Abstract
Plant peptide signaling is an upcoming topic in many areas of plant research. Our recent findings show that the tyrosine sulfated peptide receptors PSKR1 and PSY1R are not only involved in growth and development but also in plant defense. They modulate salicylate- and jasmonate-dependent defense pathways in an antagonistic manner and this phenomenon might be dependent on the age and developmental stage of the plant. Here we discuss how the endogenous peptides might integrate growth, wounding, senescence and the opposing defense pathways against biotrophic and necrotrophic pathogens for increased fitness of the plant.
Keywords: LRR-RLK, plant immunity, sulfated peptide, phytosulfokine, PSKR1, PSY1R, hormone homeostasis
Introduction
In recent years the function of several receptor kinases was revealed showing that members of this large gene family are involved in all aspects of plant growth, development and adaptation to the environment (for an overview see: Receptor-like Kinases in Plants-from Development to Defense, Springer).1 To uncover pathogen inducible plant receptor-like kinases (RLKs) involved in plant defense, we utilized a reverse genetic approach and identified the phytosulfokine receptor PSKR1. Phytosulfokines (PSKs) were first described as secreted cell proliferation promoting peptides found in culture media of asparagus cell cultures.2 Subsequent work demonstrated that the active bisulfated five-amino acid peptide is processed from an approximately 80-amino acid propeptide encoded by five paralogous genes in the Arabidopsis genome.3,4 The RLKs PSKR1, and to a much lesser extent PSKR2, were shown to be critical for the binding of PSKα and for physiological consequences of PSKα perception such as the promotion of root growth and the generation of callus tissue.5,6 Mutations in PSKR1 exhibit early senescence, a salicylate (SA) associated response, and are impaired in wound healing, a jasmonate (JA)-associated response.3,5 This suggests a link between phytosulfokine signaling and the regulation of SA/JA homeostasis. A second peptide with overlapping physiological roles associated with PSKα such as cellular proliferation and differentiation is PSY1, an 18-amino acid sulfated and glycosylated peptide.5 Similarly to PSKα, PSY1 was also identified as a secreted peptide purified from plant cell culture media and is perceived by a related RLK referred to as PSY1R.5 While mutations in the individual receptors results in no morphological phenotypes, mutations in all three of the above mentioned receptors results in slight dwarfism due to a reduction of cell number and size.5
Sulfation of PSKα and PSY1, which is critical for receptor binding and function, is mediated by a unique Golgi-localized tyrosylprotein sulfotransferase (TPST).7 Interestingly, loss of TPST function also results in moderate dwarfism and early senescence, further evidence supporting the importance of sulfated peptide signaling in controlling cellular homeostasis. A recent report by Igarashi, et al.8 described the involvement of PSKR1 but not PSKR2 in PAMP responses, plant defense responses induced by pathogen associated molecular patterns (PAMPs). These PAMPs are perceived by surface receptors including RLKs and receptor like proteins.9 Activation of PAMP-triggered immunity (PTI) includes the production of reactive oxygen species, accumulation of salicylic acid and pathogenesis-related genes, callose deposition and seedling growth inhibition.10,11 The SA-dependent plant defense pathway directed against biotrophic pathogens is thought to work antagonistically to a jasmonate-dependent pathway that fends off necrotrophic pathogens.12-16
PSKR1 mutants exhibited enhanced defense gene expression, increased sensitivity to seedling growth inhibition upon PAMP treatment as well as heightened resistance to the bacterial pathogen Pseudomonas syringae pv tomato (Pto) DC3000. Similarly, PAMP-related phenotypes were also enhanced in tpst mutants and were partially rescued by treatment with PSKα. These results demonstrate that PSKα sulfation and perception is indeed critical for PSKR1-mediated regulation of PAMP responses.8
PSKR1 and PSY1R Function in Plant Defense to Biotrophic and Necrotrophic Pathogens
