Abstract
Background
Obstructive sleep apnea (OSA) is a prevalent disorder with multiple consequences including negative effects on neurocognitive function. Several domains of cognitive function are impaired in OSA patients, but the mechanisms through which this sleep disorder results in impairment are not clear. Given the well-known effects of cortisol on cognitive function, in particular memory, the dysregulating effects of OSA on cortisol levels is hypothesized as a potential pathway leading to cognitive impairment.
Methods
Fifty-five participants with OSA (mean apnea-hypopnea index [AHI], 30.3) were assessed over 2 days. Over a 24-hour period, blood was collected every 2 hours to examine cortisol levels. The following night, sleep was monitored with polysomnography (PSG). Participants were given a battery of neurocognitive tests, which assessed 7 cognitive domains.
Results
OSA severity assessed by oxygen desaturation index (ODI) was associated with 24-hour cortisol levels. AHI, ODI, and nighttime cortisol levels were associated with global deficit scores (GDS) in cognitive functioning, particularly in domains of learning, memory, and working memory (P<.05 for all). Hierarchical linear regression analysis revealed that nighttime cortisol accounted for 9% to 16% of variance in learning (P=.018), memory (P=.003), and working memory (P=.016) domains, though apnea severity did not significantly predict any additional variance.
Conclusions
In our sample of patients with OSA, nocturnal cortisol levels were associated with neuropsychologic functioning above and beyond the influence of covariates and apnea severity. These findings suggest that OSA-related alterations in cortisol activity may partially explain the pathophysiology of neuropsychologic impairments in sleep apnea.
Keywords: Obstructive sleep apnea, sleep, neurocognitive function, memory, cortisol, hypothalamic-pituitary-adrenal axis
1. Introduction
Obstructive sleep apnea (OSA) is a sleep disorder involving repeated episodes of complete or partial obstruction of the upper airway, which cause transient cessations of breathing during sleep. These breathing disruptions cause intermittent hypoxia and sleep disturbances that are associated with daytime sleepiness and fatigue [1]. OSA also has been demonstrated to have negative effects on cognitive functioning, which add to the public health risks for the disease [2]. The neurobehavioral consequences of OSA extend to functional impairments, such as impaired driving, increased risk for accidents, and decreased quality of life [3,4]. Numerous studies have examined the effects of OSA on the cognitive abilities underlying these neurobehavioral consequences. Impairments in memory, vigilance, psychomotor performance, and executive function all have been reported in OSA patients, but the presence and degree of impairments is inconsistent between studies [5-12]. A recent meta-analysis [2] of systematic reviews found support for impairments in attention or vigilance, delayed verbal and visual long-term memory, visuospatial or constructional abilities, and executive function. Further, neuroimaging studies have reported structural changes in specific brain regions associated with cognitive function, and the loss of hippocampal volumes in OSA patients is consistently reported [13]. Associations between cognitive function tests and magnetic resonance imaging findings have been reported for verbal memory and information processing [14] as well as for verbal memory and executive function [15].
The mechanisms by which OSA results in neurocognitive dysfunction are not entirely clear for several reasons. The comorbidities that accompany OSA (e.g., hypertension) are associated with neural injury, making it important to control for confounding associations of OSA to neurocognitive impairment (see [1] for review). In addition, the variety of tests used to assess neurocognitive function along with the varied sample characteristics are vast, which limit the possibilities for comparing the reported cognitive sequelae of OSA [16]. Further, individual differences in the severity of sleep disturbances also may add variability to the pattern of neurocognitive deficits observed in patients with OSA, with greater deficits found in more severe OSA, particularly in the case of executive function [17,18].
The neurochemical, vascular, and structural changes accompanying hypoxemia and sleep fragmentation have been implicated in the adverse effect of OSA on cognitive functioning (see [16] for review). One potential pathway of interest might be dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis. The primary human product of the HPA axis is cortisol, a hormone that evidences a strong diurnal rhythm. The fluctuation of cortisol throughout the night is intricately related to sleep, and thus it has been proposed as an important mechanism through which sleep disorders manifest some of their physiologic changes. Further, both the hippocampus and amygdala, which are strongly involved in memory processes, express a high density of corticosteroid receptors [19]. Given the association between cortisol and cognitive function and the association between OSA and cognitive dysfunction, the role that cortisol may play in cognitive dysfunction in OSA is worthy of investigation.
The literature linking OSA and HPA function is mixed, and few studies have found differences in cortisol levels between OSA and healthy patients [20]. However, many studies are limited by the assessment of cortisol levels at a single time point [21,22]. Those investigations that have reported differences used extensive circadian sampling [23]; additionally, elevated cortisol levels appeared to be corrected after treatment with continuous positive airway pressure (CPAP) [23-25]. Cortisol levels, especially during the night, might be an important factor in the association between OSA and neurocognitive functioning. In our study, we examined the 24-hour cortisol profile in 55 patients with OSA and its association with performance in cognitive function tests. It was hypothesized that increased OSA severity would be associated with both decreased cognitive performance and elevated cortisol levels.
