Abstract
Purpose
To identify all available reconstruction methods for a total sacrectomy. Secondarily, we aimed to evaluate outcomes based on different interventions.
Methods
We searched PubMed to identify sacral resections for tumors requiring internal fixation for stabilization. Demographic information, fixation techniques and postoperative outcomes were abstracted.
Results
Twenty-three publications (43 patients) met inclusion criteria from an initial search of 856 (κ 0.93). Mean age was 37 years and follow-up was 33 months. Fixation methods included a combination of spinopelvic fixation (SPF), posterior pelvic ring fixation (PPRF), and/or anterior spinal column fixation (ASCF). For the purposes of analysis, patients were segregated based on whether they received ASCF. Postoperative complications including wound/instrument infections, GI or vascular complications were reported at a higher rate in the non-ASCF group (1.63 complications/patient vs. 0.7 complications/patient). Instrument failure was seen in 5 (16.1 %) out of the 31 patients with reported outcomes. Specifically, 1 out of 8 patients (12.5 %) with ASCF compared with 4 out of 23 patients (17.4 %) without ASCF had hardware failure. At final follow-up, 35 of 39 patients were ambulating.
Conclusion
While surgical treatment of primary sacral tumors remains a challenge, there have been advances in reconstruction techniques following total sacrectomy. SPF has shifted from intrapelvic rod and hook constructs to pedicle and iliac screw–rod systems for improved rigidity. PPRF and ASCF have adapted for deficiencies in the posterior ring and anterior column. A trend toward a lower rate of hardware failure emerged in the group utilizing anterior spinal column support. Despite a more involved reconstruction with ASCF, surgical complications such as infection rates and blood loss were lower compared to the group without ASCF. While we cannot definitively say one system is superior to the other, based on the data gleaned from this systematic review, it is our opinion that incorporation of ASCF in reconstructing the spinopelvic junction may lead to improved outcomes. However, most importantly, we recommend that the treating surgeon operate on patients requiring a total sacrectomy based on his or her level of comfort, as these cases can be extremely challenging even among experts.
Keywords: Total sacrectomy, Spinopelvic fixation, Posterior pelvic ring fixation, Anterior spinal column fixation, Systematic review
Introduction
Primary sacral tumors represent a rare entity, accounting for <7 % of all spinal tumors [1]. Chordomas, which are the most common primary malignant sacral neoplasm, along with chondrosarcomas and giant cell tumors are relatively resistant to radiotherapy and chemotherapy [2, 3]. Given the relatively mild symptoms initially, patients with sacral malignancies often present with large, advanced neoplastic disease. Intralesional resection without adequate margins may lead to higher local recurrence [1, 4–6]. Hence, en bloc resection has been demonstrated to increase the disease-free survival period in patients harboring various primary sacral neoplasms that are unresponsive to chemotherapy and radiotherapy [4–6]. Despite the obvious advantages for disease-free survival, en bloc resection and spinopelvic reconstruction for sacral tumors pose a unique challenge for the surgeon given the complex anatomical and biomechanical relationships that govern the spinopelvic junction. Although partial sacrectomy operations are usually well tolerated without the requirement of stabilization, in patients with extensive expansion of the tumor requiring total sacrectomy, the vertical and rotational instability created necessitates stabilization between the lumbar spine and the pelvis to allow for mobilization and improved function [7–9].
With advances in surgical techniques, a variety of interventions for reconstruction following total sacrectomy have been reported. However, the rarity of this unique intervention has limited these accounts to individual case reports and small case series [2, 8, 10–30]. There are no published systematic reviews that provide the surgeon with a comprehensive summary of current and past surgical reconstructive techniques with associated outcomes. The primary aim of this systematic review was to provide the treating surgeon with a comprehensive overview of available options for reconstructing the spinopelvic junction following total sacrectomy. Secondarily, we examined outcomes and complications between the various treatment techniques, identifying differences between the methods of instrumentation.
Materials and methods
A search strategy was developed with the assistance of a medical librarian to identify relevant articles in the PubMed database that reported on en bloc sacral resections for tumors requiring internal fixation for stabilization. All relevant articles in English up to March 17, 2011 were included. Conceptually, the search was constructed by combining keywords that focused on (1) sacral tumors, (2) sacral resection and (3) reconstruction (Appendix). We additionally performed hand searches of bibliographies of relevant articles as well as archives from meeting proceedings of the American Academy of Orthopaedic Surgeons (2010–2011), Musculoskeletal Tumor Society (2010–2011), Scoliosis Research Society (2009–2011), American Academy of Neurological Surgeons (archives), and Congress of Neurological Surgeons (2005–2012).
We included all studies that contained details of surgical instrumentation and fixation methods for patients who underwent en bloc resection of the tumor requiring a total sacrectomy. We excluded studies that reported on patients with partial sacrectomy, those who underwent intralesional resection, tumors without sacral involvement, those who did not report on reconstruction details, studies without rigid internal fixation, animal models, finite element modeling, and biomechanical studies.
Titles and abstracts were reviewed by two investigators to include potentially eligible articles. Full manuscripts identified were retrieved in a blinded manner. Disagreements were resolved by a third investigator. Cohen’s Kappa was used to quantify overall agreement. For all included studies, demographic information, fixation techniques and instrumentation details, as well as postoperative outcomes were abstracted using a standardized form. We considered surgical constructs that connected the lumbar spine to the ilium as ‘spinopelvic fixation’ (SPF) while constructs that connected only the ilia together without fixation to the lumbar spine were identified as ‘posterior pelvic ring fixation’ (PPRF). Constructs that aimed to strengthen the anterior spinal column were recognized as ‘anterior spinal column fixation’ (ASCF). These designations were used either individually or in combination.
All authors were contacted to inquire about any changes in patient outcomes since publication as they relate to tumor recurrence, ambulation, and the need for revision surgery. Authors of the studies were contacted in cases where patient data were presented only as an aggregate, making it difficult to tease apart the relevant data. If further clarification could not be provided, we maintained the aggregate data and noted this in the tables.
Results
Search results
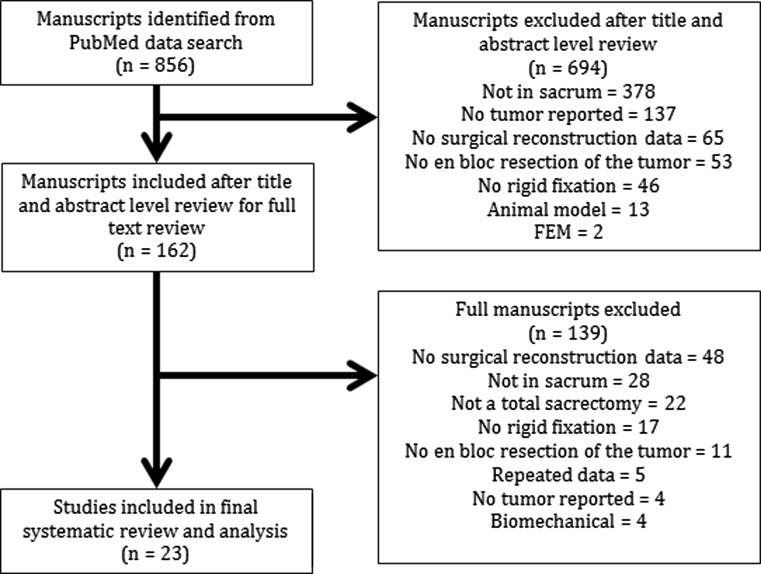
The initial search of the PubMed database located 856 publications based on our search strategy. After a title and abstract level review, 162 articles were found to be potentially eligible (κ 0.93). Of those, 139 studies did not meet our inclusion criteria—48 did not provide surgical technique or instrumentation detail, 28 were based on non-sacral tumors, 22 did not describe a total sacrectomy, 17 did not use rigid fixation for the treatment of sacral tumors, 11 reported intralesional tumor resections, 5 included repeated patient data or no patient data, 4 reported biomechanical data, and 4 did not include tumors (Fig. 1).
