Abstract
Purpose
A retrospective study of 32 patients with osteoblastoma (OBL) in the mobile spine was performed to analyze the clinical characteristics of two types of spinal OBL. We also aimed to find influential factors for OBL in the mobile spine.
Methods
Between 2002 and 2011, 32 patients with either conventional osteoblastoma (CO) or aggressive osteoblastoma (AO) in the mobile spine were treated in our center. All patients were treated with either total excision or subtotal excision + postoperative radiotherapy. The mean follow-up was 45.8 (18–128) months. Clinical data and surgery efficacy were analyzed to search for clinical characteristics of two subtypes of spinal OBL and discuss the possible factors influencing relapse.
Results
There is significant difference between CO and AO in tumor size (p < 0.0005), preoperative alkaline phosphatase (ALP, p < 0.0005) and intraoperative blood loss (p = 0.013). Multivariate logistic regression was used to find the influential factors for relapse and the results were: preoperative ALP, b = 0.023, p = 0.029; surgery protocol, b = −7.597, p = 0.007; tumor size, ≥3/<3, b = 24.805, p < 0.0005; age, b = 0.054, p = 0.632; and pathology type, b = 1.998, p = 0.34.
Conclusions
Tumor size, preoperative ALP and CT images were helpful for distinguishing AO from CO. The difference in intraoperative blood loss between CO and AO is mainly attributed to the size of the lesion. Preoperative ALP, surgery protocol and tumor size (≥3/<3) were considered to significantly influence relapse of spinal OBL.
Electronic supplementary material
The online version of this article (doi:10.1007/s00586-013-3049-1) contains supplementary material, which is available to authorized users.
Keywords: Retrospective study, Conventional osteoblastoma, Aggressive osteoblastoma, Alkaline phosphatase, Relapse
Introduction
Osteoblastoma (OBL) is a rare, benign, primary bone tumor that currently accounts for 1 % of all bone tumors and 3 % of benign bone tumors, with one-third of OBL arising from the spine [1, 2]. In 1956, Jaffe and Lichtenstein [3, 4] independently differentiated OBL from osteoid osteoma and established benign osteoblastoma as a specific term. In 1984, Dorfman and Weiss [5] reported on aggressive osteoblastoma and defined it as a borderline osteoblastic tumor entity with the histologic feature of epithelioid osteoblasts, and a higher recurrence rate and malignant transformation capability.
Since the definition of AO in 1984, some authors tried to describe this uncommon subtype of OBL. But due to its rarity, there were only some case reports in literature, let alone systematic and detailed description about clinical characteristics and therapeutic outcome of AO.
Surgical resection is generally recognized as the first choice of treatment for spinal OBL, while the efficacy of radiotherapy is still in dispute [6–8]. Relapse is not uncommon after surgery and incomplete excision of the tumor is regarded as the main reason.
In this retrospective study, we sought to identify the clinical characteristics of the two subtypes of OBL by analyzing the clinical data of 32 patients who were with either CO or AO in the mobile spine and treated in our bone tumor center between 2002 and 2011. Furthermore, clinical data and surgery efficacy were analyzed to find the factors associated with relapse. As far as we know, this study is the first large series designed to study AO, although there were some case reports about AO in the literature.
Patients and methods
A total of 40 patients with OBL in the mobile spine who received operation in our center were identified from 2002 to 2011; 8 of them had already accepted treatment at another institution. The other 32 patients were regarded as “intact” cases since they did not receive surgical intervention and any other treatment before admission into our institution and were selected to be analyzed as a series. Their clinical data, including symptoms, signs, radiographic features, surgery information, and detailed pathologic workup were recorded and preserved (Supplemental Table).
Two independent pathologists (Hongyu Yu and Bingbing Li) were invited to review all the histology slides for differentiating pathological types. Moreover, the results of the classification were confirmed by the “Shanghai clinicopathological discussion seminar” which was held in our hospital every Thursday afternoon.
Of the 32 patients with OBL in the mobile spine, 20 cases were diagnosed as conventional osteoblastoma (CO) and 12 cases were aggressive osteoblastoma (AO). Alkaline phosphatase (ALP) was assayed in 27 patients (17 CO and 10 AO) via a chemiluminescence immunoassay, with the standard normal range being 15–112 μ/l (Supplemental Table).
