Abstract
HIV-1 infection induces chronic oxidative stress. The resultant neurotoxicity has been associated with Tat protein. Here, we for the first time describe the induction of oxidative stress by another HIV-1 protein, reverse transcriptase (RT). Expression of HIV-1 RT in human embryonic kidney cells generated potent production of the reactive oxygen species (ROS), detected by the fluorescence-based probes. Quantitative RT-PCR demonstrated that expression of RT in HEK293 cells induced a 10- to 15-fold increased transcription of the phase II detoxifying enzymes human NAD(P)H:quinone oxidoreductase (Nqo1) and heme oxygenase 1 (HO-1), indicating the induction of oxidative stress response. The capacity to induce oxidative stress and stress response appeared to be an intrinsic property of a vast variety of RTs: enzymatically active and inactivated, bearing mutations of drug resistance, following different routes of processing and presentation, expressed from viral or synthetic expression-optimized genes. The total ROS production induced by RT genes of the viral origin was found to be lower than that induced by the synthetic/expression-optimized or chimeric RT genes. However, the viral RT genes induced higher levels of ROS production and higher levels of HO-1 mRNA than the synthetic genes per unit of protein in the expressing cell. The capacity of RT genes to induce the oxidative stress and stress response was then correlated with their immunogenic performance. For this, RT genes were administered into BALB/c mice by intradermal injections followed by electroporation. Splenocytes of immunized mice were stimulated with the RT-derived and control antigens and antigen-specific proliferation was assessed by IFN-γ/IL-2 Fluorospot. RT variants generating high total ROS levels induced significantly stronger IFN-γ responses than the variants inducing lower total ROS, while high levels of ROS normalized per unit of protein in expressing cell were associated with a weak IFN-γ response. Poor gene immunogenicity was also associated with a high (per unit of protein) transcription of antioxidant response element (ARE) dependent phase II detoxifying enzyme genes, specifically HO-1. Thus, we have revealed a direct link between the propensity of the microbial proteins to induce oxidative stress and their immunogenicity.
Keywords: HIV-1 reverse transcriptase, oxidative stress, reactive oxygen species, transcription factor Nrf2, DNA immunization
Introduction
Reactive oxygen species (ROS), which include superoxide anion, hydroxyl radical and hydrogen peroxide, are formed in small amounts during various cell processes. The production of these intermediates is stimulated by “danger-signal-molecules” released by infected, stressed, and/or dying cells. Production of an excess of ROS by cells of the immune system is crucial for the clearance of viral, bacterial or parasitic infections. The role of ROS in the innate immune response was first identified in phagocytic cells as a “respiratory burst,” resulting in intracellular killing of the invading pathogens.1 ROS react with DNA, proteins and lipids, and affect their intrinsic activities, leading to apoptotic cell death as a part of the antimicrobial defense.2,3 Proliferative and effector responses of CD8+ cytotoxic T cells (their cytolytic function and cytokine production) depend on the redox balance and are markedly affected by the antioxidant regulation.4-6 Exogenous as well as endogenous sources of ROS also initiate and dictate the CD4+ T cell response.7 An uncontrolled oxidative stress can, however, be harmful: it affects activation of the immune cells, makes them refractory to growth and death stimuli and causes an overall dysfunction of the immune system.8-11
ROS act not only as effectors, but also as signaling molecules: they coordinate the efforts of the innate and adaptive immune systems and, by mediating signal transduction via cell surface receptors, orchestrate the adaptive phase of the immune response.11-13 The regulatory role of ROS is achieved through modification of the critical thiol residues, which induces a reversible activation of cellular kinases, phosphatases and transcription factors.3,9 This, among other events, results in the upregulated production of proinflammatory cytokines14 and IFN-γ.15 In its turn, INF-γ (as well as IL-2) helps to sustain oxidative stress by activating and supporting further production of ROS.16-18 In T cells, ROS-mediated modification of signaling molecules leads to the expression of a variety of genes defining T cell receptor signaling, commitment of T cells to Th1, Th2 and/or regulatory phenotypes, as well as their apoptosis.19-22
The redox-status coordination of gene expression in the immune system is mediated by multiple transcription factors, such as the nuclear factor-κB (NF-κB) and STAT families, AP-1, HIF-1α and others.23-26 The activities of pro-inflammatory transcription factors, such as NF-κB, are counteracted by an efficient defensive mechanism employing Nuclear factor E2-related factor 2 (Nrf2).27 The activity of Nrf2 is mediated through its binding to a short conservative sequence, antioxidant response element (ARE), in the promoters of the respective genes which initiates the Nrf2/ARE-pathway of oxidative stress response.28 Specifically, it switches the anti-inflammatory cytoprotective genes, down-regulates the synthesis of Th1-, and up-regulates the synthesis of Th2-cytokines.27,29,30 We and others have described a poor performance of hepatitis C virus (HCV) core in gene immunization31,32 and also found that the expression of HCV core gene in hepatoma cells induces the production of ROS as well as a strong Nrf2/ARE-mediated stress response.33 This pointed to possible causative associations of the oxidative stress induced by a gene when expressed in cell culture with a poor immunogenic performance of this gene when introduced into mice.
With this in mind, we have undertaken a study of the oxidative stress and oxidative stress response on a panel of HIV-1 reverse transcriptase (RT) genes of high to low immunogenicity.34-36 All RT genes were found to induce oxidative stress in the transfected human embryonic kidney cells. The capacity of an RT gene to induce ROS correlated with its capacity to induce RT-specific IFN-γ responses in mice. Interestingly, however, high levels of ROS normalized to the amount of protein expressed per transfected cell were predictive of a poor IFN-γ response. Weak gene immunogenicity was also associated with a high (per unit of protein in a cell) transcription of ARE-dependent phase II detoxifying enzyme genes.
Results
Design of expression-optimized genes encoding active and inactivated multidrug-resistant RTs
We have designed synthetic genes encoding multi-drug resistant reverse transcriptase (RT1.14) and RT1.14 with mutations D187N, D188N in the polymerase and E480Q in the RNase H domains. These mutations abrogate both the polymerase and the RNase H activities of the enzyme.37 Genes were optimized for the expression in human cells (http://evrogen.ru/). This optimization led to a 20-fold increase in the accumulation of the two RT1.14 variants as compared with the parental non-optimized protein encoded by the viral gene (from 20 to up-to 500–560 fg/cell; Fig. 1). We have compared the levels of RTopt and RTopt-in expression to that of the other RT gene variants designed by us earlier35,36,38,39 (Fig. S1). Equally good expression was achieved only for the fusions of RT with the lysosome-associated membrane protein I (LAMP-I) (Fig. S1).
