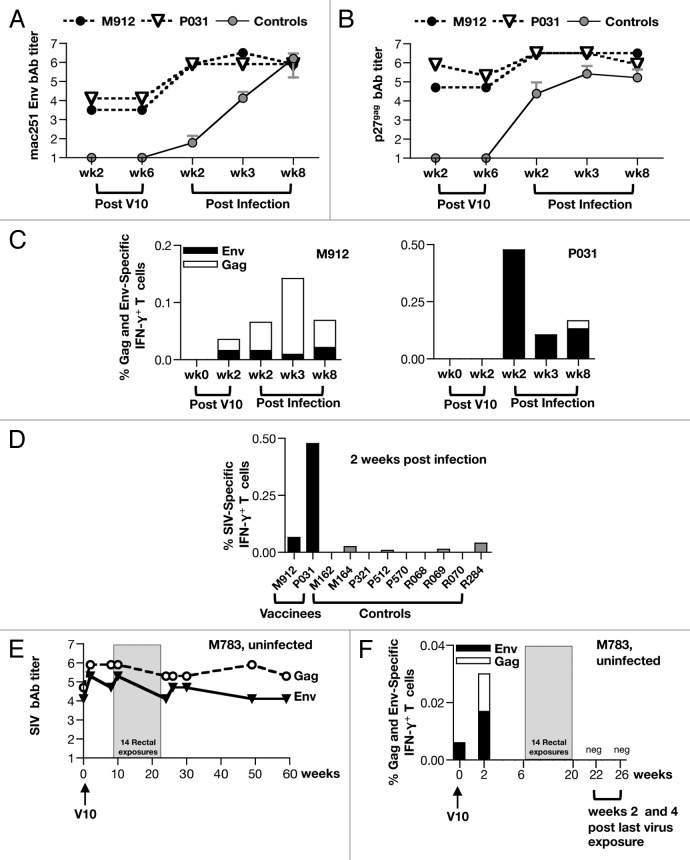
Six weeks after receiving the 3rd booster vaccination (V10) with the combined gag and env DNAs, the immunized macaques, together with 10 naïve animals, were challenged intrarectally by weekly low dose exposures using a well-characterized highly pathogenic SIVmac251 viral stock. The control animals became infected with SIVmac251 at an average rate of ~30%/exposure during the first 3 exposures (Fig. 7A). Because exposures 4 to 7 did not result in any additional new infection and 3 control animals and 2 of the 3 vaccinees remained uninfected at this point, the challenge dose was increased (from dilution 1:500 to 1:200) for subsequent exposures. After 14 exposures, one of the control animals and one vaccinee (M783) remained uninfected. Of the immunized macaques, P031 got infected at the 1st exposure and M918 resisted infection until the 13th exposure. Although the number of animals in the vaccine group did not allow for statistical analysis of virus acquisition, this study suggests a trend in delay from virus acquisition.

Figure 7. Control of viremia upon rectal challenge with low dose SIVmac251. (A) Kaplan-Meier curve shows the number of challenges to infection for the vaccine group (n = 3) and the unvaccinated control group (n = 10). (B) Viral load measurements (RNA copies/ml plasma) for the vaccinees and controls over the 24 weeks of follow-up. (C, D) Comparison of the viral loads at peak (C) and in the chronic phase of infection (D) (AUC; from weeks 6–24) of the infected vaccinees (n = 2) and the controls (n = 9). P values (unpaired t test) are shown.
The virus loads of the animals were monitored over 24 weeks of follow-up (Fig. 7B). The controls showed high peak (median ~log 8 RNA copies/ml plasma) and chronic viremia (range 5.5 to 6.6 log RNA copies/ml plasma). Analysis of the virus loads revealed potent control of viremia by the infected vaccinees with significant differences in peak VL (p = 0.0265) (Fig. 7C) and significant reduction in viremia during the chronic phase (p = 0.0242; Figure 7D), as measured by area under the curve (AUC), between weeks 6–24 post infection compared with control animals. Together our results show that immune responses elicited upon vaccination with the Vaxfectin® adjuvanted SIV DNAs are long-lasting and efficiently contribute to control infection with the highly pathogenic SIVmac251.
