Abstract
Arbuscular mycorrhizal (AM) symbiosis, established between AM fungi (AMF) and roots of higher plants, occurs in most terrestrial ecosystems. It has been well demonstrated that AM symbiosis can improve plant performance under various environmental stresses, including drought stress. However, the molecular basis for the direct involvement of AMF in plant drought tolerance has not yet been established. Most recently, we cloned two functional aquaporin genes, GintAQPF1 and GintAQPF2, from AM fungus Glomus intraradices. By heterologous gene expression in yeast, aquaporin localization, activities and water permeability were examined. Gene expressions during symbiosis in expose to drought stress were also analyzed. Our data strongly supported potential water transport via AMF to host plants. As a complement, here we adopted the monoxenic culture system for AMF, in which carrot roots transformed by Ri-T DNA were cultured with Glomus intraradices in two-compartment Petri dishes, to verify the aquaporin gene functions in assisting AMF survival under polyethylene glycol (PEG) treatment. Our results showed that 25% PEG significantly upregulated the expression of two aquaporin genes, which was in line with the gene functions examined in yeast. We therefore concluded that the aquaporins function similarly in AMF as in yeast subjected to osmotic stress. The study provided further evidence to the direct involvement of AMF in improving plant water relations under drought stresses.
Keywords: aquaporin, arbuscular mycorrhiza, monoxenic culture, Glomus intraradices, drought stress
Aquaporins, a multifunctional protein family branched from the major intrinsic protein (MIP) superfamily, are commonly present in nearly all organisms. They facilitate the transport of water and certain small molecules across biological membranes, and are fundamentally important for the osmoregulation of organisms.1-5 There are five groups of fungal aquaporins, with two groups of classical aquaporins and three groups of aquaglyceroporins. These aquaporins have been fully described in Saccharomyces cerevisiae and filamentous fungi, but not in symbiotic mycorrhizal fungi.3,6,7 Most recently, we reported “First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices” in New Phytologist.8 The study provided solid data and strong support to the direct involvements of AMF in plant drought tolerance. The aquaporins could play important roles not only in helping AM fungi themselves to resist drought stress, but also in delivering water via AM fungi to host plants.
Briefly, based on G. intraradices expressed sequence tag (EST) database we cloned two aquaporin genes, namely GintAQPF1 and GintAQPF2, by 5′-RACE and 3′-RACE techniques. The two genes had high similarities with the only two predicted aquaporin genes existing in nonredundant virtual transcripts (NRVTs) of G. intraradices (DAOM 197198) according to Tisserant et al. (2012).9 Aquaporin activities and water permeability were examined by heterologous gene overexpression in yeast. The two aquaporins showed high capacity to transport water and high activities in expose to PEG treatment, which is crucial for yeast cells to survive the osmotic stresses.
Here we conducted follow-up experiment to examine the aquaporin functions in AM fungi subjected to osmotic stress. We adopted the monoxenic culture system for AMF. In the system, carrot (Daucus carota L.) roots transformed by Ri-T DNA were cultured with sterilized spores of G. intraradices AH01 in two-compartment Petri dishes.10 The root compartment (RC) contained solid minimal medium (M medium) to initiate the monoxenic culture, and the other compartment for hyphal development (HC) was filled with liquid M medium without sucrose (M-C medium). After cultivation for around three months, osmotic stress was imposed to HC by adding a final concentration of 25% PEG 6000 for 5 d. Extraradical mycelium (ERM) in HC was then harvested for analysis of gene expressions (methods refer to Aroca et al.)11. The experimental data indicated that PEG stress significantly stimulated the expression of the two aquaporin genes in ERM (Fig. 1), which was in accordance with the heterologous system experiment. It is therefore reasonable to ratiocinate that the two aquaporin genes were responsible for AMF water absorption and tolerance to osmotic stress. Together with previous findings that the gene expressions during symbiosis were also significantly enhanced under drought stress,8 the study lent further support to the importance of AMF in balancing plant water relations.