All the patients accepted surgery in our department. Our principle of selecting the surgery protocol is that total excision is the first considered choice, and subtotal excision + postoperative radiotherapy (RT) is adopted when total excision is hard to achieve. Local conventional radiotherapy was undertaken 4–6 weeks after surgery and the total dose administered over 20 applications ranged from 30 to 50 Gy. For patients with neurologic disorders, intravenous injection of mecobalamin 0.5 mg was given once a day for continuous 7 days, followed by mecobalamin tablets 0.5 mg orally taken three times a day for 3 months.
The mean follow-up period was 45.8 months (range 18–128). Follow-up data were obtained from follow-up visits and telephone interviews. In addition, neural function was re-evaluated 3 months after surgery according to the Frankel score system (Supplemental Table).
Results
Patient features
In our series, the mean age for OBL, CO, and AO was 24.3, 26.3, and 23.5 years, respectively, with approximately 70 % of the patients under 30 years old. The patients with CO seemed older than those with AO, but there was no statistical significance (p = 0.557, Table 2). A higher male:female ratio was found in AO, with 1.86:1, 3:1 for CO and AO.
Table 2.
Clinical data contrast of two types of osteoblastoma
| CO | AO | p value | |
|---|---|---|---|
| Age | 26.3 ± 16.1 | 23.5 ± 10.5 | 0.557 |
| Duration of symptoms (m) | 20.7 ± 23.3 | 9.2 ± 8.6 | 0.113 |
| Neurologic disorders (±) | 12/8 | 11/1 | 0.054 |
| Spinal deformity (±) | 4/16 | 5/7 | 0.187 |
| Extraosseous paravertebral (layer A) involvement | 6/14 | 10/2 | 0.003 |
| Epidural space (layer D) involvement | 7/13 | 10/2 | 0.008 |
| Preoperative ALP (μ/l) | 120.6 ± 55.8 | 292.6 ± 163.5 | <0.0005 |
| ALP (+)/ALP (−) | 7/10 | 8/2 | 0.0499 |
| Intraoperative blood loss (ml) | 1172.5 ± 864.1 | 2350 ± 1669.4 | 0.013 |
| Tumor size (cm) | 2.7 ± 1 | 4.3 ± 0.8 | <0.0005 |
| Relapse (±) | 2/18 | 5/7 | 0.036* |
| Follow-up (m) | 48.5 ± 35.7 | 41.4 ± 29 | 0.548 |
Symptoms and neurological findings
Dull and localized pain in the spine was the most common initial complaint, and the mean duration of symptoms was 16.2 months for all patients with OBL, with 20.7 months for CO and 9.2 months for AO (p = 0.113, Table 2). The aggressive trend of pain was more common in CO (60 %) than in AO (33.3 %) (p = 0.236), and six patients with CO (30 %) eventually suffered intense pain. In addition, 40 % of patients with CO suffered a dull pain that worsened at night.
Neurologic disorders were found in 60 % of the patients with CO and 91.7 % of the cases with AO (p = 0.054, Table 2). Spinal deformity was also more common in AO (41.7 %) than CO (20 %) (p = 0.187, Table 2).
55 % of CO involved the cervical spine (C1–T1), while the thoracic spine (T2–T12) was infringed by 50 % of AO. As for the domain of tumor involvement, 70 % of CO infringed on one vertebral level and 50 % of AO infringed on two vertebral levels.
Laboratory blood test findings
The preoperative ALP levels were 120.6 and 292.6 μ/l for cases with CO and AO, respectively (p < 0.0005, Table 2), while the positive/negative values of ALP were 7/10 and 8/2 for cases with CO and AO, respectively (p = 0.0499, Table 2). For the six patients with both preoperative ALP and postoperative ALP, there was a significant drop after operation (p = 0.036).