We also measured changes in the antibody responses to Env and Gag as a result of SIVmac251 infection (Fig. 8). We observed anamnestic responses in both infected macaques peaking at 2 weeks post-infection with Env bAb titers ~6–6.5 log (Fig. 8A) and Gag bAb titers ~log 6.5 (Fig. 8B). In contrast, the antibody responses among the naïve controls peaked at later time points (week 3 and 8 postinfection for Gag and Env, respectively, Figure 8A and B). Of note, macaque M783, which resisted infection, showed the highest ab titers prior to challenge (Fig. 8E). Env and Gag antibody titers were also monitored in this uninfected animal after the challenge period. Macaque M783 maintained durable Gag and Env humoral responses during the 1 y of follow-up, and we did not observe any increase in neither Gag nor Env antibody titers as a result of repeated virus exposures (Fig. 8E). The lack of anamnestic responses, together with repeated negative plasma viral load measurements (Fig. 7B), confirmed that macaque M783 was uninfected, possibly as a result of the protection conferred by the vaccine-induced immunity.
Figure 8. Anamnestic humoral and cellular immune responses measured upon SIVmac251 infection. (A, B) SIV Env (A) and Gag (B) reciprocal endpoint bAb titers were measured after infection from the 2 infected vaccines (shown in log). The titers (mean+/−SEM) of the 9 infected control animals are shown. (C) Frequency of Env and Gag specific IFN-γ+ T cells is shown after V10 and post infection (weeks 2, 3, and 8) for the 2 infected macaques. (D) Comparison of the frequency of SIV-specific (Env and gag) IFN-γ+ T cells of the 2 infected vaccinees and 9 control animals at 2 weeks post-infection. (E) Reciprocal Env and Gag Env endpoint titers overtime in the uninfected macaque M783 (in log). The antibody titers are shown for the period after V10 including the period of 14 viral exposures up to week 60 weeks after V10. (F) Frequency of Env- and Gag-specific IFN-γ+ T cells of the uninfected vaccine (M783) are shown after V10 and during the 26 weeks of follow-up that included the period of the 14 virus exposures. The measurements at 2 and 6 weeks after the 14th virus exposure (week 22 and 26 post V10) are shown.
In addition, we also monitored cellular immune responses to Gag and Env after challenge. We observed anamnestic responses in both infected macaques, M912 and P031 (Fig. 8C). A comparison of the cellular immune responses of the vaccinees with those of non-vaccinated infected controls was performed at 2 weeks post-infection (Fig. 8D). We found higher frequency of SIV-specific responses in the 2 infected vaccinees compared with the 9 controls, supporting induction of recall responses in the vaccinees. Macaque M783, which resisted 14 challenges, showed detectable low cellular immune responses prior to challenge (V10wk2; V10wk6) (Fig. 8F). Although we measured cellular responses at 2 and 6 weeks after the last vaccination, we were unable to detect responses after the 14th virus exposure, in support of the lack of infection of this animal. Of note, these time points correspond to 22 and 26 weeks after V10 and this data further showed that the low responses after vaccination V10 were not maintained to detectable levels for the 6 mo of follow-up. Despite this, macaque M783 maintained stable humoral responses (Fig. 8E).
Discussion
DNA intramuscular vaccination is able to induce potent cellular immunity but lower humoral responses compared with other methods. One approach to overcome this limitation has been to use DNA as prime in combination with protein or recombinant viral vector boost or use alternative DNA delivery methods and adjuvant combinations. Improvements in DNA vaccine immunogenicity have been achieved upon inclusion of cytokine DNA as adjuvant (e.g., IL-1228,45-49), and using the IM/EP delivery method. Long-lasting immune responses (~1 y for Env; 2 y for Gag responses) have been reported after vaccination with IM/EP delivered DNA. 26,50 As an alternative method, we have performed a pilot study testing the efficacy of Vaxfectin® to deliver our optimized SIV DNA vaccine to 3 macaques via the IM route. The vaccination schedule spanning ~4 y allowed us to monitor the development and durability of the Gag and Env antibody responses upon sequential and simultaneous DNA vaccinations. This vaccine regimen induced very high binding and neutralizing antibody titers. Importantly, the induced bAb showed cross-reactivity against tier 1A-like and 1B-like viruses and are able to cross-neutralize heterologous SIVsmE660 Env, which differs by ~20% from the SIVmac251 Env sequences, similar to the diversity among HIV clades.