In our studies we found that plants lacking PSKR1 have heightened resistance to the biotrophic pathogen Pto DC3000.17 Conversely, inoculation of pskr1 mutant plants with the necrotrophic fungal pathogen Alternaria brassicicola leads to enhanced susceptibility compared with wild-type plants. In testing the role of additional sulfated peptide receptors, we found that plants carrying mutations in PSY1R but not PSKR2 also display similarly altered defense responses compared with pskr1 mutants, including enhanced PAMP responsiveness. In pskr1/psy1r double and pskr1/pskr2/psy1r triple receptor mutants, the defense-related phenotypes were found to be additive, suggesting a partially redundant role for PSKα and PSY1 signaling. Molecular analysis revealed that upon Pto DC3000 inoculation, pskr1 mutants, and to a greater extent pskr1/pskr2/psy1r mutants, accumulate elevated levels of salicylate and SA-responsive PR-gene transcripts. However, the JA-responsive genes PDF1.2 and OPR3 were significantly repressed. These findings correlate enhanced levels of SA signaling with increased resistance to the biotrophic pathogen Pto DC3000 and, antagonistically, decreased levels of JA signaling with the loss of resistance to the necrotrophic pathogen Alternaria brassicicola. Taken together, these results suggest a scenario where sulfated peptide signaling shifts the balance of defense signaling toward JA responses. We sought to provide additional data demonstrating that the above mentioned findings stem from the loss of PSKαperception rather than developmental differences of the receptor mutants compared with wild-type plants independent of PSKα signaling. Therefore, we tested plants overexpressing the PSK propeptide-encoding genes PSK2 and PSK4 and found heightened susceptibility to Pto DC3000 infection and enhanced resistance to Alternaria brassicicola. tpst-1 mutants that are thought to lack tyrosine sulfated peptides phenotypically mimicked the defense responses associated with the triple receptor mutant, suggesting that no additional sulfated peptides are associated with these responses. Importantly, pretreatment of tpst-1 plants with PSKα lead to a partial complementation of the Pto DC3000 resistance phenotype, implying a direct effect of PSKα on plant defense and leaving space for the potential additional involvement of PSY1.
New Insights into PSKR1 and PSY1R Functions as Modulators of Plant Defense, Senescence and Wounding
While PSKα and PSY1 signaling was initially linked to plant growth and development, studies have begun to underscore the important role these signaling molecules play in modulating stress responses such as the repression of stress-related gene induction, wound repair and PAMP signaling.3,8,17-19 We observed apparent differences in FRK1 expression in adult leaves17 compared with the results presented by Igarashi, et al.8 They report that seedlings treated with flg22, a flagellin derived elicitor of PTI, does not lead to a significant increase in FRK1 expression in pskr1 mutants. As we were clearly detecting significant differences after flg22 treatment in all assays we concluded that the differences were most likely the consequence of the different tissues used (seedlings8 vs. mature leaves17). To confirm this, we tested seedlings using our experimental conditions to quantify expression levels of FRK1 and found that we also could not detect any significant differences between pskr1 and wild-type plants after flg22 treatment in young tissue (Fig. 1). This result indicates that the observed experimental differences are indeed attributable to the developmental stage of tissue being used. This finding correlates well with the observation that PSK precursor genes PSK2, PSK4 and PSK5 are predominantly expressed in mature leaf tissue and are induced during senescence.3 This correlation indicates that the enhanced FRK1 expression after flg22 treatment in pskr1 plants is a result of heightened PSKα signaling in mature (or early senescent) leaf tissue. However, pskr1 plants did show enhanced FRK1 expression upon treatment with elf18,8 an elongation factor Tu-derived elicitor of PTI. This suggests that flg22- and elf18-induced defense responses might be differentially sensitive to the tissue or age specific PSKα/PSY1-regulated predisposition for altered PAMP responses.
Figure 1.