Radiological findings
The typical circular high-density shadow imaging was found in 35 % of patients with CO, but normal imaging appeared in 50 % of the CO cases and 25 % of AO cases. There was obvious difference in CT images that all AO showed lytic imaging and 55 % of CO appeared in osteogenic imaging, with 10 % of CO showing lytic imaging and the others in mixed imaging (Fig. 1). Furthermore, typical MRI imaging (low T1 signal and high T2 signal) was found in 60 % of patients with CO and 83.3 % of the AO cases.
Fig. 1.
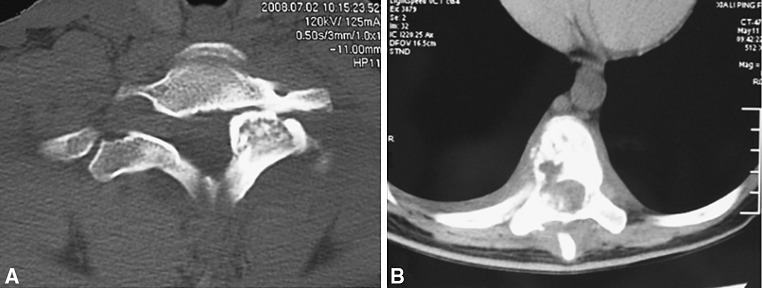
CT images of OBL. a CT images of a patient with CO presenting with an expansile lesion with peripheral hardening. b CT images of a patient with AO presenting mainly lytic imaging
Staging findings
There was significant difference between CO and AO in extraosseous paravertebral (layer A) involvement and epidural space (layer D) involvement (p = 0.003, 0.008, Table 2). Moreover, the size of AO (4.3 cm) was much bigger than that of CO (2.7 cm) (p < 0.0005, Table 2).
Treatment and outcome
Needle biopsy was performed in all patients before surgery and the result was OBL without further classification. Two types of surgery protocol were undertaken to treat the tumors: total excision (including en bloc resection) and subtotal excision + postoperative radiotherapy (Table 1). There was more intraoperative blood loss in patients with AO (2,350 ml) than those with CO (1,172.5 ml) (p = 0.013, Table 2). Local relapse occurred in seven patients (2 CO and 5 AO); two-thirds of the patients accepting subtotal excision + postoperative radiotherapy suffered relapse, while only one patient with AO who accepted total excision suffered relapse. Logistic regression was used to analyze possible factors influencing recurrence (age and preoperative ALP used as covariate, surgery protocol, pathology and tumor size (≥3/<3) used as factors) and the results were that preoperative ALP (b = 0.023, p = 0.029), surgery protocol (b = −7.597, p = 0.007) and tumor size (≥3/<3) (b = 24.805, p < 0.0005) could significantly influence relapse (Table 3). Although Chi-square test showed that patients with AO had higher relapse rate (p = 0.036, Table 2), it was not supported by logistic regression.
Table 1.
Treatment protocols and recurrence in two types of osteoblastoma in the mobile spine
| CO | AO | |||||
|---|---|---|---|---|---|---|
| n | Relapse | % | n | Relapse | % | |
| Treatment | ||||||
| Subtotal + RT total | 3 | 2 | 66.7 | 6 | 4 | 66.7 |
| Total | 15 | 0 | 0 | 4 | 1 | 16.7 |
| En bloc | 2 | 0 | 0 | 2 | 0 | 0 |
Table 3.
The results of multivariate logistic regression
| b | p | |
|---|---|---|
| Age (years) | 0.054 | 0.632 |
| Preoperative ALP (μ/l) | 0.023 | 0.029 |
| Pathology type | 1.998 | 0.34 |
| Surgery protocol | −7.597 | 0.007 |
| Tumor size (≥3/<3) | 24.805 | <0.0005 |
Pathology findings
The existence of epithelioid osteoblasts that are plump and larger than the osteoblasts of CO is the main pathological feature of AO, together with tumor invasion of cortical bone. These features formed our standard for the diagnosis of AO. Eosinophilic cytoplasm, more prominent nucleoli, and larger and irregular trabeculae were found in AO. In addition, osteoclast-like cells were more frequently found in AO than CO. Mitoses and mild cellular pleomorphism that do not appear in CO were occasionally found in AO.