We were also able to monitor the persistence of humoral immune responses for extended periods of time (1.8 y for Gag; 1.2 y for Env) and report remarkable durability of the bAb responses, which showed a decline of only ~1 log during the 1st year of follow-up. DNA-only vaccination (using up to 3 mg each of gag and env DNA) by needle and syringe has not been able to induce immune responses of this magnitude, breadth, and longevity (ref. 22 and our unpublished observation).
In addition to systemic humoral responses, we also detected mucosal Env-specific IgG both in saliva as well as in rectal fluid. Importantly, these responses also showed potent durability and could be detected in 2 of the 3 macaques at 1.6 y after vaccination. These are promising findings since the ability of induce long-lasting immunity has been a major challenge in the field of HIV/SIV vaccine. The limited efficacy of the RV144 trial1 has been attributed to the fact that the vaccine-induced responses waned over time, suggesting that improved vaccine designs are needed to achieve long-lasting cross-clade specific immune responses able to prevent infection.
Interestingly, we found that Vaxfectin® adjuvanted DNA induced antibodies recognize both linear peptides covering V1 and V2 of gp120 (using plasma samples) as well as scaffolded gp70-V1/V2 (saliva samples). Since IgG antibodies against the V1/V2 region correlated with protection from infection42-44 in the Thai trial, our findings suggest the immunization with this method may contribute to the generation of protective Ab.
Importantly, vaccination with Vaxfectin® adjuvanted DNA induced immune responses that confer protection upon SIVmac251 challenge. One vaccinated animal remained uninfected after 14 exposures and the other two macaques showed significant reduction of both acute and chronic viremia upon infection with the highly pathogenic SIVmac251. Thus, in this report, we show that immune responses generated upon vaccination with the SIV DNA adjuvanted with Vaxfectin® are long-lasting, broad, and able to control SIVmac251 viremia. These promising results warrant future expansion of this pilot study. The Vaxfectin® platform has been reported to enhance immunogenicity and effectiveness of several vaccine candidates.34-37 Our data expand these reports and suggest Vaxfectin® as a promising HIV DNA vaccine adjuvant.
Material and Methods
Plasmids
All plasmids were expression-optimized and contain RNA/codon optimized gag and env coding sequences51-54 cloned in the CMVkan mammalian expression vector.29,30,52,55 SIVmac239 p39gag is expressed as fusion to MCP3 (plasmid 209S).29,30 Four plasmids expressing different SIVmac gp160 Envs were used in these experiments: SIVmac239 (plasmid 99S), transmitted SIVmac251 Env sequences (detailed elsewhere56): mac251_15 (plasmid 217S; GenBank accession #KF003436), 35014_7 (plasmid 220S; GenBank accession #KF003434) and 35014 (plasmid 221S; GenBank accession #KF003433J; also referred to as M766, GenBank accession #JQ086004). Highly purified endotoxin-free DNAs were prepared (Vical).
Mouse immunogenicity
BALB/c mice (6 to 8 weeks old) were obtained from Charles River Laboratories, Inc. and housed at the National Cancer Institute in a temperature-controlled, light-cycled facility. Mice were immunized with 100 µg of SIV gag plasmid DNA adjuvanted in Vaxfectin® or in PBS. Mice were immunized via intramuscular route using needle and syringe at week 0 and week 4 and were sacrificed 2 weeks post 2nd immunization. Spleen and plasma were recovered and analyzed for immune responses. Cellular immune responses were evaluated by IFN-γ ELISPOT assay30 after incubation of splenocytes with an SIV gag peptide pool (15-mer peptides overlapping by 11 amino acids (Infinity Inc. Biotech Research and Resource) at a final concentration of 1 µg/ml of each peptide. The spots were counted on a C.T.L. ELISPOT reader (Cellular Technology Ltd., BD Biosciences) and analyzed using ImmunoSpot software version 2.06. The cut-off was defined as the average spots obtained in the negative control wells (triplicates) plus 2 standard deviations. Gag-specific spots were calculated by subtracting the cut-off value and adjusted to the number of spot-forming cells per million splenocytes. End-point antibody titers to p27gag were measured by ELISA (Advanced BioScience Laboratory, Inc.).