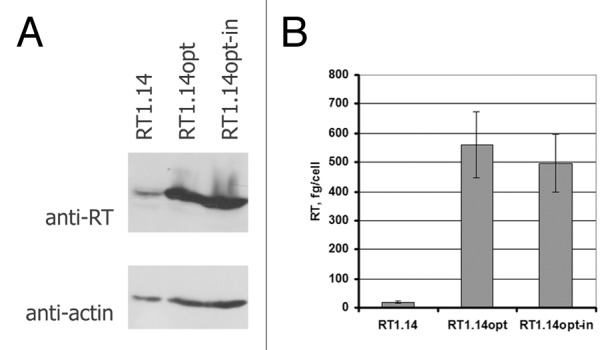
Figure 1. Expression in HEK293 cells of the viral gene encoding multi-drug resistant HIV reverse transcriptase (RT1.14) and humanized genes encoding active (RT1.14opt) and inactivated (RT1.14opt-in) RT variants. (A) Cells were harvested 48 h post transfection and lysed. Accumulation of RT proteins was assessed by western blotting with the polyclonal rabbit antibodies equally well recognizing diverse variants of RT protein.58 For normalization, blots were stripped and re-stained with anti-actin antibodies. (B) Quantification of RT expression per cell (done as described previously36).
Expression of RT genes leads to generation of reactive oxygen species (ROS)
In the next step we have assessed the capacity of RT genes to induce oxidative stress. This was done by treating the RT-expressing cells with an oxidation-sensitive dye, 2’,7’-dichlorodihydrofluorescein diacetate (DCFH), a fluorescence-based probe commonly utilized to detect the total production of ROS.40 Endogenous esterases deacetylate DCFH to dichlorofluorescein, which reacts with various ROS to form the fluorophore DCFH registered by microplate fluorimetry. Expression of all RT variants induced considerable ROS production, 7 to 11 times greater than the levels of ROS in the vector-transfected cells (Fig. 2A; data are presented as a fold-increase relative to the effect of the empty vector). ROS production induced by the expression of RT genes was similar in potency to that observed earlier for the nucleocapsid (core) and NS5A proteins of hepatitis C virus.33 Induction of ROS was completely inhibited by pretreatment of cells with a ROS scavenger, pyrollidine dithiocarbamate (PDTC; data not shown). Cell transfections with serial dilutions of an RT gene (investigated for RT1.14opt) led to proportional decreases in the RT protein content per cell and in total ROS production (Fig. S2), indicating that the generation of ROS was RT-specific. Interestingly, the effect was not due just to the high protein expression (protein overload), since it was observed in response to both highly and poorly expressed RT gene variants (Fig. 2A; Fig. S1), and had not been earlier observed for a highly (up to 600–700 fg/cell) expressed gene of the RNA-dependent RNA-polymerase of HCV.33
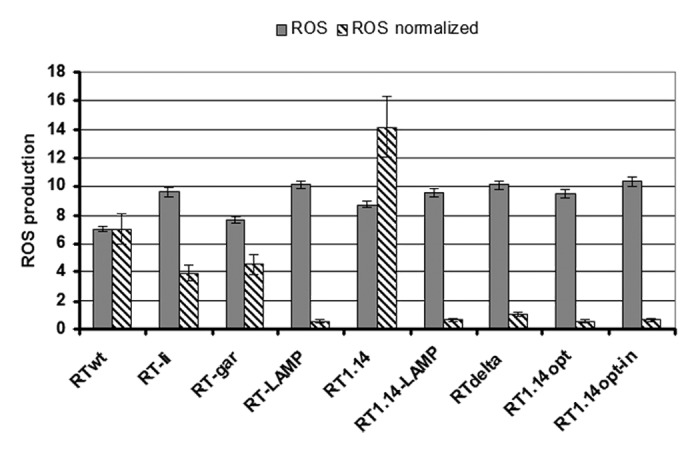
Figure 2. Generation of ROS in HEK293 cells transiently transfected with RT genes. Total ROS production (gray bars) and ROS level normalized to the content of RT variant per expressing cell (stripped bars) are both given in relation to the production of ROS in response to transfection with the empty vector. Data represent the results of two to three independent transfection experiments, each done in duplicate.
As noted above, up to 20-fold differences in the expression levels of RT protein variants (Fig. S1) did not lead to a similar difference in total ROS production, indicating that RT gene variants might differ in their capacity to induce oxidative stress (Fig. 2). To characterize the effect of one protein unit, we divided total ROS production by the level of the respective RT protein accumulation in the expressing cell as compared to the wild-type RT (Fig. S1) which yielded the normalized levels of ROS (per protein unit per cell; Fig. 2). RT genes of the viral origin (such as RTwt and RT1.14) were found to generate significantly higher levels of the normalized ROS than the expression-optimized synthetic RT genes (RT1.14opt, RT1.14opt-in, or RTdelta38; Fig. 2).
Expression of RT genes induces transcription of phase II detoxifying enzymes
Next we investigated whether the RT-induced ROS production is followed by an oxidative stress response. Specifically, using quantitative RT-PCR, we studied the effects of RT variants on the transcription of genes for the phase II detoxifying enzymes human NAD(P)H:quinone oxidoreductase (Nqo1) and heme oxygenase 1 (HO-1). Expression of RT variants stimulated a 10- to 15-fold increased transcription of these genes as compared with the effect of the empty vector (Fig. 3A). The effect of the RT variants was similar to what we have earlier observed for the core protein of HCV.33 Using the data presented in Figure S1, we have normalized the transcription of Nqo1 and HO-1 modulated by RT variants to the level of the respective RT protein accumulation in the expressing cell as compared to the wild-type RT. As in the case of ROS, RT genes of the viral origin (RTwt, RT1.14) were found to induce markedly higher normalized levels of Nqo1 and HO-1 transcription than the synthetic or chimeric RT genes (Fig. 3B).
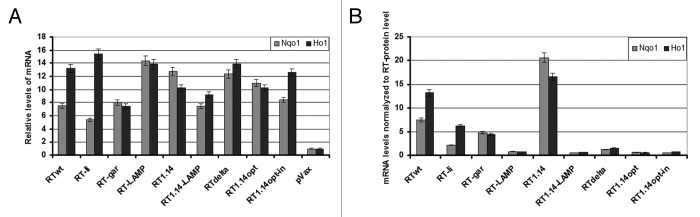
Figure 3. Transient expression of RT gene variants in HEK293 cells activates the transcription of NAD(P)H:quinone oxidoreductase (Nqo1) and heme oxygenase 1 (HO-1). (A) HO-1 and Nqo1 mRNA levels were quantified by RT-qPCR and related to the respective mRNA levels after transfection of HEK293 cells with the empty vector. (B) Relative levels of HO-1 and Nqo1 mRNA were normalized to the level of the respective RT protein accumulation per expressing cell as compared to the wild-type RT (given in Fig. S1).
