Abstract
Hodgkin Lymphoma (HL) prognostic models based on factors measured at time of autologous hematopoietic cell transplantation (AHCT) are limited by small sample sizes. Models based on information at diagnosis are often not uniformly collected or available at transplantation. We propose an easily implementable prognostic model for progression-free survival (PFS) post-AHCT based on factors available at transplantation in a large international cohort of HL patients. The outcomes of 728 AHCT recipients for relapsed/refractory HL were studied. Patients were randomly selected for model development (n=337) and validation (n=391).
The multivariate model identified four major adverse risk factors at the time of AHCT with the following relative weights: Karnofsky performance score <90 and chemotherapy resistance at AHCT were each assigned 1 point while ≥3 chemotherapy regimens pre-AHCT and extra-nodal disease at AHCT were each assigned 2 points. Based on the total score summed for the four adverse risk factors, three risk groups were identified: Low, (score=0), Intermediate, (score=1-3) or High, (score=4-6). The 4-year PFS (95% CI) for the Low (N=176), Intermediate (N=261) and High (N=283) risk groups were 71% (63-78%), 60% (53-66%), and 42% (36-49%), respectively. The prognostic model was validated in an independent cohort. The CIBMTR Model is based on factors easily available at the time of AHCT and discriminates patients with favorable post-AHCT outcomes as well as an intermediate risk group. This model should assist in the prospective evaluation of alternative treatment strategies.
Introduction
Autologous hematopoietic cell transplantation (AHCT) is standard therapy for relapsed and refractory Hodgkin Lymphoma (HL).1, 2 While survival post-AHCT for HL has improved significantly over time,3, 4 the primary cause of AHCT failure is due to HL relapse or progression.5 To date, efforts to improve disease control post-AHCT for HL have had limited success.6-10
Several validated prognostic models have been developed to predict treatment outcomes for HL patients and to help guide initial treatment decisions. However, these models are targeted for prognosis of newly diagnosed HL patients, and were not designed for prognosis of HL patients after relapse or for assessment of AHCT outcomes.11-17 There are six published prognostic models of progression-free survival (PFS) for relapsed/refractory HL based on risk factors assessed at time of AHCT, instead of at diagnosis.18-23 We could not attempt to validate three of these six models due to either a high rate of missing data (diagnostic albumin, hemoglobin, white blood cell count, absolute lymphocyte count; duration of first complete remission) or data not collected (e.g. relapse in a previously radiated field) on the standard Center for International Blood and Marrow Transplant Research (CIBMTR) report forms for HL.18-20 We were able to independently validate the other three published models21-23 which were able to differentiate low and high risk groups, but all lacked discrimination of an intermediate risk group.24 Two additional prognostic models have assessed patient characteristics at time of relapse to guide salvage therapy options.25, 26 We sought to develop, and validate, a prognostic model using factors that are easily and widely available at time of AHCT in the largest cohort of HL patients treated with AHCT.
Materials and Methods
CIBMTR
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR) and the National Marrow Donor Program (NMDP) established in 2004, which comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplants to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for discrepancies, physicians' review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority, and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Boards of the National Marrow Donor Program and the Medical College of Wisconsin since 1985.
Study Population
The selection criteria for this study were: HL patients receiving a first AHCT from 1996-2007 reported to the CIBMTR (N=1026), excluding HL patients in first complete remission pre-AHCT (N=160 excluded), those with a planned second transplant (N=9 excluded), and those with missing data on potential prognostic factors (N=129 excluded). Thus, the study cohort yielded 728 AHCT recipients with relapsed or refractory HL with complete data reported to the CIBMTR. Eight cases were missing information on relapse post-AHCT and are excluded from analyses of PFS, but are included in analyses of overall survival (OS).
Statistical Methods
Progression was defined as an increase of ≥ 25% in the sites of lymphoma or development of new sites of lymphoma. Relapse was defined as recurrence of lymphoma after a complete response (CR). Patients alive without evidence of disease relapse or progression were censored at last follow up and the PFS event was summarized by a survival curve.
