Abstract
Bacterial adhesion to other bacteria, to eukaryotic cells, and to extracellular matrix proteins is frequently mediated by cell surface-associated polymers (fimbriae) consisting of one or more subunit proteins. We have found that polymerization of curlin to fimbriae-like structures (curli) on the surface of Escherichia coli markedly differs from the prevailing model for fimbrial assembly in that it occurs extracellularly through a self-assembly process depending on a specific nucleator protein. The cell surface-bound nucleator primes the polymerization of curlin secreted by the nucleator-presenting cell or by adjacent cells. The addition of monomers to the growing filament seems to be driven by mass action and guided only by the diffusion gradient between the source of secreted monomer and the surface of monomer condensation.
Full text
PDF


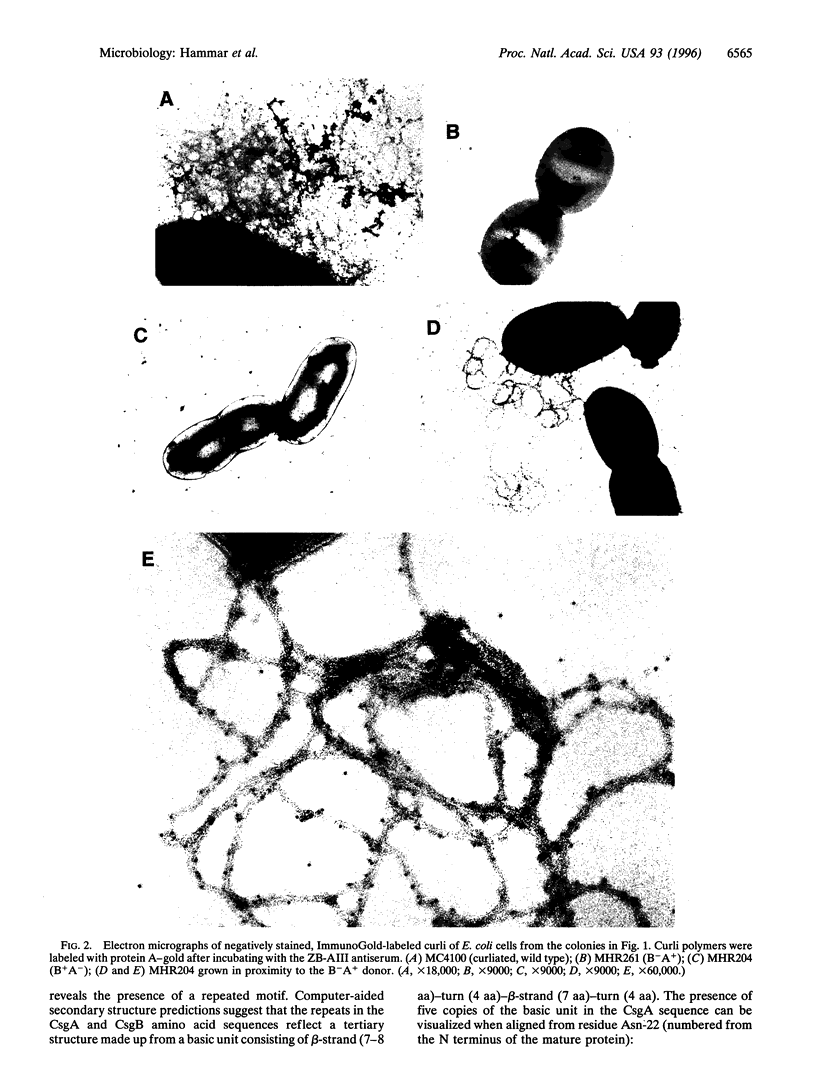

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., NOSSAL G. J., PYE J., ABBOT A. BEHAVIOUR OF ACTIVE BACTERIAL ANTIGENS DURING THE INDUCTION OF THE IMMUNE RESPONSE. I. PROPERTIES OF FLAGELLAR ANTIGENS FROM SALMONELLA. Nature. 1963 Sep 28;199:1257–1259. doi: 10.1038/1991257a0. [DOI] [PubMed] [Google Scholar]
- Aizawa S. I., Vonderviszt F., Ishima R., Akasaka K. Termini of Salmonella flagellin are disordered and become organized upon polymerization into flagellar filament. J Mol Biol. 1990 Feb 20;211(4):673–677. doi: 10.1016/0022-2836(90)90064-S. [DOI] [PubMed] [Google Scholar]
- Arnqvist A., Olsén A., Pfeifer J., Russell D. G., Normark S. The Crl protein activates cryptic genes for curli formation and fibronectin binding in Escherichia coli HB101. Mol Microbiol. 1992 Sep;6(17):2443–2452. doi: 10.1111/j.1365-2958.1992.tb01420.x. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Namba K. Switching in the self-assembly of tobacco mosaic virus. Adv Biophys. 1990;26:157–185. doi: 10.1016/0065-227x(90)90011-h. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Pan K. M., Huang Z., Baldwin M., Fletterick R. J., Prusiner S. B. Structural clues to prion replication. Science. 1994 Apr 22;264(5158):530–531. doi: 10.1126/science.7909169. [DOI] [PubMed] [Google Scholar]
- Collinson S. K., Doig P. C., Doran J. L., Clouthier S., Trust T. J., Kay W. W. Thin, aggregative fimbriae mediate binding of Salmonella enteritidis to fibronectin. J Bacteriol. 1993 Jan;175(1):12–18. doi: 10.1128/jb.175.1.12-18.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson S. K., Emödy L., Müller K. H., Trust T. J., Kay W. W. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J Bacteriol. 1991 Aug;173(15):4773–4781. doi: 10.1128/jb.173.15.4773-4781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran J. L., Collinson S. K., Burian J., Sarlós G., Todd E. C., Munro C. K., Kay C. M., Banser P. A., Peterkin P. I., Kay W. W. DNA-based diagnostic tests for Salmonella species targeting agfA, the structural gene for thin, aggregative fimbriae. J Clin Microbiol. 1993 Sep;31(9):2263–2273. doi: 10.1128/jcm.31.9.2263-2273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRINGTON W. F., SCHACHMAN H. K. Studies on the alkaline degradation of tobacco mosaic virus. I. Ultracentrifugal analysis. Arch Biochem Biophys. 1956 Nov;65(1):278–295. doi: 10.1016/0003-9861(56)90194-1. [DOI] [PubMed] [Google Scholar]