Macaque vaccination and challenge
This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Rhesus macaques were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International at the Advanced BioScience Laboratories Inc., and were approved by the Institutional Animal Care and Use Committee (OLAW assurance number A3467–01 and USDA Certificate number 51-R-0059). Indian rhesus macaques were sequentially vaccinated with SIV gag DNA (4 times), followed by SIV env DNA (3 times), followed by simultaneous vaccination with the combination of SIV gag and env DNAs (3 times). The DNA was formulated with Vaxfectin®34, 36 and was administered intramuscularly into arms and legs using the needle-free Biojector 2000 injection system (V1–3) and subsequently using needle and syringe (V4-V10). Gag DNA (1 mg) was delivered at 2 separate sites (500 ml/site). Four different env DNAs (0.5 mg each) were separately formulated with Vaxfectin® and injected at different sites (500 ml/site; arms and legs). For the combination vaccine, 1 mg gag DNA and 2 mg of the same 4 env DNAs were injected into 6 sites (2 sites for gag DNA and 4 sites for env DNA). Historical data from macaques (ref. 28 and our unpublished data) vaccinated with gag and env DNA using IM/EP route are added to some figures. Blood, saliva and rectal washes were collected at the indicated time points.
The macaques vaccinated with the Vaxfectin® formulated SIV DNAs were challenged intrarectally using a titrated low dose SIVmac251 (1 ml of a 1:500 dilution of SIVmac251–2010; harvested at day 9; R. Desrosiers). A highly related stock, harvested 1 d earlier has been described as 251-RD (SIVmac251–7/9/2010).57 After 7 exposures the dose of challenge virus was increased (dilution 1:200) for another 7 exposures. Macaques were monitored for 24 weeks post-infection.
Humoral and cellular immune responses
Endpoint binding antibody titers to SIV Gag and SIVmac251 Env, were determined by ELISA (Advanced BioScience Laboratories, Inc.). Endpoint titers were considered positive when OD450 was greater than the mean +3SD of normal mouse or monkey plasma. Antibody titers to SIVmac239 and antibody avidity upon treatment with 1.5 M sodium thiocyanate (NaSCN; Sigma-Aldrich) were measured as described.58,59 The avidity index (%) was calculated by taking the ratio of the NaSCN-treated plasma dilution giving an absorbance of 0.5 to the TBS-treated plasma dilution giving an OD of 0.5 and multiplying by 100. Peptide scan analysis was performed using 20-mer peptides overlapping by 14 AA derived from SIVmac251. Neutralizing antibody titers were determined using the M7-luc assay for the TCLA-SIVmac251/H9 and the TZM-bl assay for the transmitted SIVmac251 Env 35014_7, 35014 (M766), and the heterologous SIVsmE660 Env proteins CG7G and CG7V.60 Cellular immune responses were measured from live-frozen PBMCs stimulated with Gag peptide pools (15-mer overlapping by 11 AA at a final concentration of 1 µg/ml). Immunostaining and flow cytometric analysis was performed as described.7,26,29 These data were internally controlled because every sample was run in the absence and in the presence of the Gag-specific peptide pool. Only peptide-stimulated samples giving a frequency of IFN-γ+ T cells at least 2-fold higher than the negative controls (same samples without peptide stimulation) were considered positive. The background in the absence of peptides was subtracted from the peptide-stimulated samples.
Analysis of mucosal tissues
SIV-specific IgG in saliva and rectal washes were determined by custom SIV multiplex assay as previously described.7,61,62 Mucosal specimens were filtered and concentrated to equal volumes before measurement of total and specific antibody. Specific activity was calculated by MFI (linear range of standard curve)/µg/ml total macaque IgG. Antibodies against native V1/V2 epitopes were quantitated by binding assays against native SIV V1/V2 antigens expressed as gp70- fusion proteins related to the CaseA2 antigen used in the RV144 correlate study.44 These proteins contained the glycosylated, disulfide-bonded V1/V2 regions of SIVmac239, SIVmac251 and SIVSME660 (corresponding to AA 120–204 of HXB2 Env), fused to residue 263 of the SU (gp70) protein of Fr-MuLV.
