Abstract
Phytohormones are essential regulators of various processes in plant growth and development. Several phytohormones are also known to regulate plant responses to environmental stress and pathogens. Only recently, cytokinins have been demonstrated to play an important role in plant immunity. Increased levels of cytokinins such as trans-zeatin, which are considered highly active, induced resistance against mainly (hemi)biotrophic pathogens in different plant species. In contrast, cis-zeatin is commonly regarded as a cytokinin exhibiting low or no activity. Here we comparatively study the impact of both zeatin isomers on the infection of Nicotiana tabacum by the (hemi)biotrophic microbial pathogen Pseudomonas syringae. We demonstrate a biological effect of cis-zeatin and a differential effect of the two zeatin isomers on symptom development, defense responses and bacterial multiplication.
Keywords: cytokinin, phytohormone, plant immunity, plant pathogen interaction, Pseudomonas syringae, plant defense, plant resistance, tobacco, zeatin
Various phytohormones are known to mediate specific plant responses to pathogen attack. Depending on the plant-pathogen interaction, such defense reactions are mainly regulated by ethylene, jasmonic and/or salicylic acid (SA) and their signaling components.1,2 Over the past years, the involvement of other phytohormones such as abscisic acid, auxin or gibberellins to modulate plant immunity became increasingly evident.1,2 Only recently, several studies focused on the role of the classic phytohormone cytokinin (CK) in plant immunity.
CKs have been demonstrated to modulate defense responses of several plant species to mainly (hemi)biotrophic,3-5 but also to necrotrophic pathogens.3,6 Depending on the plant species, various mechanisms such as the formation of antimicrobial phytoalexins4,7 or regulation of defense genes and other phytohormones such as SA3-5,8 have been shown to be CK responsive. The effects of CKs on plant immunity have been shown in different pathosystems to act in a dose dependent manner.4,5 These studies focused on the application of CKs known as highly active such as benzyl adenine, kinetin or trans-zeatin (tZ).3-5,8 Although cis-zeatin (cZ) is generally described as lowly or non-active in comparison to the trans-isomer, cZ is ubiquitously present in plants and recent studies propose physiological functions for it.9-11 Since various phytopathogens such as Rhodococcus fascians,12 Colletotrichum graminicola13 or Magnaporthe oryzae8 are capable of producing and modulating cZ-type CKs at infection sites, also a potential impact of cZ on plant immunity must be considered. Here, we comparatively study the differential impact of cZ and tZ treatment on tobacco-Pseudomonas syringae pv tabaci (Pst) interaction, a well-established pathosystem to analyze CK effects on plant immunity.4
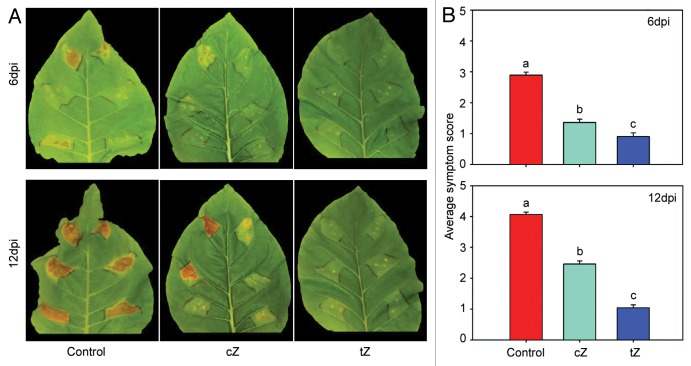