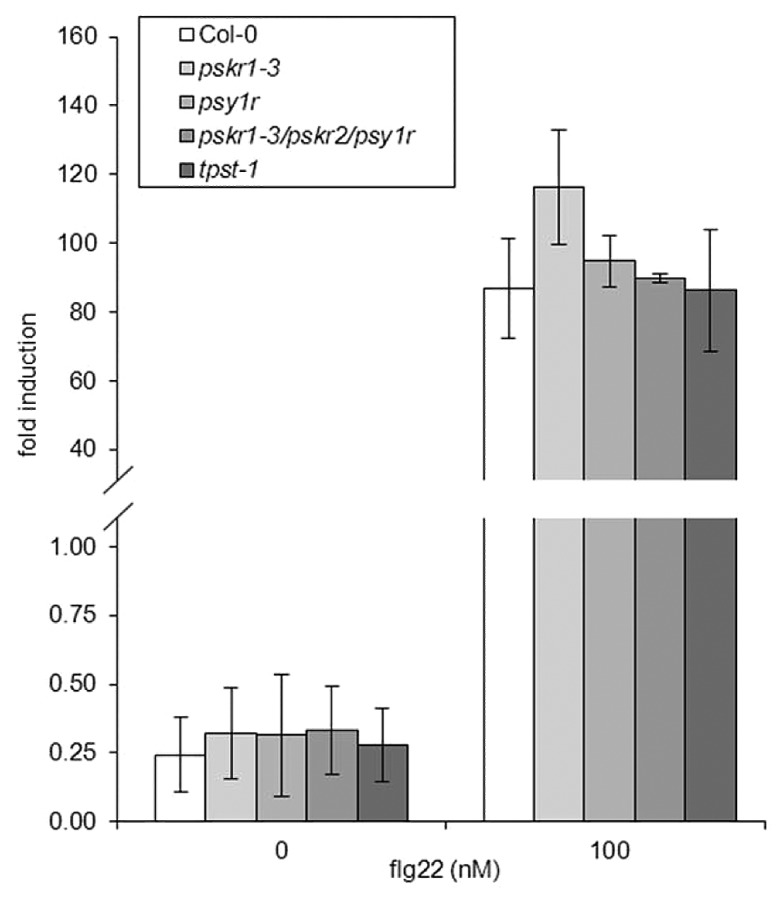
FRK1 gene expression in seedlings treated with flg22. After treatment of 10-d-old seedlings with 100 nM flg22 or water, quantitative RT-PCR experiments were performed on cDNA generated from three independent biological replicates to analyze FRK1 gene expression as described by Mosher, et al.17 Expression values were calculated as a ratio to the housekeeping gene EF1α and presented as fold induction. Bars represent mean ratios +/− SEM.
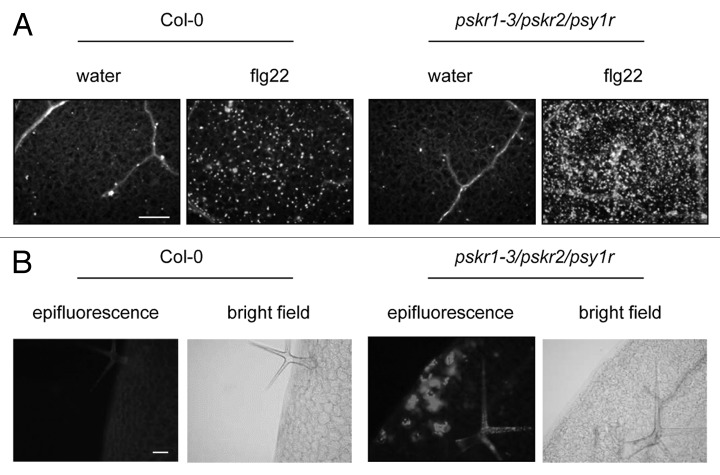
We observed increased callose deposition in pskr1/pskr2/psy1r triple receptor mutants as compared with wild-type plants. Upon infiltration with flg22 callose deposition is strongly induced in wild-type and triple mutant plants with significantly higher responses in the triple mutants (Fig. 2A). Interestingly, callose staining was also observed along the leaf margin even in untreated tissue (Fig. 2B). In agreement with this observation, Amano, et al.5 reported particularly high levels of PSY1 expression in the marginal regions of leaf tissue — the area where senescence processes tend to start.20 Further investigations of the effect of phytosulfokine signaling on wound responses and senescence will increase the understanding of the role of sulfated peptides PSKα and PSY1 in stress responses.
Figure 2.
