Abstract
Autism spectrum disorders (ASD) consist of a spectrum of neurodevelopmental diseases with three salient features: reduced social interactions, impaired communication and repetitive/stereotyped behaviors. In a recent study we found that increased eIF4E (eukaryotic initiation factor 4E)-dependent protein synthesis as a result of genetic deletion of Eif4ebp2 (eIF4E-binding protein 2) in mice, stimulates the production of neuroligins (Nlgns, synaptic cell-adhesion molecules important for synapse regulation) and engenders an imbalance of excitatory to inhibitory synaptic transmission (E/I) in CA1 pyramidal neurons. This imbalance is accompanied with deficits in social interaction, communication and repetitive/stereotyped behaviors in Eif4ebp2−/− mice. Using a compound that blocks cap-dependent translation or by knocking down Nlgn1, we restored the E/I balance and reversed the autism-like social deficits.
Keywords: translational control, ASD, excitation-inhibition balance, autism-like behaviors, mouse models
Modeling ASD behaviors using mice has been an extremely challenging task.1 However, several mouse models of ASD “risk-genes” display autism-like phenotypes in three domains: social interaction, impaired communication and repetitive/stereotyped behaviors, accompanied by alterations in the E/I balance of synaptic transmission.2-9 Moreover, mouse models of fragile-X syndrome (FXS) and tuberous sclerosis (TSC) display autism-like behaviors and altered synaptic plasticity as a result of exaggerated protein synthesis.10 It has also been hypothesized that several signaling pathways, including PI3K (phosphoinositide 3-kinase)/Akt/mTOR (mammalian/mechanistic target of rapamycin) and ERK (extracellular signal-regulated kinase), which have a common endpoint of exaggerated translation, are dysregulated in ASD.11
In our recent study, we examined a mouse model where cap-dependent translation is elevated as a result of genetic deletion of Eif4ebp2 in mice. The 4E-BP2 protein encoded by this gene is a master regulator of translation initiation, as it binds to eIF4E and disrupts the formation of the eIF4F initiation complex, which consists of: eIF4E, the cap-binding protein; eIF4G, a scaffolding protein that bridges the mRNA to the ribosome; and eIF4A, an RNA helicase.11 4E-BPs repress translation by competing with eIF4G for binding to the convex dorsal surface of eIF4E.12 Genetic removal of the 4E-BP2 “brake” on translation initiation, allows eIF4E to preferentially translate a subset of mRNAs.13 In the Eif4ebp2−/− mouse brain we identified neuroligins9 and AMPA-glutamate receptor subunits14 as eIF4E-sensitive mRNAs. Exaggerated translation of those mRNAs engenders an imbalance of excitatory to inhibitory synaptic transmission in CA1 pyramidal neurons of the hippocampus, favoring excitation. Neuroligins are present in excitatory and inhibitory synapses and are important for the maintenance of the E/I balance.15 Increased excitatory synaptic transmission was associated with autism-like phenotypes in the Eif4ebp2−/− mouse: impaired social interaction, as measured by the three-chamber paradigm and direct social interaction tests; enhanced isolation induced ultrasonic vocalizations in pups; and increased grooming and marble-burying behavior. Synaptic plasticity and behavioral deficits were reversed by: (1) an inhibitor of cap-dependent translation, 4EGI-1,16 and (2) using lentiviruses to deliver short-hairpin RNAs against neuroligin 1.