Fig. 1.
Outline of the review process
23 Articles representing 43 patients met our inclusion criteria and were included in our final analysis. 15 studies of the 23 included were single case reports and 8 studies were case series. Eight studies reported on multiple patients, where only a portion of the patients met our inclusion criteria. Patients not meeting our criteria in those studies were excluded and the eligible patient data were isolated.
A total of 107 abstracts were identified as relevant from the annual meeting archives of relevant academic organization using the search terms “sacrum”, “sacral”, and “sacrectomy”. Of these, 23 studies were identified as potentially relevant; however, all were excluded. 16 abstracts did not provide sufficient reconstruction technique or instrumentation details, 5 studies presented data on partial sacrectomy, and 2 studies reported on patients who were presented in studies included from our original search.
Study characteristics
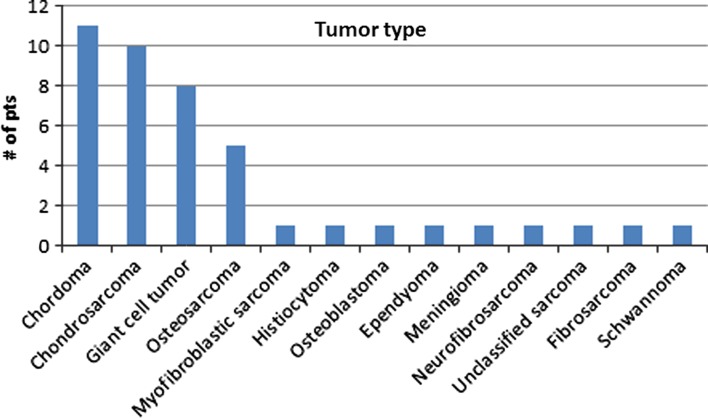
For the 43 patients included in our review, the mean age of patients was 37 years (range 13–61), with 25 being male and 18 female. The tumor types are displayed in Fig. 2. 32 patients were reported to have neurological deficits preoperatively, while no data were reported for the other 11 patients (Table 1).
Fig. 2.
Tumor diagnoses for patients included in this review
Table 1.
Patient demographics and tumor type
| Author | Year | # of Pts | Age | Sex | Tumor type | Neurological deficit |
|---|---|---|---|---|---|---|
| Humphries et al. [16] | 2010 | 1 | 15 | F | Myofibroblastic sarcoma | Y |
| Gallia et al. [17] | 2010 | 1 | 52 | F | Malignant fibrous histiocytoma | Y |
| Varga et al. [10] | 2010 | 1 | 42 | M | Chordoma | Y |
| Newman et al. [11] | 2009 | 1 | 35 | M | Chondrosarcoma | Y |
| McLoughlin et al. [12] | 2008 | 1 | 57 | M | Osteoblastoma | Y |
| Shen et al. [18] | 2006 | 1 | 44 | M | Chordoma | NR |
| Gallia et al. [21] | 2005 | 1 | 52 | M | Chordoma | Y |
| Fourney et al. [2] | 2005 | 3 | 35a | M (3) | Chordoma (2), chondrosarcoma (1) | NR |
| Dickey et al. [20] | 2005 | 6 | 40a | M (2), F (4) | Ependymoma, osteosarcoma, chordoma, chondrosarcoma, osteosarcoma, meningioma | Y |
| Min et al. [29] | 2005 | 1 | 35 | F | Neurofibrosarcoma | Y |
| Zileli et al. [24] | 2003 | 3 | 28a | M (1), F (2) | Chondrosarcoma (2), osteosarcoma (1) | Y |
| Ohata et al. [13] | 2004 | 1 | 13 | M | Unclassified sarcoma | Y |
| Doita et al. [8] | 2003 | 3 | 61a | M (2), F (1) | Giant cell tumor, chordoma (2) | NR |
| Mooney et al. [14] | 1999 | 1 | 10 | M | Fibrosarcoma | Y |
| Sar et al. [25] | 2002 | 3 | 18a | M (2), F (1) | Giant cell tumor, chondrosarcoma (2) | NR |
| Wuisman et al. [19] | 2001 | 1 | 42 | F | Osteosarcoma | Y |
| Jackson et al. [22] | 2000 | 5 | 45b | M (8), F (5)b | Giant cell tumor (2), chordoma (2), chondrosarcoma (1) | Y |
| Spiegel et al. [15] | 1999 | 1 | 16 | M | Osteosarcoma | Y |
| Gokaslan et al. [26] | 1997 | 2 | 34a | F (2) | Giant cell tumor (2) | Y |
| Santi et al. [30] | 1993 | 1 | 48 | M | Schwannoma | N |
| Shikata et al. [23] | 1992 | 1 | 34 | M | Giant cell tumor | Y |
| Tomita et al. [27] | 1990 | 2 | 52a | M (1), F (1) | Giant cell tumor (1), Chordoma (1) | NR, Y |
| Shikata et al. [28] | 1988 | 2 | 35a | M (1) F (1) | Giant cell tumor (1) Chondrosarcoma (1) | Y |
NR not reported, Y yes
a Average of patient with total sacrectomy, b average of all patients reported in article
Reconstruction methods
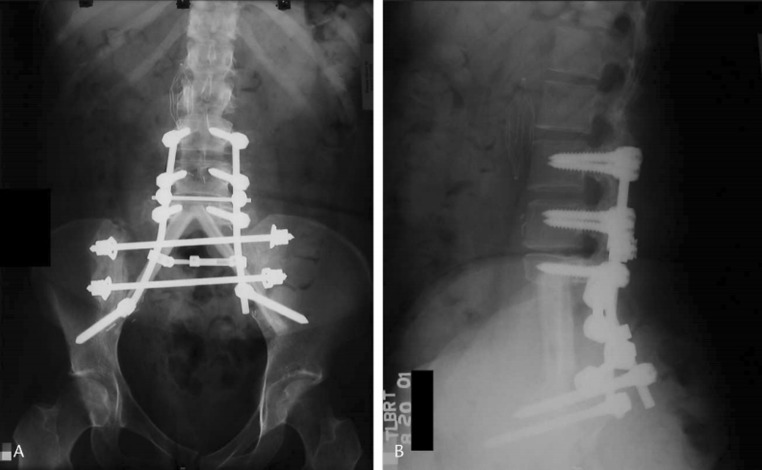
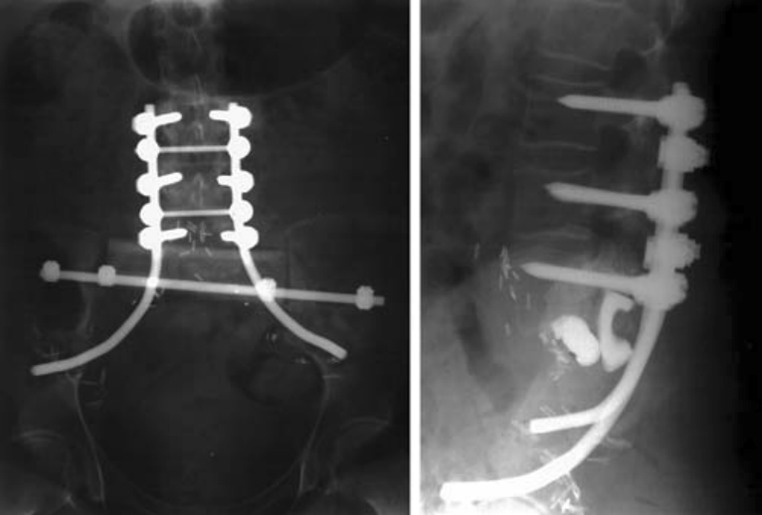
Fixation methods were stratified by the techniques of reconstruction (SPF, PPRF, and ASCF). All patients received SPF along with some form of additional fixation: 1 patient (2.3 %) had additional ASCF (Fig. 3), 34 (79.1 %) received additional PPRF (Fig. 4), and 8 (18.6 %) received both ASCF and PPRF in conjunction with SPF (Fig. 5). For the purpose of our evaluation, we separated those who received ASCF (9 patients, 20.9 %) from those that did not (34 patients, 79.1 %).