Callose deposition is heightened in pskr1/pskr2/psy1r mutant plants. Adult plants were stained as described by Mosher, et al.17 with aniline blue for visualization of callose deposition after infiltration with (A) 100nM flg22 or water and (B) in untreated leaf areas at the leaf margin. Bright field pictures show the position of the shown area in the leaf. Scale bar represents 0.2 mm.
PSKα and PSY1 signaling mediated by the respective receptors PSKR1 and PSY1R shifts the hormone homeostasis in favor of the JA pathway and negatively regulates SA accumulation and signaling.17 An additional degree of complexity is added by the fact that PSKα induced growth promotion is mediated by auxin.21 Integration of PSKα and PSY1 signaling in growth promotion, development and defense may include crosstalk between different phytohormones including auxin. Importantly, auxin was shown to have a suppressive effect on SA responses and vice versa.22-25 Loivamaki, et al.18 report reduced crown gall sizes induced by Agrobacterium infection in pskr1 mutants. This is in line with the fact that auxin has a major role in Agrobacterium/Arabidopsis interaction.26 Auxin has also been shown to have a negative impact on senescence which would link another aspect of PSKα action to auxin.27-29 As all consequences of PSKα/PSY1-signaling can be explained by direct or indirect actions of auxin, auxin might be a central regulator that balances defense responses for the benefit of senescence prevention and growth. Further investigations on hormone crosstalk in PSKα/PSY1 signaling are needed to disentangle causes and consequences in this complex network.
Conclusions and Perspectives
Several recent reports have shown that sulfated peptides are important signaling molecules utilized by plants to integrate growth and development programs with stress responses. Since plants continuously undergo exposure to multiple stresses in the natural environment, it is of paramount importance to respond in a timely manner to these stresses. However, activation of stress responses comes at the cost of reduced growth. Improper regulation or prolonged activation of stress responses can lead to stunted growth and even cell death.10,30 Our recent findings support a scenario where PSKα and/or PSY1 perception leads to the downregulation of SA-related responses after biotrophic pathogen infection, thereby preventing an over-induction of this particular signaling pathway that would otherwise reduce the fitness of the plants and leave them vulnerable to necrotrophic pathogens. Investigation into the molecular mechanisms involved in phytosulfokine signaling will shed light on how diverse biological processes are integrated in a way that increases the fitness of plants living on limited resources in a dynamic environment. (Fig.3)
Figure 3.
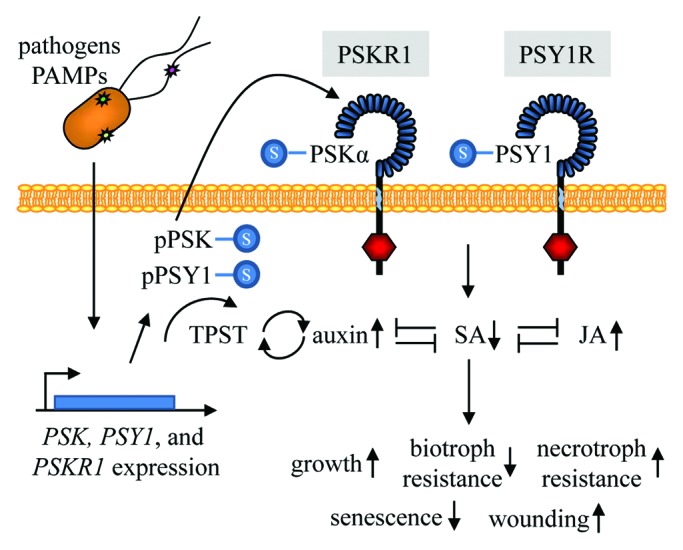
Tyrosine-sulfated peptide signaling in plant defense. PSKR1,PSK1,2 and 4, and PSY1 are induced by Pseudomonas syringae pv tomato DC3000 infection and by PAMPs (depicted as stars in a bacterial cell).17 The preproproteins pPSK and pPSY1, are tyrosine-sulfated (-S) by TPST which most likely takes place in the Golgi apparatus.7 The preproproteins are thought to be proteolytically processed in the apoplast at least in part by subtilisins.31 The fully processed PSKα-S and PSY1-S peptides are then able to bind to their respective receptors, PSKR1 and PSY1R.5,6 Receptors consist of an extracellular leucine rich repeat domain (blue), a single transmembrane domain (light blue) and a cytosolic kinase domain (red). Activation of these receptors leads to the suppression (↓) of SA signaling and consequently suppressed resistance to biotrophic pathogens17 and senescence3 as well as the upregulation (↑) of JA