Neurologic status
The pain in all patients was significantly relieved soon after surgery and was mostly absent by their 3-month follow-up visit. For patients with spinal deformity, the deformity improved or disappeared during the postoperative follow-up period. Most of the patients with neurologic disorders experienced improvement and a 1–2 grade decrease in Frankel scores. The neurologic status of all 32 cases did not worsen in the early postsurgical period.
Discussion
As rare, benign primary bone tumor, osteoblastoma (OBL) can be divided into two subtypes: conventional osteoblastoma (CO) and aggressive osteoblastoma (AO). The aims of our retrospective study were as follows: to search for the clinical characteristics helpful in distinguishing the two subtypes of OBL and to find possible factors influencing the relapse of OBL in the spine.
In our series, more than two-thirds of patients with OBL were under 30 years (mean 24.8 years) with total male:female ratio of 2.2:1, consistent with previous studies [2, 9]. Some authors believed that AO generally affected an older age group than CO, but our results did not support this point [10, 11]. Furthermore, we found a higher male: female ratio in patients with AO.
Dull and localized pain was the most common initial complaint for patients with either CO or AO. The duration of symptoms is obviously longer in patients with CO (20.7 months) than cases with AO (9.2 months), although the difference was not statistically significant. In our opinion, the difference may be due to the aggressive growth and higher growth rate of AO. The aggressive trend of pain was also more common in patients with CO than cases with AO, while intense pain and aggravated pain at night only occurred in patients with CO. But AO was more likely to cause spinal deformity and neurologic disorders than CO, because AO had a significantly larger scope of tumor involvement and size.
In our series, CO was most likely to infringe upon the cervical spine, while the thoracic spine was the most favorite position for AO. AO tended to involve more segments with half of AO encroaching on two segments and more than two-thirds of CO involving one segment.
Phosphate monoester hydrolase is bound up with bone metabolism in the human body. Alkaline phosphatase (ALP) can catalyze the hydrolysis and transfer of phosphate groups under alkaline conditions and is also regarded as an early marker of osteoblast differentiation; thus, ALP is involved in the production of a mineralized matrix [12]. Many authors have suggested that ALP might be a tumor-associated antigen and used as a monitor of drug efficacy in serum [13–16]. In our study, preoperative ALP was significantly higher in patients with AO than those with CO (p < 0.0005), and there is statistical difference between CO and AO in positive/negative value of ALP (p = 0.0499). Furthermore, a very high ALP value (>225 μ/l) was found in 70 % of the patients with AO (Supplemental Table). Therefore, ALP can serve as a clinical screening index for differentiating AO from CO. Furthermore, we found a significant drop in ALP after surgery.
Plain radiography is the most common radiological choice and is thought to have great value for diagnosing spinal OBLs [17–20]. However in our study, normal images were found in half of the patients with CO and one-fourth of cases with AO, which might cause a high number of misdiagnoses if it were the only diagnostic tool used. Therefore, we believe that plain radiography is not a reliable method and can lead to misdiagnosis of OBL in the spine.
The features of spinal OBL on MRI are often nonspecific and sometimes lead to a more aggressive or malignant diagnosis as a result of the localized inflammatory response elicited by the tumor [21–23]. Therefore, CT which has advantages in displaying bone structure change is a better radiological choice for diagnosis and can help to confirm the location, size, and extent of the tumor [21, 22]. In our series, CT showed obvious difference between CO and AO. More than half of CO appeared as osteogenic images, while all AO showed lytic images (Fig. 1). So, we think that CT might be able to provide help for distinguishing CO and AO.
Histologically, the existence of plump and large epithelioid osteoblasts as well as invasion of cortical bone were the main feature of AO, eosinophilic cytoplasm, more prominent nucleoli, larger and irregular trabeculae were found in AO. Furthermore, mitoses and mild cellular pleomorphism which did not appear in CO were occasionally found in AO.