The study cohort was randomly split into two datasets, one for model development (N=337) and one for model verification (N=391). A Cox regression method was used to identify potential risk factors which are associated with treatment failure (failure event of PFS), the model development cohort was used to build a forward stepwise model with P=0.05 to enter and remove factors from the model. The results were then confirmed using a backward elimination procedure. The following risk factors were considered in the model building procedure: ≥3 chemotherapy regimens before AHCT, Karnofsky performance score (KPS) at AHCT: <90 vs. ≥90, age >40 vs ≤40, HL histology, B symptoms at diagnosis, Relapse/Primary induction failure at AHCT, chemotherapy-resistant disease at AHCT, elevated LDH at AHCT, extranodal involvement at AHCT, ≥ 5 cm mass at AHCT, and < 12 months from diagnosis to transplant.
Based on the final multivariate model of significant prognostic factors, each factor was assigned a weighted score based on the relative risk (RR) estimates (see Table 2). The score for each of the four significant prognostic factors was summed to a total score which ranged from 0 to 6. The distribution of patients by the total risk score is: 98 patients had a total risk score = 0 (reference category), 51 patients had a total risk score = 1 (RR range 4.3–4.4), 101 patients had a total risk score = 2 (RR range 5.8– 18.5), 45 patients had a total risk score = 3 (RR range 25.0–26.3), 23 patients had a total risk score = 4 (RR range 35.5–112), 19 patients had a total risk score = 5 or 6 (RR range > 150). Based on the range of RR and the distribution of patients across the total risk score categories, we classified each patient into one of three prognostic risk groups: Low risk group (score = 0), Intermediate risk group (score = 1–3) or High risk group (score = 4– 6). Once the final model was defined, it was then tested in the independent model validation cohort. All statistical analyses were performed using SAS version 9.3 (Cary, NC).
Table 2.
Multivariate model for the risk of treatment failure (1-PFS; or the risk of relapse or death).
| Variable | Relative Risk of treatment failure (95% CI) | P | Score |
|---|---|---|---|
| Number of prior chemotherapy regimens: (3-5) vs. (1-2) | 1.80 (1.31 – 2.47) | 0.0003 | 2 |
| Extranodal involvement prior to AHCT: Yes vs. No | 1.77 (1.24 – 2.53) | 0.0018 | 2 |
| Karnofsky score prior to AHCT: <90 vs. 90-100 | 1.47 (1.04 – 2.07) | 0.0275 | 1 |
| Chemotherapy-sensitivity pre-AHCT: Resistant vs. Sensitive | 1.45 (1.01 – 2.07) | 0.0440 | 1 |
P values of additional risk factors considered in the model are: HL histology (P=0.6295), B symptoms at diagnosis (P=0.1516), Relapse/Primary induction failure at AHCT (P=0.2025), elevated LDH at AHCT (P=0.1319), ≥ 5 cm mass at AHCT (P=0.9553), age >40 (P=0.2700), and < 12 months from diagnosis to transplant (P=0.2433).
Results
Patient characteristics are summarized in Table 1. Seven-hundred twenty eight patients were reported from 162 centers world-wide.
Table 1. Patient characteristics of autologous hematopoietic cell transplantation recipients with hodgkin lymphoma.