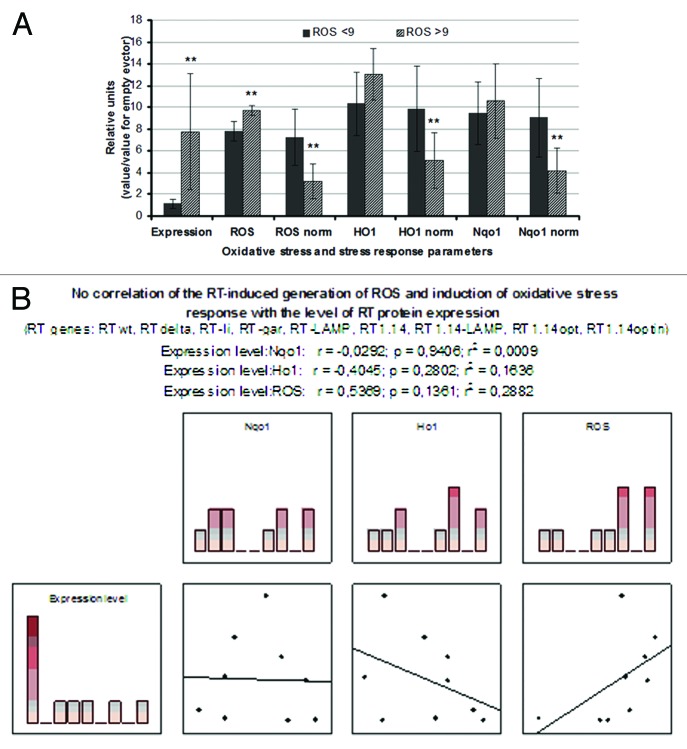
RT genes were then split into two populations, one inducing low (ROS < 9, dubbed “low ROS”) and the other high (ROS > 9, “high ROS”) total production of ROS (ROS = 8 ± 0.5, and ROS = 10 ± 0.2, respectively; p = 0.022; Fig. 4A). “High ROS” RT genes were expressed at significantly higher levels than the “low ROS” genes (p = 0.028; Fig. 4A). In principle this could be due to a cumulative effect of a higher amount of protein, since we have shown here that the increased amount of RT leads to the proportional increases in the production of ROS and in the levels of HO-1 and Nqo1 mRNA (Fig. S2). However, neither ROS, nor the levels of mRNA of the detoxifying enzymes correlated with the level of expression of the RT variants (p > 0.1 in the Spearman rank-order test; Fig. 4B). Also, RT populations characterized by high or low total ROS production did not differ in the levels of the RT-induced transcription of HO-1, or Nqo1 (p > 0.05 for the relative values; Fig. 4A). However, “high ROS” RT genes demonstrated lower levels of ROS, and of Nqo1 and HO-1 mRNA normalized to the level of the respective RT protein accumulation in the expressing cell (as compared to the wild-type RT, Fig. S1) than the “low ROS” genes (p < 0.05; Fig. 4A). Thus, RT genes differed both in their capacity to generate ROS and to induce an oxidative stress response and these phenomena were not due to the high or low levels of their expression.
Figure 4. The effect of RT protein expression on the oxidative stress and oxidative stress response. (A) RT genes based on the viral sequences generate low (ROS < 9) and codon-optimized synthetic RT genes, a high total production of ROS (ROS > 9). Data represent the results of two independent experiments, each done in three repeats; ** p < 0.05, F-test; (B) Absence of correlation between the levels of expression of different RT variants and their capacity to induce ROS or activate the oxidative stress response (p > 0.1; Spearman rank-order test).
RT genes have no effect on the viability of the expressing cells
We have further assessed whether RT-specific ROS induction was toxic to the expressing cells. The viability of expressing cells was determined by their colorimetric metabolic activity (the mitochondrial activity related to the number of viable cells; MTT test).41 The effect of all RT genes was indistinguishable from that of the empty vector, indicating that none of the RT variants affected the viability of expressing cells (data not shown).
Immunogenicity of RT variants
Our next step was to assess the immunogenicity of the RT gene series. Seven of the plasmids have been characterized by us earlier.35,36,38,39 Comparative data are presented in Figure S3. Here we assessed the immunogenicity in mice of the two newly designed genes encoding the expression-optimized active and inactivated drug-resistant RT (RT1.14opt, RT1.14opt-in). Groups of BALB/C mice (n = 5) were injected intradermally with the pVax1-based plasmids encoding RT1.14opt or RT1.14opt-in or the empty vector. Injections were followed by electroporation (Derma Vax, Cellectis). On day 23 post immunization, mice were sacrificed, splenocytes were isolated and stimulated by a peptide representing the immunodominant murine T-cell epitope of RT (aa 528–54342), recombinant RT1.14 protein or positive and negative control antigens (Fig. 5A–C and data not shown). RT1.14opt-in gene induced a significant IFN-γ, IL-2 and dual IFN-γ /IL-2, and RT1.14opt gene, a significant IL-2 response against the immunodominant mouse epitope of RT (RT 528–543)42 and the recombinant RT1.14 (Fig. 5). Cellular responses induced by both RT1.14opt and RT1.14opt-in genes well exceeded cellular responses to any other previously tested RT gene variant with the exception of the truncated RT gene (RTdelta) described recently by Hallengärd D et al.38 (RTdelta; Fig. S3).
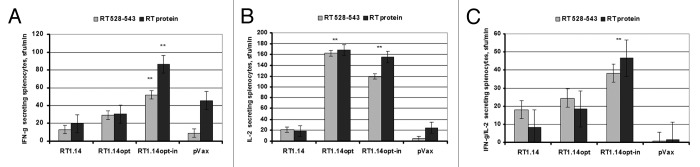
Figure 5. Cellular immune response against HIV-1 RT in BALB/c mice immunized by intradermal injections of the viral gene encoding multi-drug resistant RT (RT1.14; n = 5), a codon-optimized synthetic gene encoding enzymatically active (RT1.14opt; n = 5) or inactivated RT1.14 (RT1.14opt-in; n = 5), all followed by electroporation. Control mice (n = 5) received the empty vector. Net production of IFN-γ (A), IL-2 (B) and dual IFN-γ/IL-2 (C) by mouse splenocytes stimulated with an RT peptide (RT aa 528–543) or recombinant RT1.14 (RT protein) assessed by the dual IFN-γ/IL-2 Fluorospot. Stimulations were repeated twice. Average levels of cytokine production in response to medium alone were subtracted from the experimental values. Levels of cytokine production higher than that by the viral RT1.14 and by the empty vector are designated by ** (p < 0.05, Mann-Whitney U-test).