We showed for the first time that increased eIF4E-dependent translation of neuroligins engenders autism-like behaviors by perturbing the E/I balance of synaptic transmission and that in adult mice we can rescue these behavior pharmacologically and with neuroligin-targeting gene therapy. In a complimentary study, it was shown that mice overexpressing eIF4E display similar autism-like phenotypes and synaptic pathophysiology in the medial prefrontal cortex, striatum and hippocampus, while infusion of 4EGI-1 was sufficient to reverse these phenotypes.6
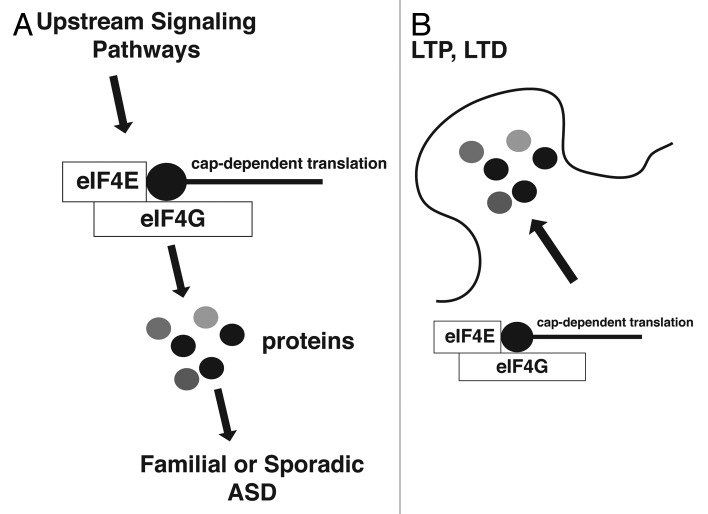
eIF4E-dependent translation has been shown in various systems to regulate gene-expression of a subset of all mRNAs, rather than a non-specific change in global translation rates.17-19 Our study links eIF4E-dependent translation of neuroligins in the brain with autism-like behaviors in mice, without any significant changes to the overall translation rates. This mode of regulation can explain the intricate regulation of synaptic strength through local translational control of synaptic mRNAs (Fig. 1).
Figure 1. A unifying theory for translational control being a common endpoint of familial and sporadic ASD-associated signaling pathways. (A) Familial or sporadic forms of autism can be linked to signaling pathways that have the translation initiation machinery (translation initiation factors eIF4E/4G) as a common endpoint. (B) Local cap-dependent translation of a subset of synaptic mRNAs affects long-term potentiation (LTP) or long-term depression (LTD).
Thus, there is an emerging important role of eIF4E-dependent translation in the development of autism-like behaviors in mouse models, which can then be extrapolated to ASD. Importantly, translational control can be now viewed as a common endpoint and a unifying explanation for ASD-linked mutations, CNVs (copy number variations) that affect signaling pathways upstream of translation, as well as for sporadic forms of autism (Fig. 1). Therefore, it is essential to identify mRNAs that are translationally regulated and study the role of translational control in different brain areas that are implicated in the development of ASDs.
Footnotes
Previously published online: www.landesbioscience.com/journals/cellularlogistics/article/24551
References
- 1.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–5. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- 3.Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–60. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 4.Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–42. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–46. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santini E, Huynh TN, MacAskill AF, Carter AG, Pierre P, Ruggero D, et al. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature. 2013;493:411–5. doi: 10.1038/nature11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlhaus R, Hines RM, Eadie BD, Kannangara TS, Hines DJ, Brown CE, et al. Overexpression of the cell adhesion protein neuroligin-1 induces learning deficits and impairs synaptic plasticity by altering the ratio of excitation to inhibition in the hippocampus. Hippocampus. 2010;20:305–22. doi: 10.1002/hipo.20630. [DOI] [PubMed] [Google Scholar]
- 8.Hines RM, Wu L, Hines DJ, Steenland H, Mansour S, Dahlhaus R, et al. Synaptic imbalance, stereotypies, and impaired social interactions in mice with altered neuroligin 2 expression. J Neurosci. 2008;28:6055–67. doi: 10.1523/JNEUROSCI.0032-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–7. doi: 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–8. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–45. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, et al. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–64. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 14.Ran I, Gkogkas CG, Vasuta C, Tartas M, Khoutorsky A, Laplante I, et al. Selective regulation of GluA subunit synthesis and AMPA receptor-mediated synaptic function and plasticity by the translation repressor 4E-BP2 in hippocampal pyramidal cells. J Neurosci. 2013;33:1872–86. doi: 10.1523/JNEUROSCI.3264-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levinson JN, El-Husseini A. Building excitatory and inhibitory synapses: balancing neuroligin partnerships. Neuron. 2005;48:171–4. doi: 10.1016/j.neuron.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Moerke NJ, Aktas H, Chen H, Cantel S, Reibarkh MY, Fahmy A, et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell. 2007;128:257–67. doi: 10.1016/j.cell.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson O, Morita M, Topisirovic I, Alain T, Blouin MJ, Pollak M, et al. Distinct perturbation of the translatome by the antidiabetic drug metformin. Proc Natl Acad Sci U S A. 2012;109:8977–82. doi: 10.1073/pnas.1201689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–13. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]