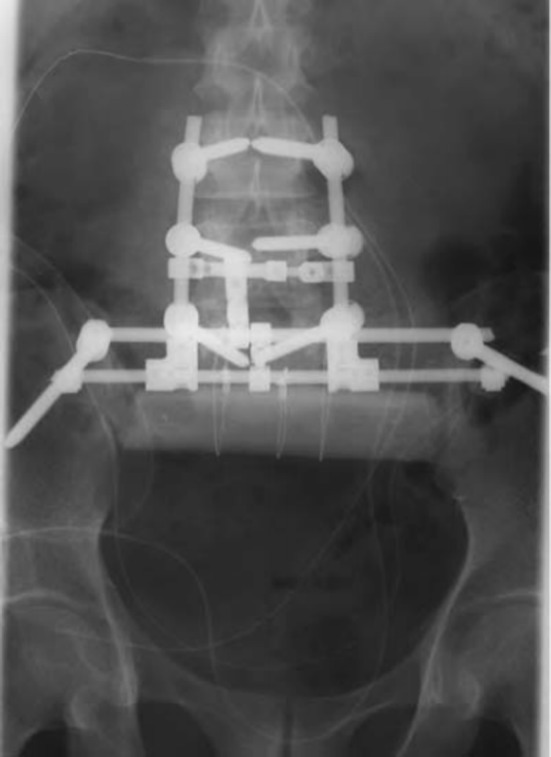
Fig. 3.
“Older” SPF methods—pedicle screws connected to ilium via Harrington rods [26]
Fig. 4.
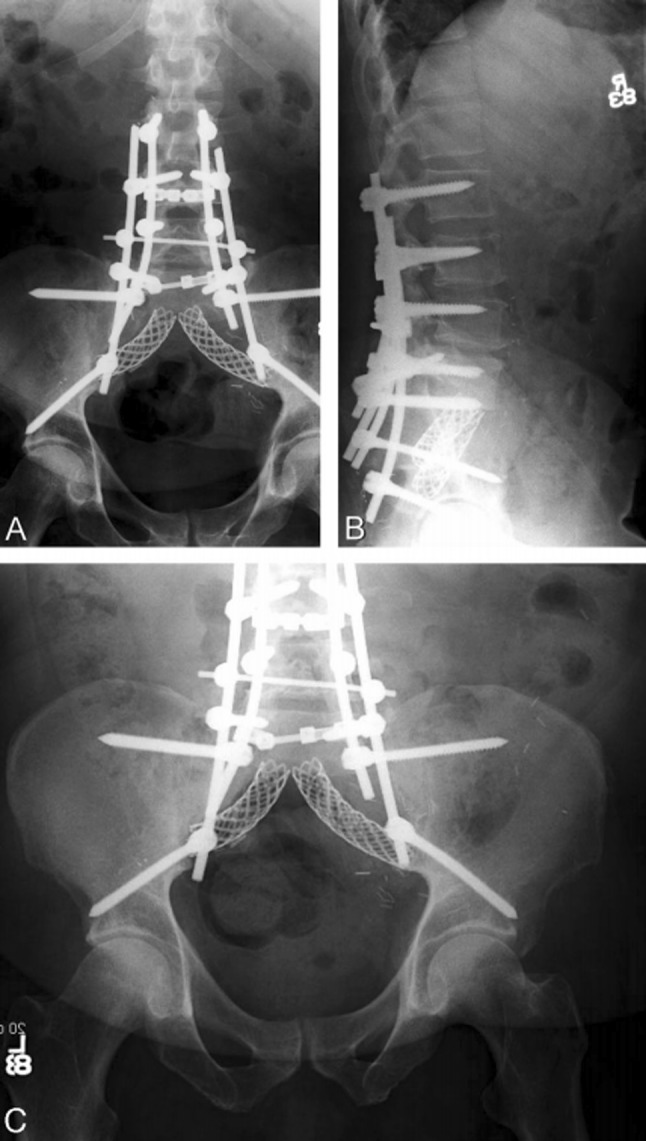
SPF with ACSF—pedicle screws connected to iliac screws by two lumbar rods on each side; two titanium mesh cages [18]
Fig. 5.
SPF and PPRF—pedicle screws connected to transiliac bar via lumbar rods and cross connectors; femoral bone graft. a Photograph of the final construct, b radiograph of the final construct [21]
A trend was recognized with regard to the evolution of SPF systems—‘older’ systems (reported up to 2005) included intrapelvic Galveston rods and hook–rod constructs connected to transiliac bars (21 patients), while ‘newer’ systems (reported starting in 2001) included pedicle screw–rod constructs connected to iliac screws, transiliac rods, or custom plates (22 patients) (Fig. 6).
Fig. 6.
SPF with PPRF and ASCF—pedicle screws connected to iliac screws via lumbar rods; two transiliac bars (not shown); two oblique fibular grafts from L5 to the anterior ilium [20]
The PPRF systems connecting ilium to the contralateral ilium included transiliac rod or plate fixation (35 patients), structural allografts (12 patients), and prosthetic cages (2 patients). ASCF systems that connected the pelvis to the anterior spinal column included iliolumbar screws (1 patient), fibular grafts (6 patients), titanium mesh and expandable cages (2 patients), and a vertical rod through the lumbar vertebral bodies (1 patient) (Table 2).
Table 2.