Viral Load
Viral loads were determined using the NASBA assay63 with a threshold of detection of 50 copies/ml (Advanced BioScience Laboratories).
Statistical Analysis
All statistical analyses were performed using GraphPad Software, Inc. (version 4.0).
Acknowledgments
We thank A. Rolland for discussions, D. Weiss, J. Treece, I. Kalisz, M. Ferrari, R. Pal and staff at Advanced Bioscience Laboratory for excellent support, W. Huang, R.G. Overman, V. Ashley, J. Lucas, and Y. Lin for contributions to humoral immunity studies, R. Desrosiers and NIAID for providing the SIVmac251 challenge stock, and T. Jones for editorial assistance.
Glossary
Abbreviations:
- EP
electroporation
- MCP-3
monocyte chemoattractant protein 3
- PMA
phorbol myristate acetate
- TCLA
T cell line-adapted
Disclosure of Potential Conflicts of Interest
GNP and BKF are inventors on US Government-owned patents and patent applications related to DNA vaccines and gene expression optimization that have been licensed to several companies. There are no further patents, products in development or marketed products to declare. SES is employed by Vical Pharmaceuticals, Inc. as such receive salary, and bonuses and stock options as compensation.
BKF, GNP, AV: designed, coordinated the study, analyzed the data, and wrote the paper. VK, MR, RJ, CA: performed experiments and analyzed the data. LY, YG: performed binding Ab and avidity assays. XS, GDT, CL, DCM: performed mucosal binding Ab assays and NAb assays. CI, RP, AP: designed, generated and purified the recombinant gp70. SES: provided Vaxfectin® adjuvant and high quality endotoxin free DNA.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25442
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Lai L, Kwa S, Kozlowski PA, Montefiori DC, Ferrari G, Johnson WE, et al. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J Infect Dis. 2011;204:164–73. doi: 10.1093/infdis/jir199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flatz L, Cheng C, Wang L, Foulds KE, Ko SY, Kong WP, et al. Gene-based vaccination with a mismatched envelope protects against simian immunodeficiency virus infection in nonhuman primates. J Virol. 2012;86:7760–70. doi: 10.1128/JVI.00599-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, et al. Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Sci Transl Med. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai L, Kwa SF, Kozlowski PA, Montefiori DC, Nolen TL, Hudgens MG, et al. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine. 2012;30:1737–45. doi: 10.1016/j.vaccine.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel V, Jalah R, Kulkarni V, Valentin A, Rosati M, Alicea C, et al. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous simian immunodeficiency virus challenge. Proc Natl Acad Sci U S A. 2013;110:2975–80. doi: 10.1073/pnas.1215393110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winstone N, Wilson AJ, Morrow G, Boggiano C, Chiuchiolo MJ, Lopez M, et al. Enhanced control of pathogenic Simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen. J Virol. 2011;85:9578–87. doi: 10.1128/JVI.05060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascola JR, Lewis MG, VanCott TC, Stiegler G, Katinger H, Seaman M, et al. Cellular immunity elicited by human immunodeficiency virus type 1/ simian immunodeficiency virus DNA vaccination does not augment the sterile protection afforded by passive infusion of neutralizing antibodies. J Virol. 2003;77:10348–56. doi: 10.1128/JVI.77.19.10348-10356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch DH, Santra S, Schmitz JE, Kuroda MJ, Fu TM, Wagner W, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–92. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 12.Barouch DH, Kunstman J, Glowczwskie J, Kunstman KJ, Egan MA, Peyerl FW, et al. Viral escape from dominant simian immunodeficiency virus epitope-specific cytotoxic T lymphocytes in DNA-vaccinated rhesus monkeys. J Virol. 