2. Methods
2.1. Participants
As part of a larger study (National Institutes of Health HL44915) examining the pathophysiology of the sympathetic nervous system in patients with OSA [26], participants with known or suspected OSA who had not received previous OSA treatment were recruited via advertisements, word of mouth referral, and referral from local medical practices in the San Diego area. Fifty-five participants with OSA were included (age range, 29–65 y). Potential participants were excluded if they (1) had a history of a major medical illness, with the exception of OSA and hypertension; (2) had current psychiatric diagnoses including alcohol or drug abuse; or (3) were receiving psychotopic medications. Two patients who were taking hypertensive medication were slowly tapered off their medications for 3 weeks prior to participation. In both cases, blood pressure (BP) remained within the inclusion range (<170/105 mmHg), and thus the patients were retained in the sample. The full details of the procedure are described elsewhere [26]. The project and all procedures were approved by the University of California, San Diego Human Subjects Committee.
2.2. Procedure
Written informed consent was obtained from all participants before participation in the study. Participants were admitted to the University of California, San Diego, General Clinical Research Center Gillin Laboratory of Sleep and Chronobiology for 2 nights. The participants were prepared for standard polysomnography (PSG) on both nights; however, the recordings were discarded from the first night due to the possibility of first-night effects on sleep metrics. A venous catheter was inserted at 5:00 PM. Starting at 6:00 PM, a blood sample was collected every 2 hours for 24 hours. Blood samples were collected in ethylenediaminetetraacetic acid, placed on ice, and spun in a refrigerated centrifuge; the plasma was then stored at −80°C until assayed. Plasma cortisol was determined using commercial sandwich enzyme-linked immunosorbent assays (Parameter assay; R&D Systems, Minneapolis, MN, USA).
Starting at 8:00 PM the following evening on completion of the 24-hour blood sampling, participants were prepared for PSG. The PSG setup began at 9:00 PM and lights-off time occurred between 10:00 PM and midnight. Parameters measured during PSG included electroencephalography, electrocardiography, electrooculography, chin and tibialis anterior electromyography, pulse oximetry, nasal-oral airflow by nasal cannula pressure transducer and thermistor, and thoracic and abdominal respiratory effort recorded on a Grass Heritage digital PSG (Model PSG36-2, Astro-Med, Inc, West Warwick, RI). The next morning, participants were awakened at 6:00 AM and PSG recording equipment was removed. Experienced PSG technicians scored PSG sleep records according to the criteria of Rechtschaffen and Kales [27]. OSA can be quantified in several ways; each of these indices captures a slightly different aspect of breathing cessation. The most commonly used assessments are recording the number of apneas and hypopneas, oxygen desaturation events, and total sleep time (TST). Apneas were defined as decrements in airflow of ≥90% from baseline lasting for ≥10 seconds. Hypopneas were defined as decrements in airflow of ≥50% but <90% from baseline lasting for ≥10 seconds regardless of the presence or absence of associated desaturation or arousal. Significant transient oxyhemoglobin desaturations were defined as transient drops in oxyhemoglobin saturation by ≥3% from baseline lasting for >10 seconds but <3 minutes. The oxygen desaturation index (ODI) was calculated as the number of transient oxygen desaturations per hour of sleep. TST was computed, and the numbers of apneas and hypopneas per hour of sleep were calculated to obtain the apnea-hypopnea index (AHI). Different criteria for OSA diagnosis exist; in our study, we used a cutoff of AHI ≥5 events per hour for diagnosis of OSA and inclusion into the study, which is in accordance with the criteria of the American Academy of Sleep Medicine [28]. The mean AHI for the included patients was 30.3 events per hour, with an interquartile range of 30.2 events per hour, thus showing moderate to severe OSA.
2.3. Neuropsychologic battery
Participants were given the following fixed battery, which assessed 7 cognitive domains: (1) Wechsler Adult Intelligence Scale-Revised [29], (2) Digit Symbol, Digit Span, Letter-Number Sequencing Test, Symbol Search Test; (3) Brief Visuospatial Memory Test-Revised (BVMT) [30]; (4) Hopkins Verbal Learning Test-Revised (HVLT) [31]; (5) Trail Making Test parts A and B [32]; (6) Digit Vigilance Test [33]; (7) Stroop Color-Word Test [34]; and (8) Word Fluency Test [35]. These tests produced 15 subscale scores per participant and assessed the following cognitive domains: (1) processing speed (Digit Symbol Test, Symbol Search Test, Digit Vigilance Test, Trail Making Test part A, Stroop Color-Word Test); (2) working memory (Letter-Number Sequencing Test, Digit Span test, Digit Vigilance Test); (3) executive functions (Trail Making Test part B, Digit Symbol Test, Symbol Search Test, Letter-Number Sequencing Test, Stroop Color-Word Test); (4) attention (Digit Vigilance Test); (5) learning (HVLT-total, BVMT-total), and (6) memory (HVLT-recall, BVMT-recall).