Surgical resection is recognized as the most effective treatment for OBL in the spine [6–8]. Although few researchers believed that subtotal excision (curettage) or resection without a wide margin was sufficient for spinal OBL because of its benign nature and limited growth, the vast majority of experts held the view that radical surgical resection was the best possible treatment [2, 4, 6, 8, 17, 24–28]. In our series, total excision had a much better outcome than subtotal excision + postoperative RT, which had a quite high relapse rate in both CO and AO. The results of multivariate logistic regression also confirmed that total excision could significantly reduce relapse rate. The result that none of the 17 patients with CO accepting total excision suffered relapse enabled us to consider that total excision was adequate for CO. In patients with AO, one out of four patients with total excision and none of two patients with en bloc resection suffered relapse. Does en bloc resection have better outcome for AO than total excision? Obviously we could not come to the conclusion according to our series, but we also think en bloc resection is a good proposal for AO when it is possible.
Because OBL is a high vascular tumor, surgery is often accompanied by much intraoperative blood loss. In our study, intraoperative blood loss was 1,614 ml for all patients with spinal OBL and patients with AO suffered much more blood loss than those with CO, which could be attributed to the larger scope and subsequent larger resection range in the treatment of AO. Preoperative embolization has been reported to reduce intraoperative bleeding significantly and can be used as an adjunctive therapy for highly vascular spinal tumors, such as giant cell tumor (GCT) and aneurysmal bone cysts (ABC) [37–39]. Although we did not use it in this series, we still consider preoperative embolization a meaningful attempt for spinal OBL, which is a high vascular tumor and often accompanied by a huge amount of intraoperative blood loss.
The effect of radiotherapy is still in dispute. Some experts insisted that radiotherapy was of no benefit for spinal OBL, while others believed that it was an effective adjuvant treatment when total excision was not possible [4, 8, 17, 24, 29–34]. We clearly found that the protocol of subtotal excision + postoperative RT resulted in quite high relapse rate, so radiotherapy might provide little help for reducing relapse.
On analyzing the possible factors influencing relapse, we found that preoperative ALP, surgery protocol and tumor size (≥3/<3) were effective factors for relapse, while age and pathology type were not. Higher preoperative ALP, subtotal excision and tumor size (≥3) could result in higher relapse rate. Dorfman and some authors considered that AO had a higher recurrence rate and malignant transformation capacity, but there have been only a few case reports in literature [5, 11, 35, 36]. However, in our study, the pathology type could not significantly influence the relapse rate and no malignant transformation was found in patients with AO.
Conclusion
Compared with CO, AO is associated with relatively mild pain, but more spinal deformity and neurologic disorders, shorter duration of symptoms, wider range of involvement and larger size. Preoperative ALP and CT images can also provide help for distinguishing AO from CO.
As subtotal excision easily leads to relapse, total excision should be strived for whenever possible. Preoperative ALP, surgery protocol and tumor size (≥3/<3) can significantly influence relapse, while age and pathology type cannot.
Electronic supplementary material
Conflict of interest
None.
Footnotes
H. Yin, W. Zhou and H. Yu contributed equally to this work, and all the three authors can be regarded as first authors.
References
- 1.Greenspan A. Benign bone-forming lesions: osteoma, osteoid osteoma, and osteoblastoma. Clinical, imaging, pathologic, and differential considerations. Skeletal Radiol. 1993;22(7):485–500. doi: 10.1007/BF00209095. [DOI] [PubMed] [Google Scholar]