| Variable | N (%) |
|---|---|
| Number of patients | 728 |
| Number of centers | 162 |
| Age at transplant, years | |
| ≤10 | 4 ( 1) |
| 11-20 | 98 (13) |
| 21-30 | 225 (31) |
| 31-40 | 197 (27) |
| 41-50 | 111 (15) |
| 51-60 | 50 ( 7) |
| ≥61 | 43 ( 6) |
| Age at transplant, median (range), years | 33 (7-74) |
| Male gender | 434 (60) |
| Karnofsky Performance Score at AHCT | |
| <90 | 192 (26) |
| HL Histology | |
| Nodular sclerosis | 542 (74) |
| Mixed cellularity | 100 (14) |
| Lymphocyte-rich | 47 ( 6) |
| Lymphocyte depleted | 8 ( 1) |
| Other-not specified | 31 ( 4) |
| HL stage at diagnosis | |
| I | 41 ( 6) |
| II | 300 (42) |
| III | 219 (30) |
| IV | 158 (22) |
| Extranodal involvement at diagnosis | 246 (34) |
| B Symptoms at diagnosis | 414 (57) |
| Number of pre-AHCT chemotherapy regimens | |
| 1 | 53 ( 7) |
| 2 | 381 (53) |
| 3 | 201 (28) |
| 4 | 75 (10) |
| 5 | 17 ( 2) |
| Interval from diagnosis to 1st CR, median (range), months | 7 (<1-123) |
| Interval from 1st CR to transplant, median (range), months | 16 (<1-136) |
| Interval from last CR to transplant, median (range), months | 6 (<1-117) |
| Disease status at AHCT | |
| Chemotherapy-sensitive Relapse | 263 (36) |
| Complete Remission (CR) | 200 (27) |
| 1st Partial Response (No prior CR) | 136 (19) |
| Chemotherapy-resistant/unknown Relapse | 87 (12) |
| Primary induction failure (No prior CR, less than partial response to induction) | 42 ( 6) |
| Chemotherapy-sensitivity at AHCT | |
| Sensitive | 579 (80) |
| Resistant | 119 (16) |
| Untreated/not evaluable | 30 ( 4) |
| LDH at AHCT | |
| Normal | 426 (59) |
| Abnormal | 196 (27) |
| Missing | 106 (15) |
| Extranodal involvement at AHCT | 139 (19) |
| ≥5 cm mass prior to AHCT | 71 (27) |
| ≥12 mo from diagnosis to AHCT | 602 (83) |
| Interval from diagnosis to AHCT, median (range), months | 22 (3-368) |
| Conditioning regimen | |
| BEAM or similar | 517 (71) |
| CBV or similar | 98 (13) |
| BuMel or BuCy | 34 ( 5) |
| TBI-based | 28 ( 4) |
| Others | 19 ( 3) |
| Cy+Thiotepa or similar | 14 ( 2) |
| Melphalan alone | 18 ( 2) |
| Year of transplant | |
| 1996-1999 | 437 (60) |
| 2000-2003 | 146 (20) |
| 2004-2007 | 145 (20) |
| Median follow-up of survivors, months | 43 (1 - 131) |
Legend: HL: Hodgkin Lymphoma; CIBMTR: Center for International Blood and Marrow Research; AHCT: Autologous Hematopoietic Cell Transplantation; LDH: Lactate Dehydrogenase; CR: Complete Remission; PR: Partial Remission; BEAM: BCNU, Etoposide, Ara-C, Melphalan; CBV: Cyclophosphamide, BCNU, Etoposide; BuMel: Busulfan, Melphalan; BuCy: Busulfan, Cyclophosphamide; TBI: Total Body Irradiation.
The final multivariate model of significant risk factors is summarized in Table 2. A poor KPS (<90) or chemotherapy-resistant disease pre-AHCT yielded Relative Risk (RR) estimates of 1.47 and 1.45, respectively, and were assigned a weighted score of 1. Three or more chemotherapy treatment regimens before AHCT, and presence of extranodal disease at time of AHCT, had higher RR estimates of 1.8 and 1.77, respectively, and were assigned a weighted score of 2. Patients with no risk factors (score=0) were assigned to the Low risk group (N=98), those with a score = 1-3 were assigned to an Intermediate Risk group (N=197), and those with a score = 4-6 were assigned to a High Risk group (N=42).
Table 3 summarizes the prognostic model's performance in the development and validation datasets. We identified three distinct risk groups including an Intermediate risk group. Statistical significance was reached for the intermediate risk group after combining the model development and validation datasets (Figure 1). The 4-year PFS estimates and 95% confidence intervals for the Low, Intermediate and High risk groups for the entire cohort were 71% (63-78%), 60% (53-66%), and 42% (36-49%), respectively. At a median follow-up of 43 months, the primary causes of death post-AHCT were recurrent HL (N=154, 64% of all deaths), organ failure (N=28, 12%), interstitial pneumonitis or adult respiratory distress syndrome (N=15, 6%), second malignancy (N=8, 3%), hemorrhage (N=5, 2%), infection (N=4, 2%) or other/missing (N=28, 12%).