Raw scores were calculated for each neuropsychologic subtest. To investigate how many patients with OSA had neuropsychologic impairment and in what domains, t scores were calculated for each of the neuropsychologic subtests, controlling for ethnicity, sex, age, and education. Higher t scores indicated better performance. Domain-wise t scores were generated by averaging the scores on the tests that contributed to each domain. A deficit score was computed for each of the 15 individual test scores according to the convention below, in which t scores were collapsed into groups from 0 to 5 (with a t score of ≥40, the deficit score was 0; with a score of ≥35 but <40, the deficit score was 1; with a score of ≥30 but <35, the deficit score was 2; with a score of ≥25 but <30, the deficit score was 3; with a score of ≥20 but <25, the deficit score was 4; and with a score <19, the deficit score was 5). The average of those scores was the global deficit score (GDS). A GDS cutoff point of ≥0.5 was used to classify individuals as having neurocognitive impairment, as it yielded the optimal balance between sensitivity and specificity. A detailed explanation of GDS is described elsewhere [36].
2.4. Psychologic assessment
To assess depressive symptoms, participants completed the Center for Epidemiologic Studies Depression Scale, a 20-item self-report scale [37]. Scores of ≥16 indicated a likely diagnosis of major depression [38].
2.5. Statistical analysis
Data were analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL). Area under curve (AUC) values were calculated for cortisol levels based on the trapezoid rule, with 24-hour nighttime (10:00 PM–6:00 AM) and daytime (8:00 AM–8:00 PM) values adjusted to a per-hour basis. Bivariate associations between neurocognitive measures, cortisol levels, and respiratory variables were investigated using Pearson product moment correlation coefficients. A series of hierarchical linear regressions were then used to investigate if significant associations between neurocognitive measures, cortisol levels, and respiratory variables persisted after potentially confounding covariates were considered (i.e., step 1: body mass index [BMI], smoking status); other potential cofounders were already adjusted for in calculation of t scores (i.e., ethnicity, sex, age, education). Potential predictors (ODI for OSA severity; and nighttime cortisol for HPA activity) were sequentially entered in steps 2 and 3 to allow for the determination of ΔR2 to which each variable belonged. In all analyses, 2-sided t tests were used. A level of P≤.05 was accepted as level of significance and η2 was calculated as a measure of effect size.
3. Results
3.1. Participant characteristics
Fifty-five participants qualified for the study (80% men). Participants were middle-aged (mean age, 50 y; standard deviation [SD], 9.1) and overweight (mean BMI, 29.6 kg/m2; SD, 5.2). BP was mildly elevated (mean systolic BP, 131.4 mmHg [SD, 17.2 mmHg]; mean diastolic BP, 78.8 mmHg [SD, 9.1 mmHg]) and 25% of participants scored in the depressed range (Center for Epidemiologic Studies Depression Scale ≥16). Six participants were current smokers. Participants had severe OSA on average (mean AHI, 30.3; SD, 21.7), Table 1 describes the sleep characteristics of the participants.
Table 1.
Sleep characteristics.
| Sleep recording measures | Mean (SD) |
|---|---|
| AHI, events/h | 30.3 (21.7) |
| ODI, events/h | 21.7 (21.9) |
| Total sleep time, min | 372.7 (45.3) |
| Total arousal index, events/h | 22.2 (18.0) |
| Wake after sleep onset, min | 53.6 (29.4) |
| Stage 1 sleep, % | 12.6 (8.3) |
| Stage 2 sleep, % | 55.86 (10.0) |
| Slow-wave sleep (stage 3 and 4), % | 12.01 (10.6) |
| REM sleep, % | 20.3 (7.13) |
Abbreviations: AHI, apnea-hypopnea index; ODI, oxygen desaturation index; h, hour; min, minutes; REM, rapid eye movement; SD, standard deviation.
The AHI, ODI, and total arousal index were calculated as events per hour of total sleep time.
3.2. Neurocognitive function
Participants' raw scores were converted to t scores for each domain of cognitive function (speed of information processing; verbal, learning, memory, executive function; and working memory) and a GDS was calculated. All t and GDS scores were adjusted for gender, age, education, and ethnicity during calculations. In a healthy population, a neurocognitive deficit (GDS, ≥0.5; t score, ≤40) would be expected in 15% of participants [39]. In our sample, 31% were impaired according to the GDS, showing a higher rate of impairment in our group. Table 2 shows average t scores and percent impairment for each domain.