- 2.Arkader A, Dormans JP. Osteoblastoma in the skeletally immature. J Pediatr Orthop. 2008;28(5):555–560. doi: 10.1097/BPO.0b013e31817bb849. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe H. Benign osteoblastoma. Bull Hosp Joint Dis. 1956;17(2):141–151. [PubMed] [Google Scholar]
- 4.Lichtenstein L. Benign osteoblastoma; a category of osteoid and bone-forming tumors other than classical osteoid osteoma, which may be mistaken for giant-cell tumor or osteogenic sarcoma. Cancer. 1956;9:1044–1052. doi: 10.1002/1097-0142(195609/10)9:5<1044::AID-CNCR2820090523>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Dorfman H, Weiss S. Borderline osteoblastic tumors: problems in the differential diagnosis of aggressive osteoblastoma and low-grade osteosarcoma. Semin Diagn Pathol. 1984;1:215–234. [PubMed] [Google Scholar]
- 6.Zileli M, Çagli S, Basdemir G, Ersahin Y. Osteoid osteomas and osteoblastomas of the spine. Neurosurg Focus. 2003;15(5):E5. [PubMed] [Google Scholar]
- 7.Pettine KA, Klassen RA. Osteoid osteoma and osteoblastoma of the spine. J Bone Joint Surg Am. 1986;68(3):354–361. [PubMed] [Google Scholar]
- 8.Ozaki T, Liljenqvist U, Winkelmann W, et al. Osteoid osteoma and osteoblastoma of the spine: experiences with 22 patients. Clin Orthop Relat Res. 2002;397:394–402. doi: 10.1097/00003086-200204000-00046. [DOI] [PubMed] [Google Scholar]
- 9.Frassica FJ, Waltrip RL, McCarthy EF, Jr, et al. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27(3):559–574. [PubMed] [Google Scholar]
- 10.Lucas D, Unni KK, McLeod R, et al. Osteoblastoma: a clinicopathologic study of 306 cases. Hum Pathol. 1994;25(2):117–134. doi: 10.1016/0046-8177(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 11.Dorfman HD, Czerniak B. Bone cancers. Cancer. 1995;75(1):203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::AID-CNCR2820751308>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Harris H. The human alkaline phosphatases: what we know and what we don’t know. Clin Chim Acta. 1990;186(2):133–150. doi: 10.1016/0009-8981(90)90031-M. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson EE, Westfall SD, McDonald C, et al. An in vivo mouse reporter gene (human secreted alkaline phosphatase) model to monitor ovarian tumor growth and response to therapeutics. Cancer Chemother Pharmacol. 2002;49(2):93–100. doi: 10.1007/s00280-001-0396-0. [DOI] [PubMed] [Google Scholar]
- 14.Rassam MB, al-Bashir NN, al-Salihi AR, et al. Heat-stable alkaline phosphatase. A putative tumor marker of head and neck squamous cell carcinoma. Acta Oncol. 1995;34(1):49–52. doi: 10.3109/02841869509093638. [DOI] [PubMed] [Google Scholar]
- 15.Bao R, Selvakumaran M, Hamilton TC. Use of a surrogate marker (human secreted alkaline phosphatase) to monitor in vivo tumor growth and anticancer drug efficacy in ovarian cancer xenografts. Gynecol Oncol. 2000;78:373–379. doi: 10.1006/gyno.2000.5925. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Gao J, Xia YF, et al. Increased pretreatment levels of serum LDH and ALP as poor prognostic factors for nasopharyngeal carcinoma. Chin J Cancer. 2012;31(4):197–206. doi: 10.5732/cjc.011.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burn SC, Ansorge O, Zeller R, Drake JM. Management of osteoblastoma and osteoid osteoma of the spine in childhood. J Neurosurg Pediatr. 2009;4(5):434–438. doi: 10.3171/2009.6.PEDS08450. [DOI] [PubMed] [Google Scholar]
- 18.Ball WS, Towbin RB, Kaufman RA. Pediatric case of the day. Osteoblastoma of the lateral mass of C6 on the right. Radiographics. 1988;8(1):191–194. doi: 10.1148/radiographics.8.1.3353533. [DOI] [PubMed] [Google Scholar]
- 19.Chew FS, Pena CS, Keel SB. Cervical spine osteoblastoma. AJR Am J Roentgenol. 1998;171(5):1244. doi: 10.2214/ajr.171.5.9798854. [DOI] [PubMed] [Google Scholar]