Table 3.
Predicting treatment failure by the CIBMTR prognostic model: model development cohort, model validation cohort and both cohorts combined.
| Model development cohort | Model validation cohort | Combined Cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk group | N | RR (95% CI) | P | N | RR (95% CI) | P | N | RR (95% CI) | P |
| Low risk | 98 | 1.00 | <0.0001a | 131 | 1.00 | 0.0019a | 229 | 1.00 | <0.0001 |
| Intermediate risk | 197 | 1.46 (0.98 – 2.18) | 0.0639 | 220 | 1.33 (0.93 – 1.89) | 0.1178 | 417 | 1.39 (1.07 – 1.81) | 0.0144 |
| High risk | 42 | 3.37 (2.07 – 5.50) | <0.0001 | 40 | 2.57 (1.61 – 4.09) | <0.0001 | 82 | 2.95 (2.11 – 4.13) | <0.0001 |
=2 d.f. test.
Figure 1. Progression-free survival for the model development, model validation, and combined cohorts.
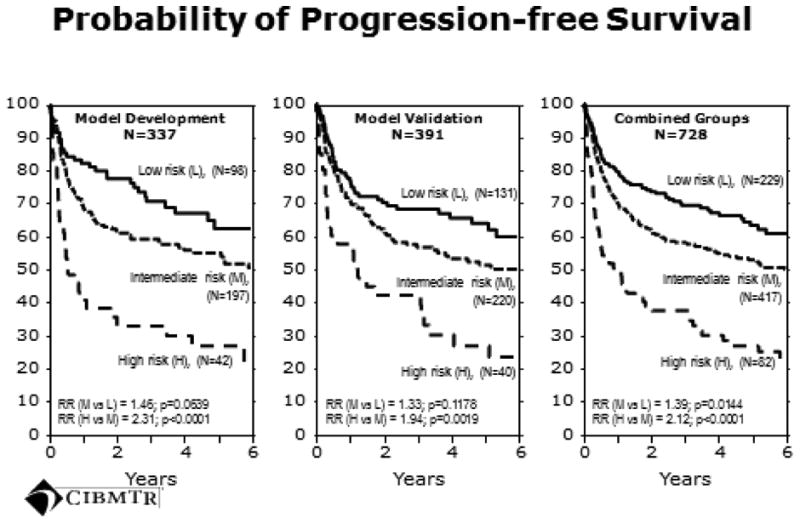
The rate of a second allogeneic HCT after failed AHCT was 9/229 (4%) for the Low, 16/417 (4%) for the Intermediate and 4/82 (5%) for the High risk groups. Hence the rate of second allogeneic HCT to rescue a failed AHCT did not differ by risk group. In addition, it did not affect the PFS curves for our analysis since all patients had an event (disease progression) which was included in the PFS analysis and which occurred before the allogeneic HCT. Hence the attempted rescue using an allogeneic HCT is not reflected in the PFS analysis.