signaling and consequently increased resistance to necrotrophic pathogens17 and increased wounding responsiveness.3,5 PSKα and PSY1 signaling also promotes plant growth2,3,5 and this growth is dependent on auxin.21 TPST expression is induced by auxin signaling and TPST activity promotes the upregulation of auxin signaling,32 suggesting that auxin and TPST pathways are tightly linked. Mutually antagonistic regulation (-|) of auxin, SA and JA have been reported.33 The interplay of these three phytohormones may explain all reported PSKR1- and PSY1R-related phenotypes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24119
References
- 1.Tax FE, Kemmerling B, eds. Receptor-like Kinases in Plants - From Development to Defense. Springer Berlin Heidelberg, 2012. [Google Scholar]
- 2.Matsubayashi Y, Sakagami Y. Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cells of Asparagus officinalis L. Proc Natl Acad Sci USA. 1996;93:7623–7. doi: 10.1073/pnas.93.15.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsubayashi Y, Ogawa M, Kihara H, Niwa M, Sakagami Y. Disruption and overexpression of Arabidopsis phytosulfokine receptor gene affects cellular longevity and potential for growth. Plant Physiol. 2006;142:45–53. doi: 10.1104/pp.106.081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Matsubayashi Y, Nakamura K, Sakagami Y. Diversity of Arabidopsis genes encoding precursors for phytosulfokine, a peptide growth factor. Plant Physiol. 2001;127:842–51. doi: 10.1104/pp.010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amano Y, Tsubouchi H, Shinohara H, Ogawa M, Matsubayashi Y. Tyrosine-sulfated glycopeptide involved in cellular proliferation and expansion in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:18333–8. doi: 10.1073/pnas.0706403104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsubayashi Y, Ogawa M, Morita A, Sakagami Y. An LRR receptor kinase involved in perception of a peptide plant hormone, phytosulfokine. Science. 2002;296:1470–2. doi: 10.1126/science.1069607. [DOI] [PubMed] [Google Scholar]
- 7.Komori R, Amano Y, Ogawa-Ohnishi M, Matsubayashi Y. Identification of tyrosylprotein sulfotransferase in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:15067–72. doi: 10.1073/pnas.0902801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Igarashi D, Tsuda K, Katagiri F. The peptide growth factor, phytosulfokine, attenuates pattern-triggered immunity. Plant J. 2012;71:194–204. doi: 10.1111/j.1365-313X.2012.04950.x. [DOI] [PubMed] [Google Scholar]
- 9.Mazzotta S, Kemmerling B. Pattern recognition in plant innate immunity. J Plant Pathol. 2011;93:7–17. [Google Scholar]
- 10.Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 11.Segonzac C, Zipfel C. Activation of plant pattern-recognition receptors by bacteria. Curr Opin Microbiol. 2011;14:54–61. doi: 10.1016/j.mib.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–27. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 13.Koornneef A, Pieterse CM. Cross talk in defense signaling. Plant Physiol. 2008;146:839–44. doi: 10.1104/pp.107.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spoel SH, Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe. 2008;3:348–51. doi: 10.1016/j.chom.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Vlot AC, Dempsey DA, Klessig DF. Salicylic Acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 16.von Saint Paul V, Zhang W, Kanawati B, Geist B, Faus-Kessler T, Schmitt-Kopplin P, et al. The Arabidopsis glucosyltransferase UGT76B1 conjugates isoleucic acid and modulates plant defense and senescence. Plant Cell. 2011;23:4124–45. doi: 10.1105/tpc.111.088443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosher S, Seybold H, Rodriguez P, Stahl M, Davies KA, Dayaratne S, et al. The Tyrosine-sulfated peptide receptors PSKR1 and PSY1R modify Arabidopsis immunity to biotrophic and necrotrophic pathogens in an antagonistic manner. Plant J. 2013;73:469–82. doi: 10.1111/tpj.12050. [DOI] [PubMed] [Google Scholar]