- 20.Laban MM, Riutta JC. “Occult” roentgenographic osteoblastoma of the cervical spine. Am J Phys Med Rehabil. 2003;82(10):820–823. doi: 10.1097/01.PHM.0000087457.02231.FE. [DOI] [PubMed] [Google Scholar]
- 21.Kroon HM, Bloem JL, Holscher HC, et al. MR imaging of edema accompanying benign and malignant bone tumors. Skeletal Radiol. 1994;23(4):261–269. doi: 10.1007/BF02412359. [DOI] [PubMed] [Google Scholar]
- 22.McLeod RA, Dahlin DC, Beabout JW. The spectrum of osteoblastoma. AJR Am J Roentgenol. 1976;126(2):321–325. doi: 10.2214/ajr.126.2.321. [DOI] [PubMed] [Google Scholar]
- 23.Yamamura S, Sato K, Sugiura H, et al. Prostaglandin levels of primary bone tumor tissues correlate with peri tumoral edema demonstrated by magnetic resonance imaging. Cancer. 1997;79(2):255–261. doi: 10.1002/(SICI)1097-0142(19970115)79:2<255::AID-CNCR8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 24.Boriani S, Capanna R, Savini R, et al. Osteoblastoma of the spine. Clin Orthop Relat Res. 1992;278:37–45. [PubMed] [Google Scholar]
- 25.Nowparast B, Mesgarzadeh A, Lassemi I. Benign osteoblastoma of the mandible. A clinical–pathologic review and report of a case. Int J Oral Surg. 1979;8(5):386–390. doi: 10.1016/S0300-9785(79)80069-1. [DOI] [PubMed] [Google Scholar]
- 26.Bruneau M, Cornelius JF, George B. Osteoid osteomas and osteoblastomas of the occipitocervical junction. Spine (Phila Pa 1976) 2005;30(19):567–571. doi: 10.1097/01.brs.0000180489.50171.ee. [DOI] [PubMed] [Google Scholar]
- 27.Janin Y, Epstein JA, Carras R, Khan A. Osteoid osteomas and osteoblastomas of the spine. Neurosurgery. 1981;8(1):31–38. doi: 10.1227/00006123-198101000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Loh JK, Lin CK, Howng SL, et al. Primary spinal tumors in children. Clinical study. J Clin Neurosci. 2005;12(3):246–248. doi: 10.1016/j.jocn.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Boriani S, Amendola L, Bandiera S, et al. Staging and treatment of osteoblastoma in the mobile spine: a review of 51 cases. Eur Spine J. 2012;21(10):2003–2010. doi: 10.1007/s00586-012-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirwan EO, Hutton PA, Pozzo JL, et al. Osteoid osteoma and benign osteoblastoma of the spine. Clinical presentation and treatment. J Bone Joint Surg Br. 1984;66(1):21–26. doi: 10.1302/0301-620X.66B1.6693472. [DOI] [PubMed] [Google Scholar]
- 31.Tuy BE, John TK, Uglialoro AD, et al. Tumoral calcinosis presenting as neck pain and mass lesion of the cervical spine. Am J Orthop (Belle Mead NJ) 2008;37(11):191–195. [PubMed] [Google Scholar]
- 32.Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol. 1998;27(suppl 1):S91–S97. doi: 10.1016/S0720-048X(98)00049-7. [DOI] [PubMed] [Google Scholar]
- 33.Seki T, Fukuda H, Ishii Y, et al. Malignant transformation of benign osteoblastoma: a case report. J Bone Joint Surg. 1975;57:424–426. [PubMed] [Google Scholar]
- 34.Schajowicz F, Lemos C. Malignant osteoblastoma. J Bone Joint Surg. 1976;58:202–211. doi: 10.1302/0301-620X.58B2.932083. [DOI] [PubMed] [Google Scholar]
- 35.Harrington C, Accurso B, Kalmar JR, et al. Aggressive osteoblastoma of the maxilla: a case report and review of the literature. Head Neck Pathol. 2011;5(2):165–170. doi: 10.1007/s12105-010-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Britt JD, Murphey MD, Castle JT. Epithelioid osteoblastoma. Head and Neck Pathol. 2012;6(4):451–454. doi: 10.1007/s12105-012-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dick HM, Bigliani LU, Michelsen WJ, et al. Adjuvant arterial embolization in the treatment of benign primary bone tumors in children. Clin Orthop Relat Res. 1979;139:133–141. [PubMed] [Google Scholar]
- 38.Green JA, Bellemore MC, Marsden FW. Embolization in the treatment of aneurysmal bone cysts. J Pediatr Orthop. 1997;17(4):440–443. [PubMed] [Google Scholar]
- 39.Hess T, Kramann B, Schmidt E, et al. Use of preoperative vascular embolisation in spinal metastasis resection. Arch Orthop Trauma Surg. 1997;116(5):279–282. doi: 10.1007/BF00390053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