Discussion
To our knowledge, this represents the largest multi-center international cohort of AHCT for relapsed/refractory HL. Our large data set helped us develop a robust prognostic model that identifies three different risk groups. The highest risk group which had a 42% PFS at 4 years and identified those with the lowest likelihood of cure from AHCT. They should be considered candidates for trials of additional or alternative therapy. These could include maintenance/consolidation therapy post-AHCT or alternative therapies such as brentuximab vedotin or reduced intensity allogeneic HCT.27, 28 A randomized multi-center trial of brentuximab vedotin after AHCT is on-going in patients classified as “high risk” according to our prognostic model, and may provide evidence of a new treatment strategy for relapsed/refractory HL.29
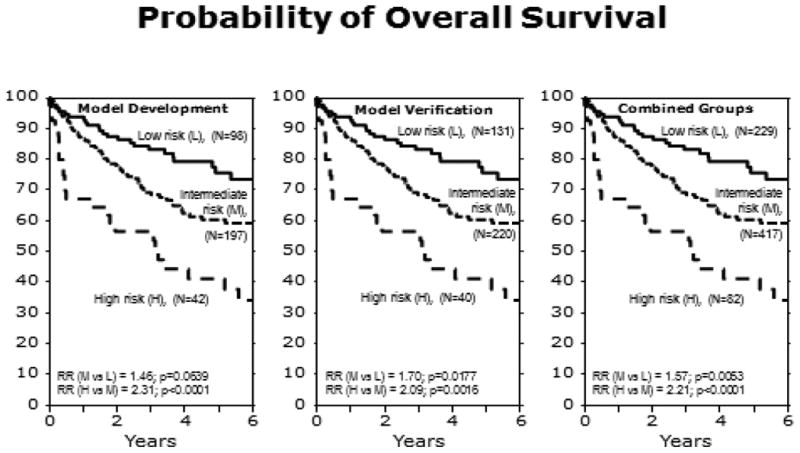
Three previously published AHCT prognostic models all yielded three risk factors, each of which were evenly weighted.21-23 One model discriminated two risk groups (low, high),21 while the other two models discriminated three risk groups (low, intermediate, high).22, 23 However, we were not able to validate the prognostic significance of the intermediate risk groups in these two models.24 In contrast, our new prognostic model contains four risk factors which were given a weighted score or 1 or 2 based on the RR estimate. Summing the scores across all risk factors generated a final score to assign patients to a risk category. One of the risk factors in our model (chemotherapy-resistant disease pre-AHCT) was a significant risk factor in all three prior models whereas the other three risk factors were reported in only one or two of the prior models. Hence, all four of our risk factors have been previously reported as prognostic of PFS after AHCT. The large size of our cohort enabled us to refine and enhance these prior models, and to significantly distinguish and validate an intermediate risk group for OS. However, the intermediate risk group was only significantly different from the low and high risk groups for PFS after combining both the development and validation cohorts.
Recent studies have reported the value of pre-transplant positron emission tomography (PET) functional imaging which provides prognostic information in the setting of AHCT for HL.30-33 PET was not approved for HL staging until 2004 in the US, which is near the end of our cohort (2006). The majority of patients in our study (73%) were not in CR pre-AHCT and therefore should have been PET-positive. Even in more recently treated patients from 2007-2010 in the same registry, about 77% had a PET scan pre-AHCT and the majority of those (81%) were positive, leaving few patients with a negative PET pre-AHCT to evaluate in a multivariate prognostic model. However, our attempt was to tease out higher risk groups in patients with detectable disease by standard CT criteria, hence the addition of PET scan information would not be discriminatory in this setting. While our prognostic model is inexpensive and easy to use, especially in countries where there is limited access to PET imaging, the future use of PET scans may further define extranodal involvement and chemotherapy sensitivity pre-AHCT and could further refine our model.
A significant proportion of patients were missing LDH at diagnosis and sizing of tumor masses during therapy. These reflect the importance of obtaining an LDH measurement by all oncologists and the need for a standardized approach to reading and sizing tumor masses during therapy. Lastly, 84% of patients who were not in CR pre-AHCT were missing information on whether post-AHCT radiation therapy was planned or not. Therefore it is unknown if post-AHCT radiation, or any other planned post-AHCT treatment, can improve prognosis or response rates, or overcome the pre-AHCT poor risk factors. It is also unknown if post-AHCT radiation therapy may have been applied differently to the three prognostic groups. However, this question can only be answered in prospective trials, and pre-AHCT prognostic models are crucial in the design and stratification of such approaches.
Our model is based on a large multicenter international cohort of HL patients undergoing AHCT and utilizes easily collectible information at the time of AHCT. We expect these features to improve its applicability and generalizability. We expect this model to assist clinicians in obtaining important prognostic features during the pre-AHCT timeline to identify those patients who may need additional post AHCT therapy, alternative transplant or novel approaches.
Figure 2. Overall survival for the model development, model validation, and combined cohorts.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children's Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Financial Disclosure Statement: The authors declare no competing financial interests.