Levels of ROS predict cytokine response in RT gene immunization
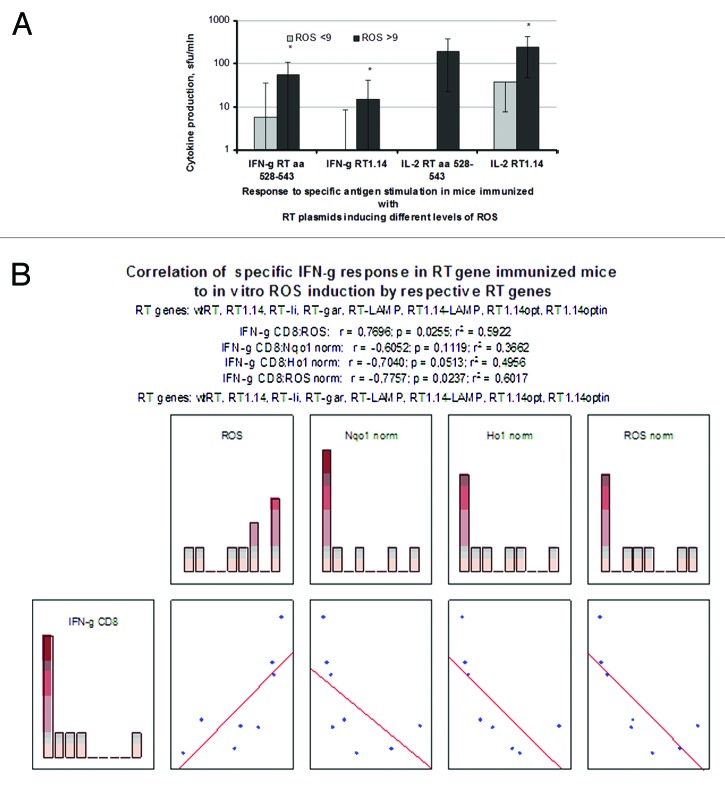
Next, we have related the in vitro parameters of stress and stress response induced by the RT genes to the capacity of these genes to induce RT-specific IFN-γ and IL-2 responses in mice. RT variants inducing high total ROS (ROS > 9) were found to mount a significantly stronger Th1 cytokine response than the variants inducing low total ROS (ROS < 9). This could be seen from the levels of IFN-γ and IL-2 secretion by the splenocytes of RT-gene immunized mice after in vitro stimulation with the recombinant RT1.14 or with the RT peptide representing the immunodominant T-cell epitope of BALB/c mice (RT 528–54342; Fig. 6A). Specific IFN-γ secretion of mice immunized with RT gene variants was found to correlate to the total ROS production induced by these genes in cell culture (p < 0.05; Fig. 6B)
Figure 6. In vitro tests predicting the capacity of the RT gene variants to induce IFN-γ response in mice, based on total ROS production and ROS production normalized to the protein content per expressing cell. The average specific IFN-γ and IL-2 production by the splenocytes of mice immunized with “high ROS” (ROS > 9) and “low ROS” (ROS < 9) RT gene variants after the in vitro stimulation with the recombinant RT1.14 and with the peptide representing aa 528–543 of RT (A). Correlation of IFN-γ production by splenocytes stimulated in vitro with RT aa 528–543 to the total ROS production (B) and to the normalized levels of ROS (C), and HO-1 mRNA (D). Analysis was done by the Spearman rank-order test using Statistica AXA 10.1.
The level of gene immunogen expression is an important determinant of gene immunogenicity.43 In view of this, we tested whether the immunogenic performance of RT genes is defined by the level of immunogen expression and/or by the levels of ROS, Nqo1 and/or HO-1 mRNA normalized to the level of the respective RT protein accumulation in the expressing cell as compared to the wild-type RT (as in Fig. S1). Interestingly, IFN-γ production in the RT gene immunized mice was found to inversely correlate to the normalized levels of ROS and of HO-1 mRNA (p < 0.05; Fig. 6C and D). No correlations were observed for the RT-specific production of IL-2 (data not shown). The cytokine response did not correlate to the level of RT expression, the total levels of Nqo1 or HO-1, or the normalized levels of Nqo1 mRNA (data not shown).
We have used the linear equations of IFN-γ dependence on the total or the normalized production of ROS, obtained on the series of eight RT genes (RT, RT1.14, RTIi, RTgar, RT-LAMP, RT1.14-LAMP, RT1.14opt, RT1.14opt-in) to predict the immunogenicity of the gene immunogen encoding the truncated HIV-1 RT (RTdelta).38 The immunogenicity of this RT gene variant was assessed in an independent experiment performed similarly to what was described above for RT1.14opt and RT1.14optin genes (Fig. S3). The capacity of the RTdelta gene to induce IFN-γ responses in mice could be predicted with an accuracy of 80%.
Discussion
HIV-1 infection induces chronic oxidative stress thought to be responsible for its neurotoxicity. Tissues of HIV-1 patients demonstrate an enhanced protein and lipid oxidation (peroxidation) and a loss of the total antioxidant capacity, simultaneously with perturbations in the levels of superoxide dismutase, glutathione, ascorbic acid, tocopherols, and carotenoids.44,45 Oxidative stress contributes to several aspects of HIV pathogenesis, including viral replication, inflammatory response, decreased immune cell proliferation, loss of immune function, apoptosis, increased sensitivity to drug toxicities and dementia.44,46,47 Oxidative stress has been strongly and unequivocally associated with the Trans-Activator of Transcription (Tat) protein46-48 and recently also with the envelope glycoprotein gp120.49 Here, we for the first time describe the induction of oxidative stress by another HIV-1 protein, namely reverse transcriptase. We have shown that the expression of HIV-1 RT in human embryonic kidney cells induces strong oxidative stress, manifested by the production of ROS that is 7–10 times greater than the levels of ROS induced by cell transfection with an empty vector. The capacity to induce oxidative stress appeared to be an intrinsic feature of all RT variants tested: enzymatically active and inactivated, bearing multiple mutations of drug resistance, having different routes of processing and presentation (proteasomal as the drug-resistant RT, lysosomal as fusions of RT with LAMP-I or Ii, or fusions of RT with a Gly-Ala repeat of the EBNA 1 protein of EBV targeted to autophagosomes).34,27,28
Some of the RT variants were characterized by a high, others by a relatively low capacity to induce oxidative stress. The difference in total ROS production was not due to a varying level of protein expression. The analysis of the data obtained for the series of eight RT gene variants did not reveal any dependence of the total ROS production on the level of RT expression. Up-to 20-fold increase in the RT expression induced only a 30 to 40% increase in the overall production of ROS. Further, we have identified two RT gene populations. One, including the chimeric RT genes RT-LAMP, RT1.14-LAMP and the expression-optimized synthetic RT genes, overexpressed the encoded proteins and was capable of triggering high total ROS production, but at the same time low levels of ROS normalized to the relative amount of the respective protein per expressing cell (as compared to wtRT). The other, including viral genes encoding the wild-type and multi-drug resistant RT variants and their chimeras with signal sequences, were poorly expressed and induced lower total, but at the same time higher normalized ROS production (p < 0,05). The mechanism of the induction of oxidative stress by HIV-1 RT and the reason(s) for the observed differences are currently being investigated.