Reconstruction details along with operative complications
| Author | Year | # Pts | Extra sacral resection | Nerve sacrifice | Spinopelvic fixation (cephalad level) | Posterior pelvic ring fixation | Anterior spinal column support | Soft tissue flaps | Blood loss (mL) | Operative complications? |
|---|---|---|---|---|---|---|---|---|---|---|
| Humphries et al. [16] | 2010 | 1 | No | S1–S5 | PS (L3), connected to transverse rod between IS | (1) TI bar (2) IS with transverse rod (3) Titanium mesh cage between ilia |
– | VRAM | NR | S. epidermidis abscess, ischial decubitus ulcer |
| Gallia et al. [17] | 2010 | 1 | (B) ilia, L5 | L5–S5 | PS (L1), connected to transverse rod between IS | (1) 2 TI bars (2) IS with transverse rod (3) TI femoral graft |
(1) Vertical rod through L2–L4 bodies (2) Distractable cage between L4 endplate and TI bar |
Paraspinal and partial LD | 800 (stage 1), 8,500 (stage 2) | Abdominal hematoma, wound dehiscence, DVT |
| Varga et al. [10] | 2010 | 1 | No | S1–S5 | PS (L2), connected to IS with U-shaped rod | (1) Bone graft between ilium and L5 | – | GM | NR | NR |
| Newman et al. [11] | 2009 | 1 | (L) ilium | S1–S5 | PS (L1), connected to transverse rod between IS | (1) IS with two transverse rods (2) Stacked, carbon-fiber cages |
– | RAM + (R) GM | 1,500 | Flap hematoma |
| McLoughlin et al. [12] | 2008 | 1 | (B) ilia | S1–S5 | PS (L3), connected to IS; two vertical rods connected to PS rod and TI bar | (1) TI bar (2) TI femoral graft |
– | Alloderm sling | 6,000 | NR |
| Shen et al. [18] | 2006 | 1 | No | NR | PS (L2), connected to IS–two rods/side | – | (1) Bilateral titanium mesh cages from L5 to SI joint | (R) RAM | 1,100 (stage 1), 2,900 (stage 2) | Ileus |
| Gallia et al. [21] | 2005 | 1 | (B) ilia | S1–S5 | PS (L3), connected to transverse rod between IS | (1) TI bar (2) IS with transverse rod (3) TI femoral graft |
– | RAM | NR | Ileus, vocal cord paralysis, UTI, infection (8 mo) |
| Fourney et al. [2] | 2005 | 3 | No | S1–S5 | PS (L3), connected to Galveston rods | (1) TI bar (2) TI tibial graft |
– | RAM | NR | SB injury, upper GI hemorrhage and major wound dehiscence |
| GM | NR | |||||||||
| RAM | NR | |||||||||
| Dickey et al. [20] | 2005 | 6 | L5 (some cases) | S1–S5 | PS (L3 or L4), connected to IS | (1) 2 TI bars | (1) Two oblique, fibular grafts | RAM | NR | None |
| RAM | None | |||||||||
| RAM | NR | |||||||||
| None | None | |||||||||
| None | None | |||||||||
| RAM | Wound infection | |||||||||
| Min et al. [29] | 2005 | 1 | (B) ilia | S1–S4 | PS (L4), connected to IS | (1) Two TI tibial grafts | – | NR | 4,300 | Wound dehiscence |
| Zileli et al. [24] | 2003 | 1 | No | S1–S5 | PS (L2 or L3), connected to custom iliac plate | (1) Custom iliac plate | – | Myocutaneous | 3,400 | Instrument infection |
| 2 | PS (L2 or L3), connected to TI bar | (1) Two TI bars | NR | NR | NR | |||||
| Ohata et al. [13] | 2004 | 1 | (B) ilia | (L) L5 and S1–S5 | PS (L3), connected to IS | (1) TI bar (2) Two TI fibular grafts |
– | GM | 12,000 | UTI, instrument infection |
| Doita et al. [8] | 2003 | 3 | No | S1–S5 | PS (L5), connected to TI bar | (1) TI bar | – | NR | 9250 | NR |
| (B) ilia | PS (L3), connected to IS; fibular graft between posterolateral spine and ilia | (1) TI bar | – | NR | 7,500 | NR | ||||
| No | (R) sciatic, S1–S5 | NR | 9,600 | Wound infections (debridement) | ||||||
| Mooney et al. [14] | 1999 | 1 | No | S1–S5 | PS (L3), connected to Galveston rods | (1) TI bar (2) TI fibular graft |
(1) Two oblique, iliolumbar screws | GM | NR | NR |
| Sar et al. [25] | 2002 | 3 | NR | S1–S4 | PS (L3), connected to Galveston rods or IS | (1) one or two TI fibular graft | – | NR | NR | NR |
| Deep wound infection | ||||||||||
| NR | ||||||||||
| Wuisman et al. [19] | 2001 | 1 | (B) ilia | S1–S5 | PS (L2), connected to custom plate | (1) Custom iliac plate | – | GM | 8,500 | Right lateral wound dehiscence |
| Jackson et al. [22] | 2000 | 5 | NR | NR | PS (L3), connected to Galveston rods | (1) TI bar | – | RAM | NR | None |
| RAM | NR | Seizures and UTI | ||||||||
| RAM | NR | Small intestinal perforation, sepsis, coagulopathy, C. difficile | ||||||||
| GM | NR | C. difficile | ||||||||
| RAM | NR | UTI | ||||||||
| Spiegel et al. [15] | 1999 | 1 | (R) ilia | S1–S5 | PS (L3), connected to Galveston rods | (1) Pelvic reconstruction plate | – | NR | NR | NR |
| Gokaslan et al. [26] | 1997 | 2 | No | S1–S5 | PS (L3), connected to Galveston rods | (1) TI bar | – | RAM | 21,500 | NR |
| 21,700 | ||||||||||
| Santi et al. [30] | 1993 | 1 | No | S1–S5 | Hooks and CD rods, connected to TI bar (L4) | 1) Two TI bars | – | NR | 6,500 | NR |
| Shikata et al. [23] | 1992 | 1 | (B) ilia | L5–S5 | Two compression rods, connected to TI bar (L3) | (1) Two TI bars | – | NR | 6,300 | NR |
| Tomita et al. [27] | 1990 | 2 | No | S1–S5 | 2 Harrington rods; fibular grafts between L4 pedicles and ipsilateral ilium (NR) | (1) TI bar | – | NR | 7,500 | NR |
| No | (R) L5, S1–S5 | CD Instrumentation (T12) | (1) AO 16-hole broad plate (2) AO Reconstruction plate |
– | NR | 17,000 | Torn iliac vein | |||
| Shikata et al. [28] | 1988 | 2 | (B) ilia | S1–S5 | Two Harrington rods (L3); fibular grafts between L3, L4, and ipsilateral ilium | (1) Two TI bars | – | NR | 10,000 | NR |
B bilateral, L left, R right, N no, N/A not applicable, NR not reported, PS pedicle screw, IS iliac screw, TI transiliac, CD Cotrel–Dubousset, RAM rectus abdominis myocutaneous, LD latissimus dorsi, GM gluteus maximus
Instrumentation complications
Revision surgery or instrument failure was seen in 5 (16.1 %) out of the 31 patients with reported outcomes (Table 3). The need for revision surgery was not reported for 12 (27.9 %) of the 43 patients included in this review. Instrumentation failure was reported in 1 out of 8 patients (12.5 %) who received ASCF compared with 4 out of 23 patients (17.4 %) in the group without ASCF (Table 4).
Table 3.