2003;77:7367–75. doi: 10.1128/JVI.77.13.7367-7375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barouch DH, Fu TM, Montefiori DC, Lewis MG, Shiver JW, Letvin NL. Vaccine-elicited immune responses prevent clinical AIDS in SHIV(89.6P)-infected rhesus monkeys. Immunol Lett. 2001;79:57–61. doi: 10.1016/S0165-2478(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 14.Chong SY, Egan MA, Kutzler MA, Megati S, Masood A, Roopchard V, et al. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques. Vaccine. 2007;25:4967–82. doi: 10.1016/j.vaccine.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 15.Egan MA, Charini WA, Kuroda MJ, Schmitz JE, Racz P, Tenner-Racz K, et al. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J Virol. 2000;74:7485–95. doi: 10.1128/JVI.74.16.7485-7495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belisle SE, Yin J, Shedlock DJ, Dai A, Yan J, Hirao L, et al. Long-term programming of antigen-specific immunity from gene expression signatures in the PBMC of rhesus macaques immunized with an SIV DNA vaccine. PLoS One. 2011;6:e19681. doi: 10.1371/journal.pone.0019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyer JD, Maciag PC, Parkinson R, Wu L, Lewis MG, Weiner DB, et al. Rhesus macaques with high levels of vaccine induced IFN-gamma producing cells better control viral set-point following challenge with SIV239. Vaccine. 2006;24:4498–502. doi: 10.1016/j.vaccine.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Boyer JD, Robinson TM, Kutzler MA, Vansant G, Hokey DA, Kumar S, et al. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proc Natl Acad Sci U S A. 2007;104:18648–53. doi: 10.1073/pnas.0709198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haigwood NL, Pierce CC, Robertson MN, Watson AJ, Montefiori DC, Rabin M, et al. Protection from pathogenic SIV challenge using multigenic DNA vaccines. Immunol Lett. 1999;66:183–8. doi: 10.1016/S0165-2478(98)00156-4. [DOI] [PubMed] [Google Scholar]
- 20.Muthumani K, Bagarazzi M, Conway D, Hwang DS, Manson K, Ciccarelli R, et al. A Gag-Pol/Env-Rev SIV239 DNA vaccine improves CD4 counts, and reduce viral loads after pathogenic intrarectal SIV(mac)251 challenge in rhesus Macaques. Vaccine. 2003;21:629–37. doi: 10.1016/S0264-410X(02)00571-6. [DOI] [PubMed] [Google Scholar]
- 21.Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, et al. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008;26:5223–9. doi: 10.1016/j.vaccine.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M, et al. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J Virol. 2005;79:8480–92. doi: 10.1128/JVI.79.13.8480-8492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin J, Dai A, Lecureux J, Arango T, Kutzler MA, Yan J, et al. High antibody and cellular responses induced to HIV-1 clade C envelope following DNA vaccines delivered by electroporation. Vaccine. 2011;29:6763–70. doi: 10.1016/j.vaccine.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23:421–9. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutnick NA, Myles DJ, Bian CB, Muthumani K, Weiner DB. Selected approaches for increasing HIV DNA vaccine immunogenicity in vivo. Curr Opin Virol. 2011;1:233–40. doi: 10.1016/j.coviro.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel V, Valentin A, Kulkarni V, Rosati M, Bergamaschi C, Jalah R, et al. Long-lasting humoral and cellular immune responses and mucosal dissemination after intramuscular DNA immunization. Vaccine. 2010;28:4827–36. doi: 10.1016/j.vaccine.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand V, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol. 2007;81:5257–69. doi: 10.1128/JVI.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalah R, Patel V, Kulkarni V, Rosati M, Alicea C, Ganneru B, et al. IL-12 DNA as molecular vaccine adjuvant increases the cytotoxic T cell responses and breadth of humoral immune responses in SIV DNA vaccinated macaques. Hum Vaccin Immunother. 2012;8:1620–9. doi: 10.4161/hv.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosati M, Bergamaschi C, Valentin A, Kulkarni V, Jalah R, Alicea C, et al. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci U S A. 2009;106:15831–6. doi: 10.1073/pnas.0902628106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni V, Jalah R, Ganneru B, Bergamaschi C, Alicea C, von Gegerfelt A, et al. Comparison of immune responses generated by optimized DNA vaccination against SIV antigens in mice and macaques. Vaccine. 2011;29:6742–54. doi: 10.1016/j.vaccine.2010.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox KS, Clair JH, Prokop MT, Sykes KJ, Dubey SA, Shiver JW, et al. DNA gag/adenovirus type 5 (Ad5) gag and Ad5 gag/Ad5 gag vaccines induce distinct T-cell response profiles. J Virol. 2008;82:8161–71. doi: 10.1128/JVI.00620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalams SA, Parker S, Jin X, Elizaga M, Metch B, Wang M, et al. NIAID HIV Vaccine Trials Network Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One. 2012;7:e29231. doi: 10.1371/journal.pone.0029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan SM, Doukas J, Hartikka J, Smith L, Rolland A. Vaxfectin: a versatile adjuvant for plasmid DNA- and protein-based vaccines. Expert Opin Drug Deliv. 2010;7:1433–46. doi: 10.1517/17425247.2010.538047. [DOI] [PubMed] [Google Scholar]
- 34.Hartikka J, Bozoukova V, Ferrari M, Sukhu L, Enas J, Sawdey M, et al. Vaxfectin enhances the humoral immune response to plasmid DNA-encoded antigens. Vaccine. 2001;19:1911–23. doi: 10.1016/S0264-410X(00)00445-X. [DOI] [PubMed] [Google Scholar]
- 35.Locher CP, Witt SA, Ashlock BM, Levy JA. Evaluation of genetic immunization adjuvants to improve the effectiveness of a human immunodeficiency virus type 2 (HIV-2) envelope DNA vaccine. DNA Cell Biol. 2004;23:107–10. doi: 10.1089/104454904322759911. [DOI] [PubMed] [Google Scholar]
- 36.Reyes L, Hartikka J, Bozoukova V, Sukhu L, Nishioka W, Singh G, et al. Vaxfectin enhances antigen specific antibody titers and maintains Th1 type immune responses to plasmid DNA immunization. Vaccine. 2001;19:3778–86. doi: 10.1016/S0264-410X(01)00090-1. [DOI] [PubMed] [Google Scholar]
- 37.Porter KR, Ewing D, Chen L, Wu SJ, Hayes CG, Ferrari M, et al. Immunogenicity and protective efficacy of a vaxfectin-adjuvanted tetravalent dengue DNA vaccine. Vaccine. 2012;30:336–41. doi: 10.1016/j.vaccine.2011.10.085. [DOI] [PubMed] [Google Scholar]
- 38.Hartikka J, Bozoukova V, Morrow J, Rusalov D, Shlapobersky M, Wei Q, et al. Preclinical evaluation of the immunogenicity and safety of plasmid DNA-based prophylactic vaccines for human cytomegalovirus. Hum Vaccin Immunother. 2012;8:1595–606. doi: 10.4161/hv.21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veselenak RL, Shlapobersky M, Pyles RB, Wei Q, Sullivan SM, Bourne NA. A Vaxfectin(®)-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine. 2012;30:7046–51. doi: 10.1016/j.vaccine.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin WH, Vilalta A, Adams RJ, Rolland A, Sullivan SM, Griffin DE. Vaxfectin adjuvant improves antibody responses of juvenile rhesus macaques to a DNA vaccine encoding the measles virus hemagglutinin and fusion proteins. J Virol. 2013;87:6560–8. doi: 10.1128/JVI.00635-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith LR, Wloch MK, Ye M, Reyes LR, Boutsaboualoy S, Dunne CE, et al. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine. 2010;28:2565–72. doi: 10.1016/j.vaccine.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 42.Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, et al. MOPH TAVEG Collaboration The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses. 2012;28:1444–57. doi: 10.1089/aid.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490:417–20. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, et al. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J Med Primatol. 2005;34:262–70. doi: 10.1111/j.1600-0684.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- 46.Schadeck EB, Sidhu M, Egan MA, Chong SY, Piacente P, Masood A, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine. 2006;24:4677–87. doi: 10.1016/j.vaccine.