- 18.Loivamäki M, Stührwohldt N, Deeken R, Steffens B, Roitsch T, Hedrich R, et al. A role for PSK signaling in wounding and microbial interactions in Arabidopsis. Physiol Plant. 2010;139:348–57. doi: 10.1111/j.1399-3054.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- 19.Motose H, Iwamoto K, Endo S, Demura T, Sakagami Y, Matsubayashi Y, et al. Involvement of phytosulfokine in the attenuation of stress response during the transdifferentiation of zinnia mesophyll cells into tracheary elements. Plant Physiol. 2009;150:437–47. doi: 10.1104/pp.109.135954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quirino BF, Noh YS, Himelblau E, Amasino RM. Molecular aspects of leaf senescence. Trends Plant Sci. 2000;5:278–82. doi: 10.1016/S1360-1385(00)01655-1. [DOI] [PubMed] [Google Scholar]
- 21.Eun C-H, Ko S-M, Matsubayashi Y, Sakagami Y, Kamada H. Phytosulfokine-alpha requires auxin to stimulate carrot non-embryogenic cell proliferation. Plant Physiol Biochem. 2003;41:447–52. doi: 10.1016/S0981-9428(03)00052-4. [DOI] [Google Scholar]
- 22.Navarro L, Bari R, Achard P, Lisón P, Nemri A, Harberd NP, et al. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol. 2008;18:650–5. doi: 10.1016/j.cub.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 23.Park CM. Auxin homeostasis in plant stress adaptation response. Plant Signal Behav. 2007;2:306–7. doi: 10.4161/psb.2.4.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert-Seilaniantz A, Grant M, Jones JD. Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol. 2011;49:317–43. doi: 10.1146/annurev-phyto-073009-114447. [DOI] [PubMed] [Google Scholar]
- 25.Kazan K, Manners JM. Linking development to defense: auxin in plant-pathogen interactions. Trends Plant Sci. 2009;14:373–82. doi: 10.1016/j.tplants.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Lee CW, Efetova M, Engelmann JC, Kramell R, Wasternack C, Ludwig-Müller J, et al. Agrobacterium tumefaciens promotes tumor induction by modulating pathogen defense in Arabidopsis thaliana. Plant Cell. 2009;21:2948–62. doi: 10.1105/tpc.108.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim PO, Kim HJ, Nam HG. Leaf senescence. Annu Rev Plant Biol. 2007;58:115–36. doi: 10.1146/annurev.arplant.57.032905.105316. [DOI] [PubMed] [Google Scholar]
- 28.Osborne DJ. Control of leaf senescence by auxins. Nature. 1959;183:1459–60. doi: 10.1038/1831459a0. [DOI] [PubMed] [Google Scholar]
- 29.Kim JI, Murphy AS, Baek D, Lee SW, Yun DJ, Bressan RA, et al. YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. J Exp Bot. 2011;62:3981–92. doi: 10.1093/jxb/err094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gómez-Gómez L, Felix G, Boller T. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J. 1999;18:277–84. doi: 10.1046/j.1365-313X.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava R, Liu JX, Howell SH. Proteolytic processing of a precursor protein for a growth-promoting peptide by a subtilisin serine protease in Arabidopsis. Plant J. 2008;56:219–27. doi: 10.1111/j.1365-313X.2008.03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou W, Wei L, Xu J, Zhai Q, Jiang H, Chen R, et al. Arabidopsis Tyrosylprotein sulfotransferase acts in the auxin/PLETHORA pathway in regulating postembryonic maintenance of the root stem cell niche. Plant Cell. 2010;22:3692–709. doi: 10.1105/tpc.110.075721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazan K, Manners JM. JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci. 2012;17:22–31. doi: 10.1016/j.tplants.2011.10.006. [DOI] [PubMed] [Google Scholar]