Long-term outcomes to evaluate ambulatory status, neurological deficits, need for revision surgery, pain, and tumor recurrence
| Author | Year | SPF | PSF | PPRF | ASF | Follow up (months) | Ambulation | Bowel/bladder deficit | Revision instrumentation | Pain at final follow up | Tumor recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Humphries et al. [16] | 2010 | N | Y | Y | N | 12 | Yes (R-AFO) | NR | None | None | None |
| Gallia et al. [17] | 2010 | N | Y | Y | Y | 5 | No | Yes | None | Metastasis | Metastasis |
| Varga et al. [10] | 2010 | N | Y | Y | N | NR | Yes (cane) | NR | Nonea | Nonea | Nonea |
| Newman et al. [11] | 2009 | N | Y | Y | N | 6 | Yes (Walker) | Partial | Nonea | NR | NR |
| McLoughlin et al. [12] | 2008 | N | Y | Y | N | 24 | Yes | Yes | None | None | NR |
| Shen et al. [18] | 2006 | N | Y | N | Y | 9 | No | Yes | None | NR | NR |
| Gallia et al. [21] | 2005 | N | Y | Y | N | 12 | Yes | Yes | None | Due to metastasis | Local + Metastasis |
| Fourney et al. [2] | 2005 | O | Y | Y | N | 83 | Yes | Yes | NR | NR | Local |
| 58 | NR | NR | NR | Local + Metastasis | |||||||
| 33 | Yes | NR | NR | Local | |||||||
| Dickey et al. [20] | 2005 | N | Y | Y | Y | 8 | Yes | Yes | None | Improved | None |
| 7 | Yes | None | None | ||||||||
| 38 | Yes | Revision instrumentation (6 and 29 months) | None | ||||||||
| 42 | No | None | Local | ||||||||
| 36 | Yes | None | None | ||||||||
| 14 | No | None | Local | ||||||||
| Min et al. [29] | 2005 | N | Y | Y | N | 60 | Yes (crutches) | Yes | Lumbar rod breakage at 4 months (rods replaced) | Improveda | None |
| Zileli et al. [24] | 2003 | N | Y | Y | N | Mean 28 (range 18–42) | Yes (crutches) | NR | None | Local + Metastasis | |
| Yes (crutches) | NR | Revision instrumentation at 7 years | None | ||||||||
| Yes (crutches) | NR | None | None | None | |||||||
| Ohata et al. [13] | 2004 | N | Y | Y | N | 60 | Yes (crutches) | Yes | None | NR | None |
| Doita et al. [8] | 2003 | N | Y | Y | N | 36 | Yes (cane) | Partial | None | Improved | NR |
| 36 | Yes (crutches) | Yes | None | NR | Local | ||||||
| 36 | Yes (crutches) | Partial | None | NR | NR | ||||||
| Mooney et al. [14] | 1999 | O | Y | Y | Y | 8 | Yes (walker + R-AFO) | Yes | NR | NR | Metastasis |
| Sar et al. [25] | 2002 | O | Y | Y | N | 49 | NR | Yes | None | NR | None |
| 33 | NR | Yes | None | NR | Local | ||||||
| 47 | NR | Yes | None | NR | None | ||||||
| Wuisman et al. [19] | 2001 | N | Y | Y | N | 36 | Yes (cane + AFO) | Yes | None | None | None |
| Jackson et al. [22] | 2000 | O | Y | Y | N | 50 | Yes (walker) | NR | NR | Improved | NR |
| 47 | Yes | ||||||||||
| 31 | Yes (walker) | ||||||||||
| 11 | Yes | ||||||||||
| 8 | Yes (cane) | ||||||||||
| Spiegel et al. [15] | 1999 | O | Y | Y | N | 74 | Yes (AFO) | Yes | Ilial rod and pelvic plate breakage (no operation–patient asymptomatic) | Occasional | None |
| Gokaslan et al. [26] | 1997 | O | Y | Y | N | 12 | Yes (cane) | Yes | NR | NR | NR |
| Yes | Yes | NR | NR | NR | |||||||
| Santi et al. [30] | 1993 | O | Y | Y | N | 33 | Yes (AFO) | NR | Hardware removed due to discomfort (7 months) | None | None |
| Shikata et al. [23] | 1992 | O | Y | Y | N | 72 | Yes (cane) | Partial | None | NR | NR |
| Tomita et al. [27] | 1990 | O | Y | Y | N | 69 | Yes | Yes | None | NR | None |
| Y | Y | N | 10 | Yes (AFO) | Partial | NR | NR | None | |||
| Shikata et al. [28] | 1988 | O | Y | Y | N | 24 | Yes (brace) | Partial | None | NR | NR |
| Y | Y | N | 14 | Yes (cane) | Partial | None | NR | NR |
NR not reported, AFO ankle foot orthosis
a Based on email correspondence
Table 4.
Complications based on reconstruction method
| Without anterior column support (34 pts) | With anterior column support (9 pts) | |
|---|---|---|
| Instrumentation failure | 4 in 23 pts (NR for 11 pts) | 1 in 8 pts (NR for 1 pt) |
| General surgical complications | 26 in 16 pts (NR for 18 pts) | 5 in 7 pts (NR for 2 pts) |
| Surgical site infection | 6 (37.5 %) | 1 (14.3 %) |
| Wound dehiscence | 3 (18.8 %) | 1 (14.3 %) |
| Gastrointestinal | 6 (37.5 %) | 1 (14.3 %) |
| Urinary tract infection | 4 (25 %) | 0 (0 %) |
| Vascular | 4 (25 %) | 2 (28.6 %) |
| Other | 3 (18.8 %) | 0 (0 %) |
| Blood loss | 9,535 mL (16 pts; NR for 18 pts) | 6,650 mL (2 pts; NR for 7 pts) |
| Ambulation | 8 (24 %) independently | 4 (44 %) independently |
| 22 (65 %) with help | 1 (11 %) with help | |
| 0 cannot | 4 (44 %) cannot | |
| 4 (12 %) NR | 0 NR |
General surgical complications
Soft tissue flaps were used in the operation for 31 patients (72.1 %) to cover the void left by the en bloc sacral resection. Blood loss was reported for 24 patients (55.8 %) with an average of 9,276 mL per patient (range 1,500–21,700 mL). Postoperative complications were reported for 23 patients and included wound/instrument infections (7 patients, 30.4 %), wound dehiscence (4 patients, 17.4 %), gastrointestinal complications (hemorrhage, perforation, C. difficile infection and ileus) (7 patients, 30.4 %), urinary tract infections (4 patients, 17.4 %), vascular complications (7 patients, 30.4 %), seizures (1 patient, 4.3 %), decubitus ulcer (1 patient, 4.3 %), and none in 4 patients (Table 2).
General surgical complications were reported at a higher rate in the group without ASCF than in the group that did receive ASCF (1.63 complications per patient vs. 0.7 complications per patient). Average blood loss for the group without ASCF was 9,535 mL for 16 patients, while in the group with ASCF an average blood loss of 6,650 mL was reported in 2 patients (Table 4).
Long-term outcomes
Mean follow-up was 33 months (range 5–83 months) as reported in 22 out of the 23 articles for 42 patients (Table 3). Since all patients had S1–S5 nerve transection (4 with additional L5 transection), bowel and bladder dysfunction was common in all 32 patients with reported outcomes (25—complete, 7—partial).