Previous Presentation: This study was previously presented at an oral session of the American Society of Hematology annual meeting, December 2011: Hahn T, McCarthy PL, Carreras J, et al. A CIBMTR prognostic model for progression-free survival (PFS) after autologous hematopoietic cell transplantation (AHCT) for relapsed or refractory Hodgkin Lymphoma (HL). Blood. 2011;118 (11); 499.
Author Contribution: T.H., P.L.M. and P.N.H. designed the study and participated in interpretation of data, manuscript preparation and approval of final manuscript. J.C. and M.J.Z. did the statistical analysis. H.M.L, G.G.L. and S.M. participated in interpretation of data, manuscript preparation and approval of final manuscript. T.H., P.L.M. and P.N.H. had primary responsibility for manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cashen AF, Bartlett NL. Salvage regimens for Hodgkin lymphoma. Clin Adv Hematol Oncol. 2008;6(7):517–524. [PubMed] [Google Scholar]
- 2.Mendler JH, Friedberg JW. Salvage therapy in Hodgkin's lymphoma. Oncologist. 2009;14(4):425–432. doi: 10.1634/theoncologist.2009-0002. [DOI] [PubMed] [Google Scholar]
- 3.Colpo A, Hochberg E, Chen YB. Current status of autologous stem cell transplantation in relapsed and refractory Hodgkin's lymphoma. Oncologist. 2012;17(1):80–90. doi: 10.1634/theoncologist.2011-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy F, Sirohi B, Cunningham D. Stem cell transplantation in Hodgkin lymphoma. Expert Rev Anticancer Ther. 2007;7(3):297–306. doi: 10.1586/14737140.7.3.297. [DOI] [PubMed] [Google Scholar]
- 5.Moskowitz AJ, Perales MA, Kewalramani T, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146(2):158–163. doi: 10.1111/j.1365-2141.2009.07727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josting A, Rudolph C, Mapara M, et al. Cologne high-dose sequential chemotherapy in relapsed and refractory Hodgkin lymphoma: results of a large multicenter study of the German Hodgkin Lymphoma Study Group (GHSG) Ann Oncol. 2005;16(1):116–123. doi: 10.1093/annonc/mdi003. [DOI] [PubMed] [Google Scholar]
- 7.Cooney JP, Stiff PJ, Toor AA, et al. BEAM allogeneic transplantation for patients with Hodgkin's disease who relapse after autologous transplantation is safe and effective. Biol Blood Marrow Transplant. 2003;9(3):177–182. doi: 10.1053/bbmt.2003.50007. [DOI] [PubMed] [Google Scholar]
- 8.Anderlini P, Saliba R, Acholonu S, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin's lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93(2):257–264. doi: 10.3324/haematol.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devetten MP, Hari PN, Carreras J, et al. Unrelated donor reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2009;15(1):109–117. doi: 10.1016/j.bbmt.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18(6):1071–1079. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- 11.Gobbi PG, Cavalli C, Federico M, et al. Hodgkin's disease prognosis: a directly predictive equation. Lancet. 1988;1(8587):675–679. doi: 10.1016/s0140-6736(88)91477-8. [DOI] [PubMed] [Google Scholar]
- 12.Wagstaff J, Gregory WM, Swindell R, et al. Prognostic factors for survival in stage IIIB and IV Hodgkin's disease: a multivariate analysis comparing two specialist treatment centres. Br J Cancer. 1988;58(4):487–492. doi: 10.1038/bjc.1988.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straus DJ, Gaynor JJ, Myers J, et al. Prognostic factors among 185 adults with newly diagnosed advanced Hodgkin's disease treated with alternating potentially noncross-resistant chemotherapy and intermediate-dose radiation therapy. J Clin Oncol. 1990;8(7):1173–1186. doi: 10.1200/JCO.1990.8.7.1173. [DOI] [PubMed] [Google Scholar]
- 14.Proctor SJ, Taylor P, Donnan P, et al. A numerical prognostic index for clinical use in identification of poor-risk patients with Hodgkin's disease at diagnosis. Scotland and Newcastle Lymphoma Group (SNLG) Therapy Working Party. Eur J Cancer. 1991;27(5):624–629. doi: 10.1016/0277-5379(91)90231-2. [DOI] [PubMed] [Google Scholar]