Table 2.
Mean (standard deviation) t scores and percent of participants classified as impaired. Higher t score indicated better performance.
| t score | % Impaired | |
|---|---|---|
| Processing speed | 48.2 (8.4) | 16.3 |
| Verbal fluency | 48.6 (10.5) | 20.4 |
| Learning | 44.2 (9.5) | 36.7 |
| Memory | 45.9 (9.1) | 24.5 |
| Executive function | 48.5 (9.5) | 14.3 |
| Working memory | 50.1 (9.6) | 14.3 |
3.3. Plasma cortisol
As shown in Fig. 1, the expected circadian effect was seen in plasma cortisol levels (reference range [morning], 5–25 μg/dL; [afternoon], 2–12 μg/dL). A significant time effect was found (F[11,43]=48.2; P<.001; η2 = .925), with levels showing a nadir at 10:00 PM to 12:00 AM and a peak at 6:00 AM to 8:00 AM. Summary values showed the expected lower cortisol AUC (adjusted to per-hour basis) during the nighttime than during the daytime (t[53]=6.332; P<.001).
Fig 1.
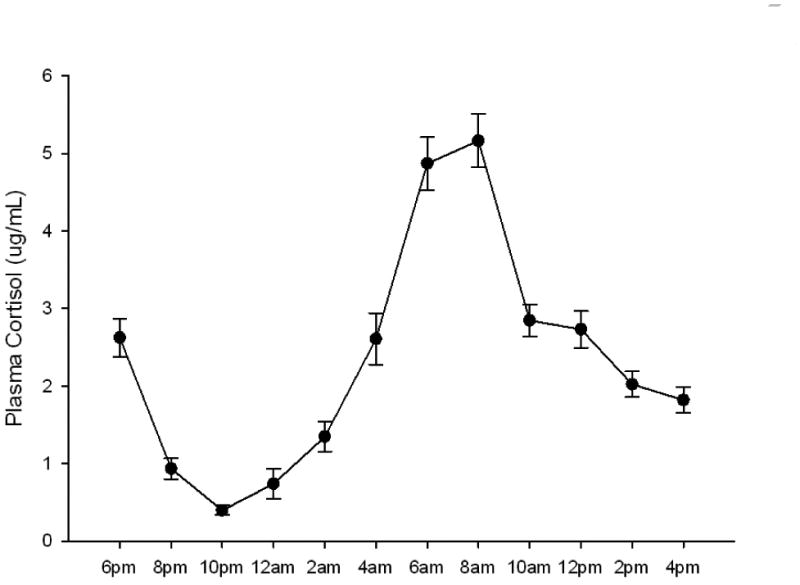
Mean (standard error) plasma cortisol levels in participants with obstructive sleep apnea. Measures were taken every 2 hours over a 24-hour period starting at 6:00 PM.
3.4. Univariate analyses
Our initial analyses examined association between OSA severity and cortisol levels. Partial correlations were performed to control for potential confounding factors of BMI and smoking with cortisol levels [40-42]. Although we found no association with AHI, ODI was significantly related to 24-hour cortisol AUC (r=.323; P=.025). However, there were no significant associations between nighttime cortisol AUC with AHI or ODI (P>.05 for both). Analyses of sleep characteristics revealed that TST was correlated with 24-hour cortisol AUC (r=−.365; P=.011) and with nighttime cortisol AUC (r=−.303; P=.036), but total arousal index; wake after sleep onset; and proportion of sleep in stage 1, stage 2, slow-wave sleep, and rapid eye movement sleep were not associated with cortisol levels. Given that sleep fragmentation indices were not related to cortisol levels, only AHI, ODI, and TST were used in the next analyses.
Next we examined associations between indices of GDS and individual cognitive domains (t scores) with both sleep characteristics (AHI, ODI, and TST) and cortisol using AUC values for nighttime and daytime measures. As expected, AHI and ODI were associated with neurocognitive function such that more severe OSA was associated with greater impairment, with significant associations with GDS and learning, memory, and working memory domains (P<.05 for all). TST was not associated with GDS or any individual cognitive domain. Daytime cortisol was not associated with any cognitive indices; however, nighttime cortisol showed a positive relationship, such that higher nighttime cortisol was associated with greater global impairment, and specifically with impaired learning, memory, and working memory domains. Table 3 describes the observed associations in detail.
Table 3.