The ability of RT genes to induce a cellular immune response varied with their ability to induce oxidative stress and oxidative stress response. The RT variants inducing low total ROS production but high levels of normalized ROS had a reduced capacity to induce RT-specific Th1-type immune response, specifically the production of IFN-γ. We were able to use the correlations between the IFN-γ response to the RT gene and the levels of ROS production recorded in cell line tests (direct for the total and inverse for the normalized levels), to predict the Th1 immunogenicity of the RT-based gene immunogens with an accuracy of up to 80%.
Interestingly, despite a potent induction of ROS, none of the RT gene variants was toxic to the expressing cells, suggesting the intervention of the oxidative stress response that counteracted the negative effects of ROS production. Indeed, all RT variants were found to activate the transcription of the genes of phase II detoxifying enzymes,50,51 specifically of heme oxygenase-1 (HO-1) and of human NAD(P)H:quinone oxidoreductase 1 (Nqo1). HO-1 transfers electrons from molecular oxygen to free heme, inducing its degradation into free iron.52 Nqo1 catalyzes the reduction of quinones, such as ubiquinone and α-tocopherone, to regenerate their antioxidant capacity and prevent lipid peroxidation of cellular membranes.53 The level of transcription of HO-1 in RT-expressing cells was increased 9–16 fold, and of Nqo1, 10–15 fold compared with the effect induced by the empty vector. Low/no toxicity (no effect of RT gene variants on the metabolic activity of NAD(P)H-dependent cellular oxidoreductase enzymes) could have been a result of this effective oxidative stress response. Interestingly, however, activation of the transcription of HO-1 or Nqo1 did not correlate to the total ROS production. RT populations generating high total ROS did not differ from that generating low total ROS in either the levels of HO-1, or of Nqo1 mRNA. This indicated that some RT variants (such as the wild-type HIV-1 RT) were able to induce a stronger oxidative stress response than other RT variants independently of the initial levels of oxidative stress.
The induction of HO-1 and Nqo1 transcription is facilitated by binding of the transcription factor Nrf2 to the antioxidant response element (ARE) in the promoters of these genes (modulated by KEAP1, a redox-sensitive binding partner of Nrf2), constituting the essential element of the Phase II response.53-55 Nrf2 regulates the transcription of several hundred genes controlling the oxidative stress response. Induction of the Nrf2/ARE pathway leads to the downregulation of IFN-γ and IL-2 synthesis in favor of the Th2-type cytokines.29,30,56 Involvement of Nrf2 would explain why the capacity of viral RT genes to induce high levels of ROS and of HO-1 mRNA normalized to the level of accumulation of respective proteins per expressing cell is associated with the decreased Th1-type immunogenicity of their genes in mice.
Conclusions
We present the first evidence that the oxidative stress produced by HIV infection and manifested by generation of ROS can be induced by HIV-1 proteins other than Tat and gp120, namely by HIV-1 reverse transcriptase. Our data suggest that HIV reverse transcriptase activates the Nrf2/ARE pathway of the oxidative stress response. Induction of ROS and of the Nrf2/ARE stress response modulates the ability of RT genes to induce the Th1-type of immune response in mice. This study points to direct links between the propensity of the microbial proteins to induce oxidative stress and the performance of their genes in immunization. These findings have direct implications for gene vaccine development by providing clues for a meaningful selection of candidate genes capable of inducing a potent Th1-type of the immune response.
Materials and Methods
Plasmids
The study employed a series of reverse transcriptase genes: RT of HIV-1 HXB2 (wild type RT; Gen Bank accession number AAB50259); RT of HIV-1 MN isolated from patient J14562 treated with 3TC, d4T, saquinavir, and ritonavir (RT1.14) both expressed in the pKCMV-based vector;57,58 RT1.14 fused to the lysosome associate membrane protein I (pKCMVRT1.14-LAMP), RT fused to LAMP I (pKCMVRT-LAMP); N-terminally fused to the first 30 amino acids of human invariant chain (Ii) (pKCMVRT-Ii); carrying a 30 amino acid-long Gly-Ala repeat of EBNA1 (pCMVRT-gar), all described previously.57-59 Plasmid encoding the truncated version of RT (pKCMVRTdelta) was kindly provided by Dr Andreas Bråve (Swedish Institute for Communicable Disease Control).
Sequence of the gene encoding RT1.14 was optimized by codon humanization; to ensure the proper protein expression, the gene was provided with an AAT-ATG-GGA sequence fused to its 5′-end, which resulted in the addition of Met-Gly to the N-terminus of the protein. The humanized gene of the expression-optimized multidrug-resistant RT (RT1.14opt) was synthesized (Evrogen) and cloned into pVax1 vector to generate pVaxRT1.14opt. The enzymatic activity of RT1.14opt was abrogated by point-mutations D187N, D188N, and E480Q, which were introduced into the RT1.14opt gene by site-directed mutagenesis (Evrogen). The latter yielded the expression-optimized gene for inactivated drug-resistant RT (RT1.14opt-in) in the pVax-1 backbone dubbed pVaxRT1.14opt-in.
The empty vectors used as controls in cell line experiments and in immunization included pKCMV60 and pVax-1 (Invitrogen Corporation).
Expression of reverse transcriptase variants in the eukaryotic cells
HEK 293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; both by Invitrogen). Cells were transfected using Lipofectamine-LTX or Lipofectamine 2000 (Invitrogen, 15338030 or 11668019) according to the manufacturer’s instructions. Cells were grown for 48 h, harvested, counted, and subjected to Western blotting or oxidative stress tests. Cells were lysed and analyzed by Western blot as previously described.36 RT-chimeras were detected using polyclonal rabbit anti-RT serum57 or monoclonal anti-RT antibodies (3F6).61 The amount of RT protein in the lysed cells was calculated on the basis of a standard curve built using serial dilutions of the recombinant wild-type RT protein62 which were analyzed together with the lysates as was described earlier.36
Measurement of reactive oxygen species
Production of the intracellular reactive oxygen species (ROS) was measured by epifluorescence. Cells were transfected as described above, growth medium was removed 40 h after transfection and cells were incubated in medium containing 25 μM 2’,7’-dichlorodihydrofluorescein diacetate (DCFH) for 30 min at room temperature. After the incubation, cells were washed 10 times with 500 μl of PBS. The fluorescence intensities (FLI) were measured using Plate CHAMELEON V reader (Hidex Ltd.) with excitation at 485 nm and emission at 535 nm.