The incidence of tumor recurrence was reported for 25 patients, of whom 14 (56 %) had no recurrence at an average of 37 months, 6 (24 %) had local recurrence at an average of 40 months, and 5 (20 %) had metastatic disease at an average of 22 months. The tumors that recurred or metastasized included chordoma (4), chondrosarcoma (3), osteosarcoma (1), histiocytoma (1), meningioma (1), and fibrosarcoma (1). Changes in pain were reported inconsistently and are presented along with other outcome data in Table 3.
At final follow-up, 35 patients (89.7 %) of the 39 with reported ambulatory status were ambulating. Of the patients who did not receive ASCF, 8 (24 %) were able to ambulate independently, 22 (65 %) with help, and the status was not reported for 4 patients (Table 4). For the group of patients that received ASCF, four (44 %) ambulated independently, one (11 %) with help, and four (37 %) could not ambulate at all.
Discussion
A variety of instrumentation techniques exist to stabilize the lumbosacral junction following total sacrectomy; however, due to the infrequency of these tumors requiring total sacrectomy, the novel instrumentation techniques are scattered across the literature in case reports and small case series. It is the relative infrequency of these operations that has limited the ability to directly compare the wide array of techniques and instrumentation approaches. Therefore, in this review we identified and summarized the various reconstructive methods available as well as examined trends in treatment outcomes and complication rates to best assist a surgeon who may be required to care for a patient with large sacral tumors.
Although our inclusion criteria of rigid fixation prevented us from including articles that reported on patients treated without reconstruction following a total sacrectomy, we do acknowledge this as a viable option. Given the high rate of major complications, many authors have found this option a better alternative for their patients [31–34]. These authors argue that the muscles and scar between the pelvis and spine form a biologic sling, eventually stabilizing the spine and often allowing ambulation [32, 35].
Through our comprehensive search, we identified 23 articles (42 patients) that reported on operative techniques for reconstruction following total sacrectomy for en bloc tumor resection. To our knowledge, this is the only systematic review that focuses on spinopelvic reconstruction. The three most common tumor types reported for patients included in this review included chordoma, chondrosarcomas, and giant cell tumors, which are known to be common sacral tumors [36].
We identified three main reconstructive strategies, namely spinopelvic fixation (SPF), posterior pelvic ring fixation (PPRF), and anterior spinal column fixation (ASCF). No single technique was used in isolation; rather, all patients received SPF in association with either one or two other techniques. The vast majority of patients (34 patients; 79.1 %) received PPRF along with SPF, while eight patients (18.6 %) had both ASCF and PPRF in conjunction with SPF. In summary, we observed an overall instrumentation failure rate of 16.1 % (5 out of 31 patients). No instrument-related complications were reported for 26 patients. Blood loss is a significant consideration for these operations, with an average of 9,276 mL reported for 24 patients. Surgical complications add significant morbidity to these large operations. 31 instances of complications were reported for 26 patients (1.35 complications per patient). Ambulation was preserved in a majority of the patients reported in literature (35 patients of 39 with reported outcomes; 90 %) with varying degrees of required assistance. However, given that all patients required at least S1–S5 nerve transection, all had some neurological deficits, including bowel and bladder dysfunction.
When comparing the various methods of reconstruction, a trend toward a lower rate of hardware failure emerged in the group utilizing anterior spinal column support when compared to the patients without ASCF (12.5 vs. 17.4 %). Despite a more involved reconstruction with ASCF, surgical complications, such as infection rates and blood loss were lower compared to the group without ASCF (mean complications: 0.7 vs. 1.63; infection: 14.3 vs. 37.5 %; blood loss: 6,650 vs. 9,535 mL).
While no obvious differences in the patient demographics were noted between the two groups, it is possible that patient risk factors and other confounders not identified in this review may have led to the counter-intuitive decreased rate of surgical complications in the group with additional ASCF.
Through our review, we identified two authors who have contributed significantly to our understanding of reconstructing the spinopelvic junction following a total sacrectomy. Gokaslan et al. [26] initially reported on the use of modified Galveston L-rods to connect the lumbar spine to the ilia, in addition to the transiliac bar that provided pelvic ring fixation. This represented a significant improvement to the Harrington rod systems of the 1980s which rarely provided the stability necessary to achieve iliolumbar fusion. Many articles included in this review have made use of this technique to establish spinopelvic fixation. Subsequent articles published by Gallia, Fourney, and McLoughlin along with Gokaslan and colleagues have evolved from the use of modified Galveston systems to lumbar pedicle screw systems cross-connecting to intricate arrangements that re-establish connection between contralateral ilia of the pelvic ring [2, 12, 17, 21]. This new spinopelvic system offers safer screw placement in the pedicles of each vertebrae that are independent of the rigid axial bar construct. The posterior pelvic ring has most commonly been reconstructed using transiliac bars, cages, and a variety of bone grafts to promote bony fusion. The fixation they provide is more rigid than previously possible. The most recent article by Gallia made use of an interconnected SPF system, two transiliac bars and femoral bone graft (PPRF), as well as a vertical cage and rod, through the bodies of lumbar vertebrae (ASCF) [17].
Dickey et al. reported the largest case series with total sacrectomies included in this review. They described their method of using pedicle screws for spinopelvic fixation, dual transiliac bars for reconstructing the pelvic ring and two fibular grafts to establish anterior spinal column fixation in a triangular fashion from the caudal end of L5 to the pelvic brim (Fig. 5).
Like all systematic reviews, this study possesses inherent limitations. Our systematic search only utilized the PubMed database and may have missed articles captured in other databases. However, to expand our search, bibliographies were manually scanned and abstracts were searched from the archives of various professional society meetings. Additionally, the assistance of a medical librarian in the development of our search strategy strengthened our ability to locate all relevant articles. Another limitation to our review was that several publications had patient and outcome data aggregated with patients receiving partial sacrectomy and other surgical treatments that did not meet our inclusion criteria of total sacrectomy followed by instrumented reconstruction. If the specific data could not be separated from the aggregated data, authors were contacted in an effort to obtain the individual data. An additional limitation was that many surgical techniques identified were from earlier articles utilizing techniques that have subsequently fallen out of favor or have been improved upon. This publication data bias may have resulted in a more guarded prognosis for recovery following treatment. It is worth acknowledging that this article lacks significant statistical pooling, which may have strengthened our arguments. However, statistical analysis was thought not to be of great value for our review given the small number of patients identified, as well as the inconsistently reported objective data. The primary aim of this article was to summarize techniques of reconstruction.
Finally, the value of any systematic review depends on the quality of the individual articles. Because total sacrectomies followed by reconstruction are uncommon, most publications were limited to retrospective small case series or case reports, representing a much lower level of evidence compared to prospective series. Nonetheless, this review remains the best available evidence we have to evaluate the surgical techniques and outcomes for this rare indication.