Pearson product moment correlation coefficients between neurocognitive functioning indices and apnea-hypopnea index, oxygen desaturation index, and nighttime cortisol.
| AHI | ODI | Nighttime cortisol | |
|---|---|---|---|
| Global deficit | r=.317; P=.018* | r=.348; P=0.010 | r=.263; P=.053 |
| Processing speed | r=−.205; P=.158 | r=−.235; P=.105 | r=−.164; P=.259 |
| Verbal fluency | r=−.231; p=.110 | r=−.232; P=−.109 | r=−.154; P=.290 |
| Learning | r=−.258; P=.073 | r=−.307; P=.032 | r=−.342; P=.016 |
| Memory | r=−.308; P=.031 | r=−.293; P=.041 | r=−.411; P=.003 |
| Executive function | r=−.205; P=.158 | r=−.227; P=.117 | r=−.032; P=.827 |
| Working memory | r=−.306; P=.033 | r=−.374; P=.008 | r=−.314; P=.028 |
Abbreviations: AHI, apnea-hypopnea index; ODI, oxygen desaturation index.
Significant associations are highlighted in bold.
3.5. Hierarchical linear regression analysis
Where significant correlational associations were found for both cortisol and OSA severity (e.g., GDS, learning, memory, and working memory), we performed hierarchical linear regression analyses to determine the strength of the associations. As in the correlations, potential cortisol confounders, BMI, and smoking status were included in step 1 (analyses were repeated including additional potential confounders depression and BP diagnosis, with all significant results remaining at P<.05). Given that ODI was more significantly associated with neurocognitive function than AHI and TST was not associated with any cognitive function variables, we chose to use ODI as our index of OSA severity. Table 4 summarizes the results. Nighttime cortisol was a significant predictor of GDS, learning, memory, and working memory, though ODI did not significantly predict any outcome after control for the other variables.
Table 4.
Regression model analyses for factors associated with neuropsychologic performance.
| Factor | β | SE | ΔR2 | F Change | Sig. F Change |
|---|---|---|---|---|---|
| Global deficit score | |||||
| Step 1: BMI, smoking | --- | --- | .196 | 5.981 | .005 |
| Step 2: ODI | .114 | .004 | .008 | .472 | .495 |
| Step 3: Cortisol | .251 | .009 | .060 | 3.863 | .055 |
|
| |||||
| Learning | |||||
| Step 1: BMI, smoking | --- | --- | .173 | 4.598 | .015 |
| Step 2: ODI | −.061 | .078 | .002 | .112 | .739 |
| Step 3: Cortisol | −.325 | .181 | .103 | 6.013 | .018 |
|
| |||||
| Memory | |||||
| Step 1: BMI, smoking | --- | --- | .182 | 4.885 | .012 |
| Step 2: ODI | −.053 | .075 | .002 | .085 | .773 |
| Step 3: cortisol | −.398 | .167 | .155 | 9.810 | .003 |
|
| |||||
| Working memory | |||||
| Step 1: BMI, smoking | --- | --- | .296 | 9.252 | <.001 |
| Step 2: ODI | −.056 | .068 | .002 | .110 | .742 |
| Step 3: cortisol | −.305 | .166 | .091 | 6.248 | .016 |
Abbreviations: SE, standard error; BMI, body mass index; ODI, oxygen desaturation index.
4. Discussion
Our study examined the associations between OSA, cognitive function, and cortisol levels. In addition to the expected finding that increasing severity of OSA was associated with reductions in neurocognitive functioning, we also found that higher levels of cortisol during the nighttime were associated with reduced neurocognitive functioning. Importantly, even after controlling for potential confounding factors, nighttime cortisol levels were a significant predictor of neurocognitive functioning, though OSA severity was not related. Several specific domains of neurocognitive functioning were particularly related to nighttime cortisol levels, with nighttime cortisol levels accounting for up to 16% of the variance in the domains of learning, memory, and working memory. The finding that nighttime cortisol level was a significant predictor of neurocognitive function over and above indices of OSA severity is important, as it allows speculation that perhaps it not apnea per say, but rather the physiologic responses to apnea (in particular HPA activity) that provide the proximal association with neurocognitive impairment.
Our data are in line with the literature reporting that chronic exposure to elevated physiologic cortisol levels is associated with a decline in neurocognitive function and hippocampal structure. The functional effects of cortisol on reduced memory function have been demonstrated by experimental cortisol treatment [43] and stress induced cortisol level increases [44]. Further, there are data that show that long-term exposure to high levels of glucocorticoids in the setting of healthy aging is associated with deficits in memory and structural changes to the hippocampus [45], and similar findings in Cushing syndrome provide further evidence of the role of excess glucocorticoids on hippocampal structure and function [46]. In this context, the finding that cortisol levels are associated with neurocognitive function in patients with OSA may be related to the possible role of cortisol in hippocampal volume loss observed in the neuroimaging studies of individuals with OSA [47]. Interestingly, we found that it was nighttime levels that were important for neurocognitive function, rather than 24-hour or daytime levels of cortisol. This finding in a sleep disorder setting is worthy of attention. The study of memory function has revealed the importance of sleep and nighttime HPA activity. Plihal and Born [48] experimentally manipulated cortisol levels during sleep and showed that elevated cortisol levels impaired declarative memory after only one single night. Given the evidence linking long-term elevated cortisol levels to atrophy in hippocampi and cerebral cortex, the examination of resting or nighttime cortisol levels on cognition is particularly informative [49-51]. The negative impact of cortisol on cognitive performance has been linked to elevated nightly nadir levels of this hormone [52]. Born and Wagner [53] showed that declarative memories, the formation of which involves the hippocampus, benefit from the inhibition of endogenous cortisol during early sleep, though an increased level of endogenous cortisol was associated with reduced emotional memory retention. The authors proposed that the effect of circulating cortisol on the glucocorticoid receptors in the hippocampus impacts memory formation during sleep [53].