MTT assay
HEK293 cells were seeded in 24-well plates at density 8 × 104 cells/well and next day transfected with 0.4 μg of plasmid using Lipofectamine 2000 (Invitrogen, 11668019). 48 h later the medium was replaced with the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) solution in cell culture medium (1 mg/ml) and the cells were incubated for additional two hours. Then the solution was removed and the resultant formazan crystals were dissolved in the 4 mM HCl in isopropanol. The absorbance at 590 nm was registered using Plate Chameleon V reader (Hidex Ldt.)
Quantitative real-time reverse transcription—PCR (QPCR)
RNA was isolated from 5 × 105 transfected cells with PerfectPure RNA Cultured Cell kit (5Prime, 2302350) and reversely transcribed using Reverse Transcription System (Promega, A3500) with random hexamer primer according to the manufacturer’s protocol. QPCR was performed using IQ5 Real-Time PCR Detection System (BioRad) using primers and probes described earlier.33 A standard reaction mixture (50 μl) contained Taqman primer/probe combination, cDNA equivalent to 100 ng total RNA, and qPCRmix-HS master mix. The real-time PCR thermal conditions for all genes were 55 °C for 5 min, 95 °C for 10 min, followed by 40 cycles each at 95 °C for 10 sec and 57 °C for 1 min (signal collection temperature). Relative quantitative analysis was performed by comparing threshold cycle number for target genes and a reference β-actin mRNA, amplified in separate tubes.
DNA immunization of mice
DNA immunization was performed as described previously.34 In brief, groups of Balb/c (H-2d) mice (n = 4–6, females, 8 weeks old) were injected intradermally with 10 μg of DNA-immunogens in 20 μl PBS. Control mice received 10 μg of the empty vector. Immediately after injection, the electroporation was performed using the Derma Vax™ DNA vaccine delivery system (Cellectis Glen Burnie). Electroporation was performed as was described earlier.63 In brief, a needle array electrode consisting of eight 2-mm pins arranged in 2 rows (1.5 × 4mm gaps) (BTX, #47-0040) was placed tightly over the skin and electric field was applied as 2 pulses of 1125 V/cm (50 μs interval) and 8 pulses of 275 V/cm (10 μs interval). Electroporation was performed in a controlled fashion by keeping the estimated (pre-pulse) skin resistance below 3000 Ω. At day 28, mice were bled and sacrificed and their spleens were collected. Main experiments were repeated twice with concordant results. All experiments were approved by the Swedish National Board for Laboratory Animals.
Assays of T-cell response
Spleens were homogenized individually in RPMI 1640 supplemented with penicillin/streptomycin (PEST), 2 mM L-Glutamine, and 5% fetal calf serum (FCS). The cells were washed once with RPMI 1640, resuspended in RPMI containing 5% FCS and used in IFN-γ /IL-2 FluoroSpot (Mabtech, FS-4142-10) according to the manufacturers instructions. In brief, 250 000 splenocytes were added per well and stimulated for 18h with recombinant RTwt or RT1.14 (10 μg/ml), peptides (10 μg/ml), medium alone, or Concanavalin A (Con A, 5 μg/ml) as a positive control. Proteins RTwt and RT1.14 were expressed in E. coli and purified by affinity chromatography as described.62 A peptide representing aa 528–543 (KEKVYLAWVP AHKGIG) of RT was purchased from GL Biochem Ltd. The number of cytokine-producing spot-forming cells (SFC) per million splenocytes was evaluated using the AID ELISpot reader system (Autoimmun Diagnostika GmbH). For each animal, a net SFC/106 cells in response to each antigen was calculated by subtracting values registered in response to stimulation with medium.
Software and data analysis
Western blot images were quantified using ImageJ software (http://rsb.info.nih.gov/ij/). Non-parametric statistics were chosen as appropriate for sample sizes < 100.65 Continuous but not normally distributed variables, such as the numbers of cytokine-producing SFCs, were compared by the nonparametric Kruskal-Wallis and Mann-Whitney U tests. Linear correlations between the variables were obtained using the Spearman rank-order test. Statistical analysis was done with Spearman rank-order test, using STATISTICA AXA 10.0 (StatSoft Inc.).
We have further used linear equations describing the correlation of IFN-γ response to the normalized ROS production to build a model of in vitro ROS/in vivo IFN-γ dependence capable of predicting IFN-γ and dual IFN-γ /IL-2 production of the RT-based gene immunogens. For this, we excluded RT variants one by one from the original set of eight genes, re-formulated the correlation equations, and used them to predict the levels of IFN-γ production induced by the RT variant excluded from the equation. Predicted values of IFN-γ production were compared with the levels registered after the RT-specific stimulation of splenocytes of the RT-gene immunized mice. RT-specific IFN-γ response could be predicted with an accuracy of 70 to 90%.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge financial support from the Swedish Research Council (grant K2011-79X-21744-01-6); the New Visby program (grant 00885/2011), and the Thematic partnership program (grant 09272/2013) of the Swedish Institute; the Russian Foundation for Basic Research (grants 13-04-01523 and 11-04-01569a); the Russian Ministry of Education and Science (agreement 8069); and the program “Molecular and Cellular Biology” of the Presidium of the Academy of Sciences of the Russian Federation.