Conclusion
While surgical treatment of primary sacral tumors remains a challenge, there has been an evolution in techniques for reconstruction of the spinopelvic junction following total sacrectomy. SPF has evolved from intrapelvic rods and hook constructs to segmental pedicle screw and iliac screw–rod systems. PPRF and ASCF have adapted for deficiencies in the posterior ring and anterior column with the use of structural allografts and/or prosthetic cages. While biomechanical and finite element analysis studies support this improved stability in the newer constructs using PPRF and ASCF [37–39], the functional outcomes and complication rates identified in this review between fixation systems identifies a lack of substantial improvement between theorized structural benefit and actual patient outcomes. While we cannot definitively say one system is superior to the other, based on the data gleaned from this systematic review, it is our opinion that incorporation of anterior spinal column fixation in reconstructing the spinopelvic junction may lead to improved outcomes with lower rates of hardware failure and other surgical complications. However, most importantly, we recommend that the treating surgeon operate on patients requiring a total sacrectomy based on his or her level of comfort, as these cases can be extremely challenging even among experts. Further investigation with larger case series and large-scale patient registries should be pursued in an effort to better distinguish differences in functional outcomes and complication rates between the instrumentation techniques.
Conflict of interest
None.
Appendix: search strategy to capture all relevant articles in the Pubmed database
General scheme
[(“tumor terminology” AND “sacral area terminology”) OR (“sacral resection terminology”)] AND [(“reconstruction terminology”)]
Search terms
Tumor terminology
(Metastasis OR (metastatic AND (tumor OR tumour OR disease OR neoplasm)) OR primary tumor OR: “neoplasm”[Mesh] OR neoplasm OR “Neoplasm metastasis”[Mesh] OR bone neoplasms OR “bone neoplasms”[mesh] OR “pelvic neoplasms”[Mesh] OR pelvic neoplasms OR “Chondrosarcoma”[Mesh] OR chondrosarcoma OR “Giant cell tumor”[Mesh] OR giant cell tumor OR “Lymphoma”[Mesh] OR lymphoma OR “Multiple Myeloma”[Mesh] OR myeloma OR “Plasmacytoma”[Mesh] OR plasmacytoma OR Ewing Sarcoma OR “Chordoma”[Mesh] OR chordoma OR “Osteosarcoma”[Mesh] OR osteosarcoma OR osteogenic sarcoma OR “Spinal Neoplasms”[Mesh] OR spinal neoplasms OR “Bone Cysts, Aneurysmal”[Mesh] OR Aneurysmal bone cysts)
Sacral area terminology
(sacrum OR sacral OR “Sacrum”[Mesh] OR “Lumbosacral Region”[Mesh] OR lumbosacral OR lumbo-sacral OR spinal pelvic OR spinal-pelvic OR spino-pelvic OR spinopelvic OR sacroiliac OR sacro-iliac OR iliosacral OR ilio-sacral OR lumbo-pelvic OR lumbopelvic OR lumboiliac OR lumbo-iliac OR lumbosacropelvic)
Sacral resection terminology
(total sacrectomy OR (en bloc resection) OR (enbloc resection) OR ((sacral OR sacrum) AND (resection)))
Reconstruction terminology
((Galveston OR galveston L-rod OR galveston rod OR L-rod) AND (instrumentation OR technique OR fixation)) OR ((sacrum OR sacral OR “Sacrum”[Mesh] OR “Lumbosacral Region”[Mesh] OR lumbosacral OR lumbo-sacral OR spinal pelvic OR spinal-pelvic OR spino-pelvic OR spinopelvic OR sacroiliac OR sacro-iliac OR iliosacral OR ilio-sacral OR lumbo-pelvic OR lumbopelvic OR lumboiliac OR lumbo-iliac OR lumbosacropelvic) AND (stabiliz* OR stabilis* OR stable OR stability OR fixation OR reconstruction OR screw)) OR (“fracture fixation”[mesh]) OR (fixation) OR (“Internal Fixators”[Mesh] OR internal fixators) OR (“Bone Screws”[Mesh] OR bone screws) OR ((transiliac OR trans-iliac) AND (bar OR rod OR screw)) OR (“Orthopedic Procedures”[Mesh] OR orthopedic procedures OR orthopaedic procedures OR “Reconstructive Surgical Procedures”[Mesh] OR reconstructive surgical procedures))
Contributor Information
S. Samuel Bederman, Phone: +1-714-4565759, FAX: +1-714-4567547, Email: sbederma@uci.edu.
Kalpit N. Shah, Email: knshah@uci.edu
Jeffrey M. Hassan, Email: hassanj@uci.edu
Bang H. Hoang, Email: bhhoang@uci.edu
P. Douglas Kiester, Email: pkiester@uci.edu.
Nitin N. Bhatia, Email: bhatian@uci.edu
References
- 1.Feldenzer JA, McGauley JL, McGillicuddy JE. Sacral and presacral tumors: problems in diagnosis and management. Neurosurgery. 1989;25:884–891. doi: 10.1227/00006123-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Fourney DR, Rhines LD, Hentschel SJ, et al. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine. 2005;3:111–122. doi: 10.3171/spi.2005.3.2.0111. [DOI] [PubMed] [Google Scholar]
- 3.Llauger J, Palmer J, Amores S, et al. Primary tumors of the sacrum: diagnostic imaging. Am J Roentgenol. 2000;174:417–424. doi: 10.2214/ajr.174.2.1740417. [DOI] [PubMed] [Google Scholar]
- 4.York JE, Kaczaraj A, Abi-Said D, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999;44:74–80. doi: 10.1097/00006123-199901000-00041. [DOI] [PubMed] [Google Scholar]
- 5.Ozaki T, Flege S, Liljenqvist U, et al. Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2002;94:1069–1077. doi: 10.1002/cncr.10258. [DOI] [PubMed] [Google Scholar]
- 6.Bergh P, Gunterberg B, Meis-Kindblom JM, Kindblom LG. Prognostic factors and outcome of pelvic, sacral, and spinal chondrosarcomas: a center-based study of 69 cases. Cancer. 2001;91:1201–1212. doi: 10.1002/1097-0142(20010401)91:7<1201::AID-CNCR1120>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Hugate RR, Jr, Dickey ID, Phimolsarnti R, et al. Mechanical effects of partial sacrectomy: when is reconstruction necessary? Clin Orthop Relat Res. 2006;450:82–88. doi: 10.1097/01.blo.0000229331.14029.44. [DOI] [PubMed] [Google Scholar]
- 8.Doita M, Harada T, Iguchi T, et al. Total sacrectomy and reconstruction for sacral tumors. Spine (Phila Pa 1976) 2003;28:E296–E301. doi: 10.1097/01.BRS.0000083230.12704.E3. [DOI] [PubMed] [Google Scholar]
- 9.Gunterberg B, Romanus B, Stener B. Pelvic strength after major amputation of the sacrum. An experimental study. Acta Orthop Scand. 1976;47:635–642. doi: 10.3109/17453677608988751. [DOI] [PubMed] [Google Scholar]