The analysis of free base cZ and tZ levels at the time-point of infection revealed a high and distinct accumulation of the according isomer applied (Table 1). In samples treated with cZ only this isomer accumulated without increasing levels of tZ compared with the untreated control (Table 1). Likewise, treatment with tZ results in elevated levels of this isomer in the tissue. Interestingly, a tendency of increased cZ levels following tZ treatment was also observed, although this accumulation was not significantly different compared with the controls. This finding excludes potential isomerization processes,11 which is in agreement with the failure to demonstrate cis-trans-isomerization in tobacco cells.9 Thus, the differential effects on Pst infection can directly be related to the specific zeatin isomer applied to the leaves. However, differences between cZ and tZ transport, degradation and conjugation processes must be assumed.9,11 These processes most likely contribute to the approximately 2-fold difference in the free base accumulation of the zeatin isomers at the end of (pre-) treatment (Table 1). Treatment with tZ increased SA levels significantly compared with the untreated controls, whereas a tendency of enhanced accumulation of SA by cZ at the time-point of infection compared with the untreated controls was evident (Table 1). In contrast, the accumulation of the phytoalexin scopoletin showed no significant differences between the treatments (Table 1). This indicates a higher activity of tZ compared with cZ to induce defense mechanisms at least in tobacco. Despite these differences in SA accumulation, both pre-treatments resulted in strongly suppressed symptom development at 6 and 12 dpi compared with the (NaOH treated) control leaves (Fig. 1A). The average symptom scores at 6 and 12 dpi indicate that the suppressive effect of tZ on Pst infection symptoms is significantly stronger compared with cZ (Fig. 1B). Although both zeatin isomers caused a significant reduction in symptom development post Pst infection, only tZ significantly restricted pathogen proliferation (Fig. 2). This efficient inhibition of pathogen growth through tZ was already visible at 48 h post infection (hpi), while cZ appeared to have no impact on Pst proliferation (Fig. 2). This difference could be at least partially explained by the diverging SA accumulation post cZ and tZ treatment and could be the reason for the stronger suppression of infection symptoms through tZ.
Table 1. Accumulation of tZ, cZ, SA and scopoletin in tobacco leaves.
| Phytohormone/scopoletin accumulation [ng/g FW] | ||||
|---|---|---|---|---|
| Sample | cZ | tZ | SA | Scopoletin |
| 0 h control | 8.44 ± 5.12b | 0.87 ± 0.65b,c | 14.74 ± 5.70c | 7.24 ± 3.38a |
| 24 h NaOH | 1.61 ± 0.84b | 0.09 ± 0.03c | 24.37 ± 11.95b,c | 28.95 ± 12.73a |
| 24 h cZ | 372.64 ± 13.57a | 0.84 ± 0.28b | 32.82 ± 10.66b | 18.21 ± 4.76a |
| 24 h tZ | 25.32 ± 17.59b | 172.65 ± 15.79a | 51.66 ± 14.25a | 24.48 ± 10.07a |
Determination of cZ, tZ, SA and scopoletin of whole leaves exclusively middle rib of indicated treatments. Mean values of three biological replicates, consisting of two technical replicates each, in ng/g fresh weight (FW) ± st. error. Letters indicate different significance groups based on paired, two-sided Student’s t-test (p < 0.05).
Figure 1. Symptom development of Pst infection in tobacco leaves after control treatment and treatment with 10 µM cZ or tZ. (A) Representative symptom development in infected tobacco leaves after indicated treatment 6 and 12 dpi. (B) Average symptom development of infected tobacco leaves after indicated treatment 6 and 12 dpi. Mean values ± st. error of three (6 dpi) and four (12 dpi) independent biological experiments consisting of ten leaves each. Letters indicate different significance groups based on paired, two-sided Student’s t-test (p < 0.05).