Glossary
Abbreviations:
- RT
reverse transcriptase of HIV-1
- ROS
reactive oxygen species
- IFN-γ
interferon gamma
- IL-2
interleukin 2
- Nrf2
nuclear factor (erythroid-derived 2)-related factor 2
- ARE
antioxidant response element
- Nqo1
NAD(P)H:quinone oxidoreductase
- HO-1
heme oxygenase 1
- NOX
NADPH oxidase
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: http://www.landesbioscience.com/journals/vaccines/article/25813/
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25813
References
- 1.Babior BM, Peters WA. The O2--producing enzyme of human neutrophils. Further properties. J Biol Chem. 1981;256:2321–3. [PubMed] [Google Scholar]
- 2.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, et al. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89:873–91. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laniewski NG, Grayson JM. Antioxidant treatment reduces expansion and contraction of antigen-specific CD8+ T cells during primary but not secondary viral infection. J Virol. 2004;78:11246–57. doi: 10.1128/JVI.78.20.11246-11257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sklavos MM, Tse HM, Piganelli JD. Redox modulation inhibits CD8 T cell effector function. Free Radic Biol Med. 2008;45:1477–86. doi: 10.1016/j.freeradbiomed.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Thayer TC, Delano M, Liu C, Chen J, Padgett LE, Tse HM, et al. Superoxide production by macrophages and T cells is critical for the induction of autoreactivity and type 1 diabetes. Diabetes. 2011;60:2144–51. doi: 10.2337/db10-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon J, Shatynski KE, Chen H, Morand S, de Deken X, Miot F, et al. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci Signal. 2010;3:ra59. doi: 10.1126/scisignal.2000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deramaudt TB, Dill C, Bonay M. Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Med Mal Infect. 2013;43:100–7. doi: 10.1016/j.medmal.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths HR. ROS as signalling molecules in T cells--evidence for abnormal redox signalling in the autoimmune disease, rheumatoid arthritis. Redox Rep. 2005;10:273–80. doi: 10.1179/135100005X83680. [DOI] [PubMed] [Google Scholar]
- 10.Larbi A, Cabreiro F, Zelba H, Marthandan S, Combet E, Friguet B, et al. Reduced oxygen tension results in reduced human T cell proliferation and increased intracellular oxidative damage and susceptibility to apoptosis upon activation. Free Radic Biol Med. 2010;48:26–34. doi: 10.1016/j.freeradbiomed.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Larbi A, Kempf J, Pawelec G. Oxidative stress modulation and T cell activation. Exp Gerontol. 2007;42:852–8. doi: 10.1016/j.exger.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Bertolotti M, Yim SH, Garcia-Manteiga JM, Masciarelli S, Kim YJ, Kang MH, et al. B- to plasma-cell terminal differentiation entails oxidative stress and profound reshaping of the antioxidant responses. Antioxid Redox Signal. 2010;13:1133–44. doi: 10.1089/ars.2009.3079. [DOI] [PubMed] [Google Scholar]
- 13.Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic Biol Med. 2004;37:1144–51. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Haddad JJ, Safieh-Garabedian B, Saadé NE, Land SC. Thiol regulation of pro-inflammatory cytokines reveals a novel immunopharmacological potential of glutathione in the alveolar epithelium. J Pharmacol Exp Ther. 2001;296:996–1005. [PubMed] [Google Scholar]
- 15.Yamada M, Gomez JC, Chugh PE, Lowell CA, Dinauer MC, Dittmer DP, et al. Interferon-γ production by neutrophils during bacterial pneumonia in mice. Am J Respir Crit Care Med. 2011;183:1391–401. doi: 10.1164/rccm.201004-0592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–27. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 17.Li X, McKinstry KK, Swain SL, Dalton DK. IFN-gamma acts directly on activated CD4+ T cells during mycobacterial infection to promote apoptosis by inducing components of the intracellular apoptosis machinery and by inducing extracellular proapoptotic signals. J Immunol. 2007;179:939–49. doi: 10.4049/jimmunol.179.2.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam GY, Huang J, Brumell JH. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin Immunopathol. 2010;32:415–30. doi: 10.1007/s00281-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 19.Chang JH, Kim YJ, Han SH, Kang CY. IFN-gamma-STAT1 signal regulates the differentiation of inducible Treg: potential role for ROS-mediated apoptosis. Eur J Immunol. 2009;39:1241–51. doi: 10.1002/eji.200838913. [DOI] [PubMed] [Google Scholar]
- 20.Gostner JM, Becker K, Fuchs D, Sucher R. Redox regulation of the immune response. Redox Rep. 2013;18:88–94. doi: 10.1179/1351000213Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesarwani P, Murali AK, Al-Khami AA, Mehrotra S. Redox regulation of T-cell function: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2013;18:1497–534. doi: 10.1089/ars.2011.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyo CW, Lee SH, Choi SY. Oxidative stress induces PKR-dependent apoptosis via IFN-gamma activation signaling in Jurkat T cells. Biochem Biophys Res Commun. 2008;377:1001–6. doi: 10.1016/j.bbrc.2008.10.103. [DOI] [PubMed] [Google Scholar]
- 23.Müller JM, Rupec RA, Baeuerle PA. Study of gene regulation by NF-kappa B and AP-1 in response to reactive oxygen intermediates. Methods. 1997;11:301–12. doi: 10.1006/meth.1996.0424. [DOI] [PubMed] [Google Scholar]
- 24.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–50. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman I. Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung diseases. J Biochem Mol Biol. 2003;36:95–109. doi: 10.5483/BMBRep.2003.36.1.095. [DOI] [PubMed] [Google Scholar]
- 26.Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21:223–44. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedruzzi LM, Stockler-Pinto MB, Leite M, Jr., Mafra D. Nrf2-keap1 system versus NF-κB: the good and the evil in chronic kidney disease? Biochimie. 2012;94:2461–6. doi: 10.1016/j.biochi.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Aleksunes LM, Manautou JE. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol Pathol. 2007;35:459–73. doi: 10.1080/01926230701311344. [DOI] [PubMed] [Google Scholar]
- 29.Natsch A. The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers--functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol Sci. 2010;113:284–92. doi: 10.1093/toxsci/kfp228. [DOI] [PubMed] [Google Scholar]
- 30.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 31.Alekseeva E, Sominskaya I, Skrastina D, Egorova I, Starodubova E, Kushners E, et al. Enhancement of the expression of HCV core gene does not enhance core-specific immune response in DNA immunization: advantages of the heterologous DNA prime, protein boost immunization regimen. Genet Vaccines Ther. 2009;7:7. doi: 10.1186/1479-0556-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidalin O, Tanaka E, Spengler U, Trépo C, Inchauspé G. Targeting of hepatitis C virus core protein for MHC I or MHC II presentation does not enhance induction of immune responses to DNA vaccination. DNA Cell Biol. 1999;18:611–21. doi: 10.1089/104454999315024. [DOI] [PubMed] [Google Scholar]