Although the level of nighttime cortisol was a significant predictor of neurocognitive function in our study, we found that ODI was positively associated with 24-hour cortisol and daytime cortisol levels. Previous reports are mixed regarding the association between OSA and cortisol levels, with studies using extensive sampling to report that an increased severity of OSA was related to elevated cortisol levels [23]. Nocturnal awakenings also were found to be associated with alterations in HPA activity, specifically increased pulsatile cortisol release [54]. Further, untreated OSA has been associated with a longer duration of cortisol secretory pulses, resulting in elevations of overall cortisol levels [24], and animal evidence has shown that hypoxia induces activation of the HPA axis [55]. Given this finding, it is possible that the mechanism of an increase in cortisol levels is specifically related to hypoxia associated with OSA, particularly noting that it was ODI and not AHI that was associated with cortisol in our data. Our results suggest a role for nighttime cortisol levels in cognitive dysfunction, but our finding that OSA severity was related to 24-hour and daytime cortisol levels suggests a complex interaction that deserves further attention.
It is not known if HPA activity alteration is uniform in OSA; if not, those patients who showed greater increases in cortisol levels with apneic events might be at greatest risk for development of neurocognitive impairment. Potentially, nighttime alterations in cortisol levels may be associated with neuropsychologic functioning outside of the setting of OSA. Indeed, future studies would do well to include healthy control participants and to examine the role of individual differences in cortisol levels within OSA, especially as our study included patients with severe OSA, though the clinical spectrum is far wider. The treatment of OSA with CPAP has been reported to show improvements in some aspects of neuropsychologic function, though findings are inconsistent [56,57]. Similarly, CPAP treatment has been reported to reduce cortisol levels [23,24]. It may yield interesting data if future studies address the possibility that CPAP treatment effects on neurocognitive function are mediated by alterations in HPA function, specifically reductions in nighttime cortisol levels.
Results from our study revealed that nocturnal cortisol levels were associated with neuropsychologic functioning above and beyond the influence of covariates and apnea severity in a sample of patients with OSA. These findings suggest that OSA-related alterations in HPA activity may play a key role in the pathophysiology of neuropsychologic impairments in OSA.
Supplementary Material
Highlights.
The association of cortisol levels with neurocognitive function in obstructive sleep apnea (OSA) patients
Nocturnal cortisol and OSA severity are both associated with cognitive function
Cortisol is associated with cognitive function even when controlling for OSA severity
In particular, learning, memory, and working memory are associated with cortisol levels
Acknowledgments
This work was supported by grants HL44915 (JED), RR 00827 (University of California San Diego General Clinical Research Center Grant), AG08415 (SAI) and UL1RR031980 (CTRI).
Footnotes
Conflict of interest: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 3.George CF, Smiley A. Sleep apnea and automobile crashes. Sleep. 1999;22:790–5. [PubMed] [Google Scholar]
- 4.Engleman HM, Douglas NJ. Sleep 4: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59:618–22. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 6.Feuerstein C, Naegele B, Pepin JL, Levy P. Frontal lobe-related cognitive functions in patients with sleep apnea syndrome before and after treatment. Acta Neurol Belg. 1997;97:96–107. [PubMed] [Google Scholar]
- 7.Salorio CF, White DA, Piccirillo J, Duntley SP, Uhles ML. Learning, memory, and executive control in individuals with obstructive sleep apnea syndrome. J Clin Exp Neuropsychol. 2002;24:93–100. doi: 10.1076/jcen.24.1.93.973. [DOI] [PubMed] [Google Scholar]
- 8.Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98:2226–34. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- 9.Bedard MA, Montplaisir J, Malo J, Richer F, Rouleau I. Persistent neuropsychological deficits and vigilance impairment in sleep apnea syndrome after treatment with continuous positive airways pressure (CPAP) J Clin Exp Neuropsychol. 1993;15:330–41. doi: 10.1080/01688639308402567. [DOI] [PubMed] [Google Scholar]