- 15.Gobbi PG, Comelli M, Grignani GE, et al. Estimate of expected survival at diagnosis in Hodgkin's disease: a means of weighting prognostic factors and a tool for treatment choice and clinical research. A report from the International Database on Hodgkin's Disease (IDHD) Haematologica. 1994;79(3):241–255. [PubMed] [Google Scholar]
- 16.Lee SM, Radford JA, Ryder WD, et al. Prognostic factors for disease progression in advanced Hodgkin's disease: an analysis of patients aged under 60 years showing no progression in the first 6 months after starting primary chemotherapy. Br J Cancer. 1997;75(1):110–115. doi: 10.1038/bjc.1997.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339(21):1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 18.Stiff PJ, Unger JM, Forman SJ, et al. The value of augmented preparative regimens combined with an autologous bone marrow transplant for the management of relapsed or refractory Hodgkin disease: a Southwest Oncology Group phase II trial. Biol Blood Marrow Transplant. 2003;9(8):529–539. doi: 10.1016/s1083-8791(03)00205-2. [DOI] [PubMed] [Google Scholar]
- 19.Sirohi B, Cunningham D, Powles R, et al. Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma. Ann Oncol. 2008;19(7):1312–1319. doi: 10.1093/annonc/mdn052. [DOI] [PubMed] [Google Scholar]
- 20.Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16(4):625–633. doi: 10.1093/annonc/mdi119. [DOI] [PubMed] [Google Scholar]
- 21.Hahn T, Benekli M, Wong C, et al. A prognostic model for prolonged event-free survival after autologous or allogeneic blood or marrow transplantation for relapsed and refractory Hodgkin's disease. Bone Marrow Transplant. 2005;35(6):557–566. doi: 10.1038/sj.bmt.1704789. [DOI] [PubMed] [Google Scholar]
- 22.Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12(10):1065–1072. doi: 10.1016/j.bbmt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler C, Eickhoff C, Elias A, et al. High-dose cyclophosphamide, carmustine, and etoposide with autologous transplantation in Hodgkin's disease: a prognostic model for treatment outcomes. Biol Blood Marrow Transplant. 1997;3(2):98–106. [PubMed] [Google Scholar]
- 24.Hahn T, McCarthy PL, Carreras J, et al. Validation of prognostic models for progression-free survival after autologous transplant for relapsed or refractory Hodgkin lymphoma. Blood. 2009;114:499. suppl; abstr 1215. [Google Scholar]
- 25.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97(3):616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 26.Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's lymphoma study group. J Clin Oncol. 2002;20(1):221–230. doi: 10.1200/JCO.2002.20.1.221. [DOI] [PubMed] [Google Scholar]
- 27.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 28.Chen R, Palmer JM, Thomas SH, et al. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2012;119(26):6379–6381. doi: 10.1182/blood-2012-03-418673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. [Accessed 7/6/12]; Available from: http://clinicaltrials.gov/ct2/show/NCT01100502.
- 30.Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119(7):1665–1670. doi: 10.1182/blood-2011-10-388058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devillier R, Coso D, Castagna L, et al. Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin's lymphoma responding to prior salvage therapy. Haematologica. 2012;97(7):1073–1079. doi: 10.3324/haematol.2011.056051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smeltzer JP, Cashen AF, Zhang Q, et al. Prognostic significance of FDG-PET in relapsed or refractory classical Hodgkin lymphoma treated with standard salvage chemotherapy and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(11):1646–1652. doi: 10.1016/j.bbmt.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sucak GT, Ozkurt ZN, Suyani E, et al. Early post-transplantation positron emission tomography in patients with Hodgkin lymphoma is an independent prognostic factor with an impact on overall survival. Ann Hematol. 2011;90(11):1329–1336. doi: 10.1007/s00277-011-1209-0. [DOI] [PubMed] [Google Scholar]