- 33.Ivanov AV, Smirnova OA, Ivanova ON, Masalova OV, Kochetkov SN, Isaguliants MG. Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PLoS One. 2011;6:e24957. doi: 10.1371/journal.pone.0024957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starodubova E, Boberg A, Ivanov A, Latyshev O, Petrakova N, Kuzmenko Y, et al. Potent cross-reactive immune response against the wild-type and drug-resistant forms of HIV reverse transcriptase after the chimeric gene immunization. Vaccine. 2010;28:1975–86. doi: 10.1016/j.vaccine.2009.10.098. [DOI] [PubMed] [Google Scholar]
- 35.Starodubova E, Krotova O, Hallengärd D, Kuzmenko Y, Engström G, Legzdina D, et al. Cellular immunogenicity of novel gene immunogens in mice monitored by in vivo imaging. Mol Imaging. 2012;11:471–86. [PubMed] [Google Scholar]
- 36.Starodubova ES, Boberg A, Litvina M, Morozov A, Petrakova NV, Timofeev A, et al. HIV-1 reverse transcriptase artificially targeted for proteasomal degradation induces a mixed Th1/Th2-type immune response. Vaccine. 2008;26:5170–6. doi: 10.1016/j.vaccine.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 37.Smith JM, Amara RR, McClure HM, Patel M, Sharma S, Yi H, et al. Multiprotein HIV type 1 clade B DNA/MVA vaccine: construction, safety, and immunogenicity in Macaques. AIDS Res Hum Retroviruses. 2004;20:654–65. doi: 10.1089/0889222041217419. [DOI] [PubMed] [Google Scholar]
- 38.Hallengärd D, Wahren B, Bråve A. A truncated plasmid-encoded HIV-1 reverse transcriptase displays strong immunogenicity. Viral Immunol. 2013;26:163–6. doi: 10.1089/vim.2012.0083. [DOI] [PubMed] [Google Scholar]
- 39.Starodubova ES, Isaguliants MG, Karpov VL. Regulation of Immunogen Processing: Signal Sequences and Their Application for the New Generation of DNA-Vaccines. Acta Naturae. 2010;2:53–60. [PMC free article] [PubMed] [Google Scholar]
- 40.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine Oxford: Oxford University Press, 2007. [Google Scholar]
- 41.van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–45. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- 42.Haas G, David R, Frank R, Gausepohl H, Devaux C, Claverie JM, et al. Identification of a major human immunodeficiency virus-1 reverse transcriptase epitope recognized by mouse CD4+ T lymphocytes. Eur J Immunol. 1991;21:1371–7. doi: 10.1002/eji.1830210607. [DOI] [PubMed] [Google Scholar]
- 43.Garmory HS, Brown KA, Titball RW. DNA vaccines: improving expression of antigens. Genet Vaccines Ther. 2003;1:2. doi: 10.1186/1479-0556-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pace GW, Leaf CD. The role of oxidative stress in HIV disease. Free Radic Biol Med. 1995;19:523–8. doi: 10.1016/0891-5849(95)00047-2. [DOI] [PubMed] [Google Scholar]
- 45.Suresh DR, Annam V, Pratibha K, Prasad BV. Total antioxidant capacity--a novel early bio-chemical marker of oxidative stress in HIV infected individuals. J Biomed Sci. 2009;16:61. doi: 10.1186/1423-0127-16-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pocernich CB, Sultana R, Mohmmad-Abdul H, Nath A, Butterfield DA. HIV-dementia, Tat-induced oxidative stress, and antioxidant therapeutic considerations. Brain Res Brain Res Rev. 2005;50:14–26. doi: 10.1016/j.brainresrev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Steiner J, Haughey N, Li W, Venkatesan A, Anderson C, Reid R, et al. Oxidative stress and therapeutic approaches in HIV dementia. Antioxid Redox Signal. 2006;8:2089–100. doi: 10.1089/ars.2006.8.2089. [DOI] [PubMed] [Google Scholar]
- 48.Song HY, Ju SM, Seo WY, Goh AR, Lee JK, Bae YS, et al. Nox2-based NADPH oxidase mediates HIV-1 Tat-induced up-regulation of VCAM-1/ICAM-1 and subsequent monocyte adhesion in human astrocytes. Free Radic Biol Med. 2011;50:576–84. doi: 10.1016/j.freeradbiomed.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 49.Silverstein PS, Shah A, Weemhoff J, Kumar S, Singh DP, Kumar A. HIV-1 gp120 and drugs of abuse: interactions in the central nervous system. Curr HIV Res. 2012;10:369–83. doi: 10.2174/157016212802138724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talalay P, Dinkova-Kostova AT, Holtzclaw WD. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv Enzyme Regul. 2003;43:121–34. doi: 10.1016/S0065-2571(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 51.Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–36. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 52.Morse D, Choi AM. Heme oxygenase-1: from bench to bedside. Am J Respir Crit Care Med. 2005;172:660–70. doi: 10.1164/rccm.200404-465SO. [DOI] [PubMed] [Google Scholar]
- 53.Ross D, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase), functions and pharmacogenetics. Methods Enzymol. 2004;382:115–44. doi: 10.1016/S0076-6879(04)82008-1. [DOI] [PubMed] [Google Scholar]
- 54.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–8. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 55.Hine CM, Mitchell JR. NRF2 and the Phase II Response in Acute Stress Resistance Induced by Dietary Restriction. J Clin Exp Pathol. 2012;S4 doi: 10.4172/2161-0681.S4-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rockwell CE, Zhang M, Fields PE, Klaassen CD. Th2 skewing by activation of Nrf2 in CD4(+) T cells. J Immunol. 2012;188:1630–7. doi: 10.4049/jimmunol.1101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Isaguliants MG, Pokrovskaya K, Kashuba VI, Pokholok D, Hinkula J, Wahren B, et al. Eukaryotic expression of enzymatically active human immunodeficiency virus type 1 reverse transcriptase. FEBS Lett. 1999;447:232–6. doi: 10.1016/S0014-5793(99)00297-5. [DOI] [PubMed] [Google Scholar]
- 58.Isaguliants MG, Belikov SV, Starodubova ES, Gizatullin RZ, Rollman E, Zuber B, et al. Mutations conferring drug resistance affect eukaryotic expression of HIV type 1 reverse transcriptase. AIDS Res Hum Retroviruses. 2004;20:191–201. doi: 10.1089/088922204773004914. [DOI] [PubMed] [Google Scholar]
- 59.Roos AK, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, et al. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS One. 2009;4:e7226. doi: 10.1371/journal.pone.0007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kjerrström A, Hinkula J, Engström G, Ovod V, Krohn K, Benthin R, et al. Interactions of single and combined human immunodeficiency virus type 1 (HIV-1) DNA vaccines. Virology. 2001;284:46–61. doi: 10.1006/viro.2001.0905. [DOI] [PubMed] [Google Scholar]
- 61.Rytting AS, Akerblom L, Albert J, Unge T, Björling E, Al-Khalili L, et al. Monoclonal antibodies to native HIV type 1 reverse transcriptase and their interaction with enzymes from different subtypes. AIDS Res Hum Retroviruses. 2000;16:1281–94. doi: 10.1089/08892220050117041. [DOI] [PubMed] [Google Scholar]
- 62.Rechinskiĭ VO, Barbashov SF, Degtiarev IL, Vorob’ev SM, Liakhov DL, Kostiuk DA, et al. [Reverse transcriptase of the human immunodeficiency virus: cloning, expression in Escherichia coli, purification of the enzyme, and production of monoclonal antibodies] Mol Biol (Mosk) 1991;25:1248–57. [PubMed] [Google Scholar]
- 63.Roos AK, Eriksson F, Walters DC, Pisa P, King AD. Optimization of skin electroporation in mice to increase tolerability of DNA vaccine delivery to patients. Mol Ther. 2009;17:1637–42. doi: 10.1038/mt.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Limberis MP, Bell CL, Wilson JM. Identification of the murine firefly luciferase-specific CD8 T-cell epitopes. Gene Ther. 2009;16:441–7. doi: 10.1038/gt.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sidney S. Statistics for the behavioral sciences. McGraw-Hill, 1956. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.