- Hammar M., Arnqvist A., Bian Z., Olsén A., Normark S. Expression of two csg operons is required for production of fibronectin- and congo red-binding curli polymers in Escherichia coli K-12. Mol Microbiol. 1995 Nov;18(4):661–670. doi: 10.1111/j.1365-2958.1995.mmi_18040661.x.. [DOI] [PubMed] [Google Scholar]
- Homma M., Iino T., Kutsukake K., Yamaguchi S. In vitro reconstitution of flagellar filaments onto hooks of filamentless mutants of Salmonella typhimurium by addition of hook-associated proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6169–6173. doi: 10.1073/pnas.83.16.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Asakura S., Kamiya R. Total reconstitution of Salmonella flagellar filaments from hook and purified flagellin and hook-associated proteins in vitro. J Mol Biol. 1989 Sep 5;209(1):109–114. doi: 10.1016/0022-2836(89)90174-5. [DOI] [PubMed] [Google Scholar]
- Lowe M. A., Holt S. C., Eisenstein B. I. Immunoelectron microscopic analysis of elongation of type 1 fimbriae in Escherichia coli. J Bacteriol. 1987 Jan;169(1):157–163. doi: 10.1128/jb.169.1.157-163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba K., Stubbs G. Structure of tobacco mosaic virus at 3.6 A resolution: implications for assembly. Science. 1986 Mar 21;231(4744):1401–1406. doi: 10.1126/science.3952490. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nguyen J., Baldwin M. A., Cohen F. E., Prusiner S. B. Prion protein peptides induce alpha-helix to beta-sheet conformational transitions. Biochemistry. 1995 Apr 4;34(13):4186–4192. doi: 10.1021/bi00013a006. [DOI] [PubMed] [Google Scholar]
- OOSAWA F., KASAI M. A theory of linear and helical aggregations of macromolecules. J Mol Biol. 1962 Jan;4:10–21. doi: 10.1016/s0022-2836(62)80112-0. [DOI] [PubMed] [Google Scholar]
- Olsén A., Arnqvist A., Hammar M., Sukupolvi S., Normark S. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol Microbiol. 1993 Feb;7(4):523–536. doi: 10.1111/j.1365-2958.1993.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Olsén A., Jonsson A., Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989 Apr 20;338(6217):652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Sjöbring U., Pohl G., Olsén A. Plasminogen, absorbed by Escherichia coli expressing curli or by Salmonella enteritidis expressing thin aggregative fimbriae, can be activated by simultaneously captured tissue-type plasminogen activator (t-PA). Mol Microbiol. 1994 Nov;14(3):443–452. doi: 10.1111/j.1365-2958.1994.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Uratani Y., Asakura S., Imahori K. A circular dichroism study of Salmonella flagellin: evidence for conformational change on polymerization. J Mol Biol. 1972 Jun 14;67(1):85–98. doi: 10.1016/0022-2836(72)90388-9. [DOI] [PubMed] [Google Scholar]
- Woodcock D. M., Crowther P. J., Doherty J., Jefferson S., DeCruz E., Noyer-Weidner M., Smith S. S., Michael M. Z., Graham M. W. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989 May 11;17(9):3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]