- 10.Bedard MA, Montplaisir J, Richer F, Malo J. Nocturnal hypoxemia as a determinant of vigilance impairment in sleep apnea syndrome. Chest. 1991;100:367–70. doi: 10.1378/chest.100.2.367. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg GD, Watson RK, Deptula D. Neuropsychological dysfunction in sleep apnea. Sleep. 1987;10:254–62. doi: 10.1093/sleep/10.3.254. [DOI] [PubMed] [Google Scholar]
- 12.Verstraeten E, Cluydts R, Verbraecken J, De Roeck J. Psychomotor and cognitive performance in nonapneic snorers: preliminary findings. Percept Mot Skills. 1997;84(3, pt 2):1211–22. doi: 10.2466/pms.1997.84.3c.1211. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J Clin Sleep Med. 2006;2:461–71. [PubMed] [Google Scholar]
- 14.Gale SD, Hopkins RO. Effects of hypoxia on the brain: neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea. J Int Neuropsychol Soc. 2004;10:60–71. doi: 10.1017/S1355617704101082. [DOI] [PubMed] [Google Scholar]
- 15.Torelli F, Moscufo N, Garreffa G, Placidi F, Romigi A, Zannino S, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54:787–93. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruce EN, Bruce MC, Vennelaganti S. Sample entropy tracks changes in electroencephalogram power spectrum with sleep state and aging. J Clin Neurophysiol. 2009;26:257–66. doi: 10.1097/WNP.0b013e3181b2f1e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sforza E, Roche F. Sleep apnea syndrome and cognition. Front Neurol. 2012;3:87. doi: 10.3389/fneur.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan SF, Parthasarathy S, Budhiraja R. Healthy sleep education—a salve for obesity? J Clin Sleep Med. 2010;6:18–9. [PMC free article] [PubMed] [Google Scholar]
- 19.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 20.Tomfohr LM, Edwards KM, Dimsdale JE. Is obstructive sleep apnea associated with cortisol levels? a systematic review of the research evidence. Sleep Med Rev. 2012;16:243–9. doi: 10.1016/j.smrv.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–14. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- 22.Lanfranco F, Motta G, Minetto MA, Baldi M, Balbo M, Ghigo E, et al. Neuroendocrine alterations in obese patients with sleep apnea syndrome. Int J Endocrinol. 2010;2010:474518. doi: 10.1155/2010/474518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bentley CM, Bixler EO, et al. Hypothalamic-pituitary-adrenal axis activity in obese men with and without sleep apnea: effects of continuous positive airway pressure therapy. J Clin Endocrinol Metab. 2007;92:4199–207. doi: 10.1210/jc.2007-0774. [DOI] [PubMed] [Google Scholar]
- 24.Henley DE, Russell GM, Douthwaite JA, Wood SA, Buchanan F, Gibson R, et al. Hypothalamic-pituitary-adrenal axis activation in obstructive sleep apnea: the effect of continuous positive airway pressure therapy. J Clin Endocrinol Metab. 2009;94:4234–42. doi: 10.1210/jc.2009-1174. [DOI] [PubMed] [Google Scholar]
- 25.Schmoller A, Eberhardt F, Jauch-Chara K, Schweiger U, Zabel P, Peters A, et al. Continuous positive airway pressure therapy decreases evening cortisol concentrations in patients with severe obstructive sleep apnea. Metabolism. 2009;58:848–53. doi: 10.1016/j.metabol.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Tomfohr LM, Ancoli-Israel S, Loredo JS, Dimsdale JE. Effects of continuous positive airway pressure on fatigue and sleepiness in patients with obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2011;34:121–6. doi: 10.1093/sleep/34.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System of Sleep Stages in Human Subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- 28.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 29.Wechsler D. Weschler Adult Intelligence Scale-III. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 30.Benedict RH. Brief Visospacial Memory Tests-Revised. Odessa, TX: Psychological Assessment Resources; 1997. [Google Scholar]
- 31.Benedict RH, Schretlen C, Groninger L, Brandt J. Hopkins verbal learning test-revised: normative data and analysis of interfrom and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 32.Boll TJ. The Halstead-Reitan neuropsychological battery. In: Friskov SB, Boll TJ, editors. Handbook of Clinical Neuropsychology. New York, NY: John Wiley & Sons; 1981. pp. 577–607. [Google Scholar]
- 33.Lewis RF. Digit Vigilance Test. Odessa, TX: Psychological Assessment Resources; 1995. [Google Scholar]
- 34.Golden CJ. A Manual for Clinical and Experimental Uses. Chicago, IL: Stoelting Co; 1978. [Google Scholar]
- 35.Lezak MD. Neuropsychological Assessment. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 36.Treanor J, Keitel W, Belshe R, Campbell J, Schiff G, Zangwill K, et al. Evaluation of a single dose of half strength inactivated influenza vaccine in healthy adults. Vaccine. 2002;20:1099–105. doi: 10.1016/s0264-410x(01)00440-6. [DOI] [PubMed] [Google Scholar]