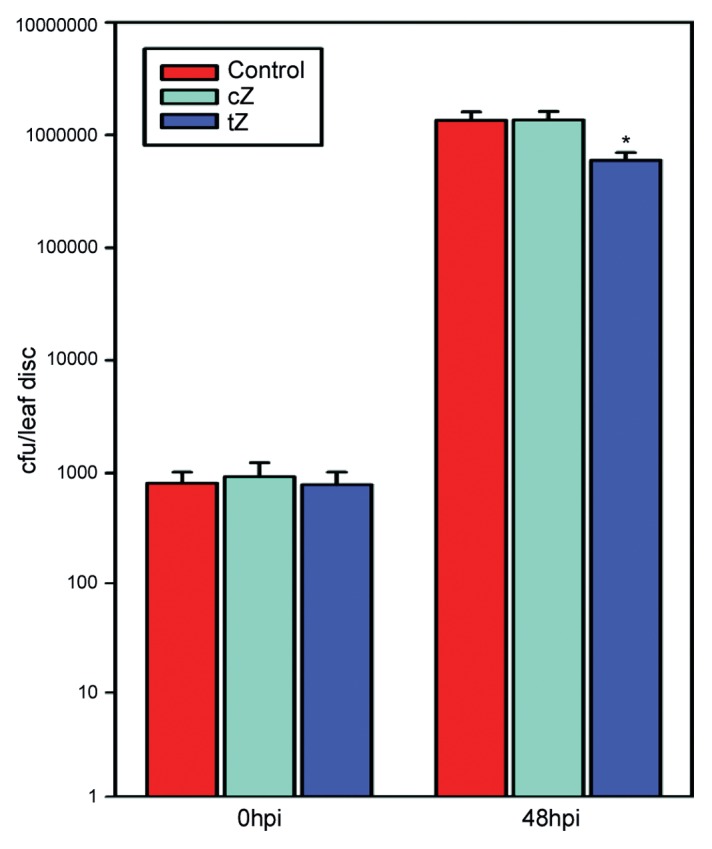
Figure 2.Pst growth in tobacco leaves after different pre-treatments 0 and 48 hpi. Mean values ± st. error of four independent biological experiments, consisting of three sets of three technical replicates each. *indicates significant difference to control and cZ treatment based on paired, two-sided Student’s t-test (p < 0.05).
Our data show that both zeatin isomers strongly suppress symptom development of Pst infection in tobacco, similarly to the reported effect of kinetin.4 However, differences in the potential of the physiological effect on plant immunity are obvious. Only tZ significantly increased SA accumulation and efficiently restricted pathogen proliferation, which is in agreement with findings of stronger biological effects of tZ compared with cZ in tobacco callus growth assays.14 Nevertheless, the suppression of infection symptoms by cZ was clearly shown, indicating a role of cZ in plant immunity, which could be even more important in plants responding stronger to cZ compared with tobacco such as rice.10 Higher levels of cZ than used in this study (maybe in combination with other CKs) could additionally have an impact on defense reactions or a stronger effect on plant fitness, which provides more time for defense reactions to efficiently act directly against the pathogen. The restriction of Pst proliferation through tZ appeared to be less distinct than reported for kinetin, while the suppression of symptoms seemed to be even stronger.4 Considering the differences in the dynamics of SA accumulation and the physiological effects (symptoms and pathogen proliferation) between the very potent CKs kinetin4 and tZ (this study), the involvement of distinct mechanisms in CK mediated resistance have to be assumed. Apparently, the combination of these different mechanisms by which the individual CKs function to restrict pathogen infection contribute to host plant and pathogen specific resistance. Thus, the efficiency of the final defense response depends on the spectrum of CKs affected. Since cZ shows neither significant impact on SA accumulation nor on Pst proliferation but still reduced symptom development, a more general CK impact on plant fitness and the physiological status has to be considered. In addition to the induction of distinct defense reactions the classical and well established physiological CK effects such as the induction of apoplasmic phloem unloading and sink metabolism15,16 as well as the delay of senescence through invertase activity,17 contribute to maintain plant tissue integrity and thereby plant fitness. The maintenance of tissue integrity integrated within the whole plant-pathogen interaction can extend the time frame during which distinct defense reactions act on the pathogen itself. This can strengthen the overall pathogen response and, thus, cZ also modulates plant immunity. An increasing number of experimental evidence is accumulating that demonstrates a close and coordinated regulation of primary carbohydrate metabolism and defense responses.18,19 Thus, the combination of different defense mechanisms regulated by the CK spectrum not only determines the efficiency by which pathogen growth is restricted, but also influences the physiological status to limit the fitness trade-off associated with defense responses. CKs are therefore of general interest for applications in plant protection as they are potent means to limit biomass losses during infections. Consequently, CK specific effects, also in correlation to other phytohormones or to other CK derivatives have to be considered in future studies on plant immunity.