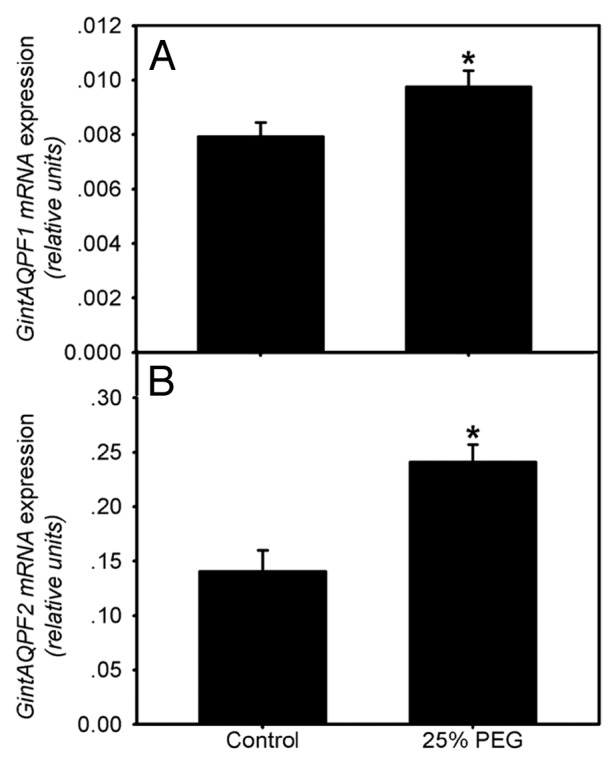
Figure 1. Expression of GintAQPF1 (A) and GintAQPF2 (B) in extraradical mycelium of Glomus intraradices in expose to 25% PEG. “*” above the column indicates significant difference between treatments by t-test (p < 0.05). The error bars represent standard deviation.
As known to us, this is the first report on functional aquaporin genes from AM fungi. We provided molecular evidences for the direct involvement of AM fungi in plant drought tolerance, and had one step forward in understanding the mechanisms underlying enhanced plant drought tolerance by AM associations. As Bonfante and Genre stated,12 there was cross talk between plants and AM fungi. Further research based on genomic and proteomic technologies are still necessary to reveal the interactions between the symbiotic partners under drought stresses.
Acknowledgments
The study was financially supported by Knowledge Innovation Program of the Chinese Academy of Sciences (Project no. KZCX2-YW-BR-17) and the State Key Laboratory of Urban and Regional Ecology (Project no. SKLURE2008-1-03).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24030
References
- 1.Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, et al. Aquaporin water channels--from atomic structure to clinical medicine. J Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–98. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 3.Pettersson N, Filipsson C, Becit E, Brive L, Hohmann S. Aquaporins in yeasts and filamentous fungi. Biol Cell. 2005;97:487–500. doi: 10.1042/BC20040144. [DOI] [PubMed] [Google Scholar]
- 4.Tanghe A, Van Dijck P, Thevelein JM. Why do microorganisms have aquaporins? Trends Microbiol. 2006;14:78–85. doi: 10.1016/j.tim.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- 6.Laizé V, Tacnet F, Ripoche P, Hohmann S. Polymorphism of Saccharomyces cerevisiae aquaporins. Yeast. 2000;16:897–903. doi: 10.1002/1097-0061(200007)16:10<897::AID-YEA583>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira R, Lages F, Silva-Graça M, Lucas C. Fps1p channel is the mediator of the major part of glycerol passive diffusion in Saccharomyces cerevisiae: artefacts and re-definitions. Biochim Biophys Acta. 2003;1613:57–71. doi: 10.1016/S0005-2736(03)00138-X. [DOI] [PubMed] [Google Scholar]
- 8.Li T, Hu YJ, Hao ZP, Li H, Wang YS, Chen BD. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013;197:617–30. doi: 10.1111/nph.12011. [DOI] [PubMed] [Google Scholar]
- 9.Tisserant E, Kohler A, Dozolme-Seddas P, Balestrini R, Benabdellah K, Colard A, et al. The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol. 2012;193:755–69. doi: 10.1111/j.1469-8137.2011.03948.x. [DOI] [PubMed] [Google Scholar]
- 10.St-Arnaud M, Hamel C, Vimard B, Caron M, Fortin JA. Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycol Res. 1996;100:328–32. doi: 10.1016/S0953-7562(96)80164-X. [DOI] [Google Scholar]
- 11.Aroca R, Bago A, Sutka M, Paz JA, Cano C, Amodeo G, et al. Expression analysis of the first arbuscular mycorrhizal fungi aquaporin described reveals concerted gene expression between salt-stressed and nonstressed mycelium. Mol Plant Microbe Interact. 2009;22:1169–78. doi: 10.1094/MPMI-22-9-1169. [DOI] [PubMed] [Google Scholar]
- 12.Bonfante P, Genre A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat Commun. 2010;1:48. doi: 10.1038/ncomms1046. [DOI] [PubMed] [Google Scholar]