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 47.Schadeck EB, Sidhu M, Egan MA, Chong SY, Piacente P, Masood A, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine. 2006;24:4677–87. doi: 10.1016/j.vaccine.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 48.Robinson TM, Sidhu MK, Pavlakis GN, Felber BK, Silvera P, Lewis MG, et al. Macaques co-immunized with SIVgag/pol-HIVenv and IL-12 plasmid have increased cellular responses. J Med Primatol. 2007;36:276–84. doi: 10.1111/j.1600-0684.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- 49.Halwani R, Boyer JD, Yassine-Diab B, Haddad EK, Robinson TM, Kumar S, et al. Therapeutic vaccination with simian immunodeficiency virus (SIV)-DNA + IL-12 or IL-15 induces distinct CD8 memory subsets in SIV-infected macaques. J Immunol. 2008;180:7969–79. doi: 10.4049/jimmunol.180.12.7969. [DOI] [PubMed] [Google Scholar]
- 50.Cristillo AD, Weiss D, Hudacik L, Restrepo S, Galmin L, Suschak J, et al. Persistent antibody and T cell responses induced by HIV-1 DNA vaccine delivered by electroporation. Biochem Biophys Res Commun. 2008;366:29–35. doi: 10.1016/j.bbrc.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 51.Nasioulas G, Zolotukhin AS, Tabernero C, Solomin L, Cunningham CP, Pavlakis GN, et al. Elements distinct from human immunodeficiency virus type 1 splice sites are responsible for the Rev dependence of env mRNA. J Virol. 1994;68:2986–93. doi: 10.1128/jvi.68.5.2986-2993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol. 1997;71:4892–903. doi: 10.1128/jvi.71.7.4892-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz S, Campbell M, Nasioulas G, Harrison J, Felber BK, Pavlakis GN. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J Virol. 1992;66:7176–82. doi: 10.1128/jvi.66.12.7176-7182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz S, Felber BK, Pavlakis GN. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol. 1992;66:150–9. doi: 10.1128/jvi.66.1.150-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jalah R, Rosati M, Kulkarni V, Patel V, Bergamaschi C, Valentin A, et al. Efficient systemic expression of bioactive IL-15 in mice upon delivery of optimized DNA expression plasmids. DNA Cell Biol. 2007;26:827–40. doi: 10.1089/dna.2007.0645. [DOI] [PubMed] [Google Scholar]
- 56.Kulkarni V, Rosati M, Bear J, Pilkington GR, Jalah R, Singh AK, et al. Comparison of Intradermal and Intramuscular Delivery of SIV Env DNA by in vivo Electroporation in Macaques. in preparation 2013. [DOI] [PMC free article] [PubMed]
- 57.Del Prete GQ, Scarlotta M, Newman L, Reid C, Parodi LM, Roser JD, et al. Comparative characterization of transfection- and infection-derived simian immunodeficiency virus challenge stocks for in vivo nonhuman primate studies. J Virol. 2013;87:4584–95. doi: 10.1128/JVI.03507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan Y, Sajadi MM, Kamin-Lewis R, Fouts TR, Dimitrov A, Zhang Z, et al. Discordant memory B cell and circulating anti-Env antibody responses in HIV-1 infection. Proc Natl Acad Sci U S A. 2009;106:3952–7. doi: 10.1073/pnas.0813392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore JP, Wallace LA, Follett EA, McKeating JA. An enzyme-linked immunosorbent assay for antibodies to the envelope glycoproteins of divergent strains of HIV-1. AIDS. 1989;3:155–63. doi: 10.1097/00002030-198903000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol 2005; Chapter 12:Unit 12 1. [DOI] [PubMed] [Google Scholar]
- 61.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–63. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolton DL, Song K, Wilson RL, Kozlowski PA, Tomaras GD, Keele BF, et al. Comparison of systemic and mucosal vaccination: impact on intravenous and rectal SIV challenge. Mucosal Immunol. 2012;5:41–52. doi: 10.1038/mi.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romano JW, Shurtliff RN, Dobratz E, Gibson A, Hickman K, Markham PD, et al. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J Virol Methods. 2000;86:61–70. doi: 10.1016/S0166-0934(99)00184-6. [DOI] [PubMed] [Google Scholar]