Taken together, our data strongly support a general role of CKs in plant immunity, which was implied by several recent studies.3-5,8 Whereas these previous studies only focused on highly active CKs, including tZ, the findings of this study also demonstrate a role of cZ in modulating plant immunity. Particularly with respect to the known cZ modulations and production by various pathogens,8,12,13 our study shows that cZ needs to be considered as additional regulator of plant-pathogen interactions. The changes in both the levels and spectrum of CKs may lead to differential modulation of plant immunity and fitness, also depending on the plant species or pathogen-interaction. Additionally, natural variations of CK content and spectrum within one plant may control the defense status of specific organs and thus contribute to the regulation of optimal defense patterns.20
Material and Methods
To address the impact of cZ and tZ on plant immunity, the experimental design was adopted from experiments using kinetin within the tobacco-Pst pathosystem.4 Detached leaves of 7–10-wk-old N. tabacum SR1 plants, grown under greenhouse conditions,4 were fed via petioles with 10 µM cZ (Olchemim Ltd.,), tZ (Duchefa), diluted from 100 mM stock solutions in 0.5 M NaOH (Roth) or accordingly diluted NaOH solvent control for 24 h prior to Pst infection.4,17 After this pre-treatment, leaves were infiltrated with 106 cfu/ml Pst in 10 mM MgCl2 and leaves were put back on water. The bacteria were derived from a exponentially growing liquid culture in LB medium supplemented with 20 mg/l tetracycline (Sigma-Aldrich).4 At the time-point of infection, levels of cZ, tZ, SA and the phytoalexin scopoletin were determined in the leaves as reported before.4 Therefore, middle ribs of leaves were excised and leaf material was frozen in liquid nitrogen. After grinding, phytohormones21,22 and scopoletin23 were extracted from leaf tissue and analyzed as described previously. For the evaluation of resistance effects, scoring of symptom development at 6 and 12 d post infection (dpi) based on a six-category scale (“0, no symptoms” to “5, maximum necrosis”) and determination of Pst growth in planta were performed.4 To evaluate pathogen growth, defined leaf discs were harvested with a cork borer (0.4 cm diameter) and ground in 10 mM MgCl2. The suspensions were plated in serial dilutions on LB plates supplemented with 20 mg/l tetracycline. After incubation at 28°C for 36 h, colonies on plates of suitable dilutions were count to determine colony forming units (cfu).
Acknowledgments
The authors are grateful to Peter Krbez and Klaus Remele for technical assistance in instrumental analyses and to Helga Hammer for perfect plant care. D.K.G. thanks the Society for the Advancement of Plant Sciences (Vienna, Austria) for funding.
Glossary
Abbreviations:
- CK
cytokinin
- cZ
cis-zeatin
- dpi
days post infection
- hpi
hours post infection
- Pst
Pseudomonas syringae pv. tabaci
- SA
salicylic acid
- tZ
trans-zeatin
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24798
References
- 1.Grant MR, Jones JDG. Hormone (dis)harmony moulds plant health and disease. Science. 2009;324:750–2. doi: 10.1126/science.1173771. [DOI] [PubMed] [Google Scholar]
- 2.Robert-Seilaniantz A, Grant M, Jones JDG. Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol. 2011;49:317–43. doi: 10.1146/annurev-phyto-073009-114447. [DOI] [PubMed] [Google Scholar]
- 3.Choi J, Huh SU, Kojima M, Sakakibara H, Paek K-H, Hwang I. The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in Arabidopsis. Dev Cell. 2010;19:284–95. doi: 10.1016/j.devcel.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Grosskinsky DK, Naseem M, Abdelmohsen UR, Plickert N, Engelke T, Griebel T, et al. Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiol. 2011;157:815–30. doi: 10.1104/pp.111.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argueso CT, Ferreira FJ, Epple P, To JPC, Hutchison CE, Schaller GE, et al. Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genet. 2012;8:e1002448. doi: 10.1371/journal.pgen.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swartzberg D, Kirshner B, Rav-David D, Elad Y, Granot D. Botrytis cinerea induces senescence and is inhibited by autoregulated expression of the IPT gene. Eur J Plant Pathol. 2008;120:289–97. doi: 10.1007/s10658-007-9217-6. [DOI] [Google Scholar]