- 10.Varga PP, Lazary A. Chordoma of the sacrum: “en bloc” total sacrectomy and lumbopelvic reconstruction. Eur Spine J. 2010;19:1039–1040. doi: 10.1007/s00586-010-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman CB, Keshavarzi S, Aryan HE. En bloc sacrectomy and reconstruction: technique modification for pelvic fixation. Surg Neurol. 2009;72:752–756. doi: 10.1016/j.surneu.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 12.McLoughlin GS, Sciubba DM, Suk I, et al. En bloc total sacrectomy performed in a single stage through a posterior approach. Neurosurgery. 2008;63:ONS115–ONS120. doi: 10.1227/01.NEU.0000312354.43020.03. [DOI] [PubMed] [Google Scholar]
- 13.Ohata N, Ozaki T, Kunisada T, et al. Extended total sacrectomy and reconstruction for sacral tumor. Spine (Phila Pa 1976) 2004;29:E123–E126. doi: 10.1097/01.BRS.0000115140.19829.4B. [DOI] [PubMed] [Google Scholar]
- 14.Mooney JF, 3rd, Glazier SS, Turner CS, DeFranzo AJ., Jr Fibrosarcoma of the sacrum in a child: management by sacral resection and reconstruction. J South Orthop Assoc. 1999;8:218–221. [PubMed] [Google Scholar]
- 15.Spiegel DA, Richardson WJ, Scully SP, Harrelson JM. Long-term survival following total sacrectomy with reconstruction for the treatment of primary osteosarcoma of the sacrum. A case report. J Bone Joint Surg Am. 1999;81:848–855. doi: 10.2106/00004623-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Humphries WE, 3rd, Satyan KB, Relyea K, et al. Low-grade myofibroblastic sarcoma of the sacrum. J Neurosurg Pediatr. 2010;6:286–290. doi: 10.3171/2010.5.PEDS09289. [DOI] [PubMed] [Google Scholar]
- 17.Gallia GL, Suk I, Witham TF, et al. Lumbopelvic reconstruction after combined L5 spondylectomy and total sacrectomy for en bloc resection of a malignant fibrous histiocytoma. Neurosurgery. 2010;67:E498–E502. doi: 10.1227/01.NEU.0000382972.15422.10. [DOI] [PubMed] [Google Scholar]
- 18.Shen FH, Harper M, Foster WC, et al. A novel “four-rod technique” for lumbo-pelvic reconstruction: theory and technical considerations. Spine (Phila Pa 1976) 2006;31:1395–1401. doi: 10.1097/01.brs.0000219527.64180.95. [DOI] [PubMed] [Google Scholar]
- 19.Wuisman P, Lieshout O, Van Dijk M, Van Diest P. Reconstruction after total en bloc sacrectomy for osteosarcoma using a custom-made prosthesis: a technical note. Spine (Phila Pa 1976) 2001;26:431–439. doi: 10.1097/00007632-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 20.Dickey ID, Hugate RR, Jr, Fuchs B, et al. Reconstruction after total sacrectomy: early experience with a new surgical technique. Clin Orthop Relat Res. 2005;438:42–50. doi: 10.1097/01.blo.0000180054.76969.41. [DOI] [PubMed] [Google Scholar]
- 21.Gallia GL, Haque R, Garonzik I, et al. Spinal pelvic reconstruction after total sacrectomy for en bloc resection of a giant sacral chordoma: technical note. J Neurosurg Spine. 2005;3:501–506. doi: 10.3171/spi.2005.3.6.0501. [DOI] [PubMed] [Google Scholar]
- 22.Jackson RJ, Gokaslan ZL. Spinal-pelvic fixation in patients with lumbosacral neoplasms. J Neurosurg. 2000;92:61–70. doi: 10.3171/spi.2000.92.1.0061. [DOI] [PubMed] [Google Scholar]
- 23.Shikata J, Yamamuro T, Shimizu K, Kotoura Y. Surgical treatment of giant-cell tumors of the spine. Clin Orthop Relat Res. 1992;278:29–36. [PubMed] [Google Scholar]
- 24.Zileli M, Hoscoskun C, Brastianos P, Sabah D. Surgical treatment of primary sacral tumors: complications associated with sacrectomy. Neurosurg Focus. 2003;15:E9. [PubMed] [Google Scholar]
- 25.Sar C, Eralp L. Surgical treatment of primary tumors of the sacrum. Arch Orthop Trauma Surg. 2002;122:148–155. doi: 10.1007/s00402-001-0356-5. [DOI] [PubMed] [Google Scholar]
- 26.Gokaslan ZL, Romsdahl MM, Kroll SS, et al. Total sacrectomy and Galveston L-rod reconstruction for malignant neoplasms. Technical note. J Neurosurg. 1997;87:781–787. doi: 10.3171/jns.1997.87.5.0781. [DOI] [PubMed] [Google Scholar]
- 27.Tomita K, Tsuchiya H. Total sacrectomy and reconstruction for huge sacral tumors. Spine (Phila Pa 1976) 1990;15:1223–1227. doi: 10.1097/00007632-199011010-00024. [DOI] [PubMed] [Google Scholar]
- 28.Shikata J, Yamamuro T, Kotoura Y, et al. Total sacrectomy and reconstruction for primary tumors. Report of two cases. J Bone Joint Surg Am. 1988;70:122–125. [PubMed] [Google Scholar]
- 29.Min K, Espinosa N, Bode B, Exner GU. Total sacrectomy and reconstruction with structural allografts for neurofibrosarcoma of the sacrum. A case report. J Bone Joint Surg Am. 2005;87:864–869. doi: 10.2106/JBJS.D.02299. [DOI] [PubMed] [Google Scholar]
- 30.Santi MD, Mitsunaga MM, Lockett JL. Total sacrectomy for a giant sacral schwannoma. A case report. Clin Orthop Relat Res. 1993;294:285–289. [PubMed] [Google Scholar]
- 31.Michel A. Total sacrectomy and lower spine resection for giant cell tumor: one case report. La Chirurgia degli organi di movimento. 1990;75:117–118. [PubMed] [Google Scholar]
- 32.Ruggieri P, Angelini A, Ussia G, et al. Surgical margins and local control in resection of sacral chordomas. Clin Orthop Relat Res. 2010;468:2939–2947. doi: 10.1007/s11999-010-1472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson AH, Porter A, Davis A, et al. Cephalad sacral resection with a combined extended ilioinguinal and posterior approach. J Bone Joint Surg Am. 1995;77:405–411. doi: 10.2106/00004623-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Capanna R, Briccoli A, Campanacci L et al (1997) Benign and malignant tumors of the sacrum. In: Frymoyer JW (ed) The adult spine: principles and practice, 2nd edn. Lippincott-Raven, Philadelphia, pp 2367–2405
- 35.Guo Y, Yadav R. Improving function after total sacrectomy by using a lumbar–sacral corset. Am J Phys Med Rehabil. 2002;81:72–76. doi: 10.1097/00002060-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Payer M. Neurological manifestation of sacral tumors. Neurosurg Focus. 2003;15:E1. doi: 10.3171/foc.2003.15.2.1. [DOI] [PubMed] [Google Scholar]
- 37.Liljenqvist U, Hackenberg L, Link T, Halm H. Pullout strength of pedicle screws versus pedicle and laminar hooks in the thoracic spine. Acta Orthop Belg. 2001;67:157–163. [PubMed] [Google Scholar]
- 38.Hackenberg L, Link T, Liljenqvist U. Axial and tangential fixation strength of pedicle screws versus hooks in the thoracic spine in relation to bone mineral density. Spine. 2002;27:937–942. doi: 10.1097/00007632-200205010-00010. [DOI] [PubMed] [Google Scholar]
- 39.Hitchon PW, Brenton MD, Black AG, et al. In vitro biomechanical comparison of pedicle screws, sublaminar hooks, and sublaminar cables. J Neurosurg. 2003;99:104–109. doi: 10.3171/spi.2003.99.1.0104. [DOI] [PubMed] [Google Scholar]