- 37.Eaton WW, Smith C, Muntaner C, Ybarra M, Tien A. The use of psychological testing for treatment planning and outcomes assessment. In: Maruish ME, editor. Center for Epidemiologic Studies Depression Scle: Review and Revision (CESD and CESDR) Mahwah, NJ: Routledge; 2003. pp. 363–77. [Google Scholar]
- 38.Bush KA, McAnulty J, McPhie K, Reynolds R, Boomer M, Clarkson LM, et al. Antiviral prophylaxis in the management of an influenza outbreak in an aged care facility. Commun Dis Intell. 2004;28:396–400. [PubMed] [Google Scholar]
- 39.Heaton RK, Grant I, Matthews CG. Comprehensive Norms for an Expanded Halstead- Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Odessa, TX: Psychological assessment resources; 1991. [Google Scholar]
- 40.Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab. 2007;92:819–24. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- 41.Kumari M, Chandola T, Brunner E, Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2010;95:4415–23. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Travison TG, O'Donnell AB, Araujo AB, Matsumoto AM, McKinlay JB. Cortisol levels and measures of body composition in middle-aged and older men. Clin Endocrinol (Oxf) 2007;67:71–7. doi: 10.1111/j.1365-2265.2007.02837.x. [DOI] [PubMed] [Google Scholar]
- 43.Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, et al. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56:527–33. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- 44.Taverniers J, Van Ruysseveldt J, Smeets T, von Grumbkow J. High-intensity stress elicits robust cortisol increases, and impairs working memory and visuo-spatial declarative memory in Special Forces candidates: a field experiment. Stress. 2010;13:323–33. doi: 10.3109/10253891003642394. [DOI] [PubMed] [Google Scholar]
- 45.Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–42. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Leon-Carrion J, Atutxa AM, Mangas MA, Soto-Moreno A, Pumar A, Leon-Justel A, et al. A clinical profile of memory impairment in humans due to endogenous glucocorticoid excess [published online ahead of print August 13, 2008] Clinical Endocrinol (Oxf) 2009;70:192–200. doi: 10.1111/j.1365-2265.2008.03355.x. [DOI] [PubMed] [Google Scholar]
- 47.Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–7. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 48.Plihal W, Born J. Memory consolidation in human sleep depends on inhibition of glucocorticoid release. Neuroreport. 1999;10:2741–7. doi: 10.1097/00001756-199909090-00009. [DOI] [PubMed] [Google Scholar]
- 49.Rothschild AJ, Benes F, Hebben N, Woods B, Luciana M, Bakanas E, et al. Relationships between brain CT scan findings and cortisol in psychotic and nonpsychotic depressed patients. Biol Psychiatry. 1989;26:565–75. doi: 10.1016/0006-3223(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 50.Sapolsky RM. Glucocorticoid toxicity in the hippocampus: temporal aspects of neuronal vulnerability. Brain Res. 1985;359:300–5. doi: 10.1016/0006-8993(85)91440-4. [DOI] [PubMed] [Google Scholar]
- 51.Karlamangla AS, Singer BH, Chodosh J, McEwen BS, Seeman TE. Urinary cortisol excretion as a predictor of incident cognitive impairment. Neurobiol Aging. 2005;(26 suppl 1):S80–4. doi: 10.1016/j.neurobiolaging.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 52.Belanoff JK, Gross K, Yager A, Schatzberg AF. Corticosteroids and cognition. J Psychiatr Res. 2001;35:127–45. doi: 10.1016/s0022-3956(01)00018-8. [DOI] [PubMed] [Google Scholar]
- 53.Born J, Wagner U. Memory consolidation during sleep: role of cortisol feedback. Ann N Y Acad Sci. 2004;1032:198–201. doi: 10.1196/annals.1314.020. [DOI] [PubMed] [Google Scholar]
- 54.Spath-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29:575–84. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- 55.Jacobson L, Dallman MF. ACTH secretion and ventilation increase at similar arterial PO2 in conscious rats. J Appl Physiol. 1989;66:2245–50. doi: 10.1152/jappl.1989.66.5.2245. [DOI] [PubMed] [Google Scholar]
- 56.Lim W, Bardwell WA, Loredo JS, Kim EJ, Ancoli-Israel S, Morgan EE, et al. Neuropsychological effects of 2-week continuous positive airway pressure treatment and supplemental oxygen in patients with obstructive sleep apnea: a randomized placebo-controlled study. J Clin Sleep Med. 2007;3:380–6. [PMC free article] [PubMed] [Google Scholar]
- 57.Bardwell WA, Ancoli-Israel S, Berry CC, Dimsdale JE. Neuropsychological effects of one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea: a placebo-controlled study. Psychosom Med. 2001;63:579–84. doi: 10.1097/00006842-200107000-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