- 7.Großkinsky DK, van der Graaff E, Roitsch T. Phytoalexin transgenics in crop protection--fairy tale with a happy end? Plant Sci. 2012;195:54–70. doi: 10.1016/j.plantsci.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Jiang CJ, Shimono M, Sugano S, Kojima M, Liu X, Inoue H, et al. Cytokinins act synergistically with salicylic acid to activate defense gene expression in rice. Mol Plant Microbe Interact. 2013;26:287–96. doi: 10.1094/MPMI-06-12-0152-R. [DOI] [PubMed] [Google Scholar]
- 9.Gajdosová S, Spíchal L, Kamínek M, Hoyerová K, Novák O, Dobrev PI, et al. Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J Exp Bot. 2011;62:2827–40. doi: 10.1093/jxb/erq457. [DOI] [PubMed] [Google Scholar]
- 10.Kudo T, Makita N, Kojima M, Tokunaga H, Sakakibara H. Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-Zeatin-O-glucosyltransferase in rice. Plant Physiol. 2012;160:319–31. doi: 10.1104/pp.112.196733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spíchal L. Cytokinins - recent news and views of evolutionally old molecules. Funct Plant Biol. 2012;39:267–84. doi: 10.1071/FP11276. [DOI] [PubMed] [Google Scholar]
- 12.Pertry I, Václavíková K, Depuydt S, Galuszka P, Spíchal L, Temmerman W, et al. Identification of Rhodococcus fascians cytokinins and their modus operandi to reshape the plant. Proc Natl Acad Sci USA. 2009;106:929–34. doi: 10.1073/pnas.0811683106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behr M, Motyka V, Weihmann F, Malbeck J, Deising HB, Wirsel SGR. Remodeling of cytokinin metabolism at infection sites of Colletotrichum graminicola on maize leaves. Mol Plant Microbe Interact. 2012;25:1073–82. doi: 10.1094/MPMI-01-12-0012-R. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz RY, Skoog F, Playtis AJ, Leonard NJ. Cytokinins: synthesis and biological activity of geometric and position isomers of zeatin. Plant Physiol. 1972;50:702–5. doi: 10.1104/pp.50.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehness R, Roitsch T. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J. 1997;11:539–48. doi: 10.1046/j.1365-313X.1997.11030539.x. [DOI] [PubMed] [Google Scholar]
- 16.Roitsch T, Ehneß R. Regulation of source/sink relations by cytokinins. Plant Growth Regul. 2000;32:359–67. doi: 10.1023/A:1010781500705. [DOI] [Google Scholar]
- 17.Balibrea Lara ME, Gonzalez Garcia MC, Fatima T, Ehness R, Lee TK, Proels R, et al. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell. 2004;16:1276–87. doi: 10.1105/tpc.018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roitsch T. Source-sink regulation by sugar and stress. Curr Opin Plant Biol. 1999;2:198–206. doi: 10.1016/S1369-5266(99)80036-3. [DOI] [PubMed] [Google Scholar]
- 19.Berger S, Sinha AK, Roitsch T. Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J Exp Bot. 2007;58:4019–26. doi: 10.1093/jxb/erm298. [DOI] [PubMed] [Google Scholar]
- 20.Meldau S, Erb M, Baldwin IT. Defence on demand: mechanisms behind optimal defence patterns. Ann Bot. 2012;110:1503–14. doi: 10.1093/aob/mcs212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novák O, Hauserová E, Amakorová P, Doležal K, Strnad M. Cytokinin profiling in plant tissues using ultra-performance liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry. 2008;69:2214–24. doi: 10.1016/j.phytochem.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Forcat S, Bennett MH, Mansfield JW, Grant MR. A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods. 2008;4:16. doi: 10.1186/1746-4811-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharan M, Taguchi G, Gonda K, Jouke T, Shimosaka M, Hayashida N, et al. Effects of methyl jasmonate and elicitor on the activation of phenylalanine ammonia-lyase and the accumulation of scopoletin and scopolin in tobacco cell cultures. Plant Sci. 1998;132:13–9. doi: 10.1016/S0168-9452(97)00260-4. [DOI] [Google Scholar]